

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123599 (ETORPHINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 25: 4689-92 (2015) Article DOI: 10.1016/j.bmcl.2015.06.092 BindingDB Entry DOI: 10.7270/Q2NV9M2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50123599 (ETORPHINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 25: 4689-92 (2015) Article DOI: 10.1016/j.bmcl.2015.06.092 BindingDB Entry DOI: 10.7270/Q2NV9M2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50189136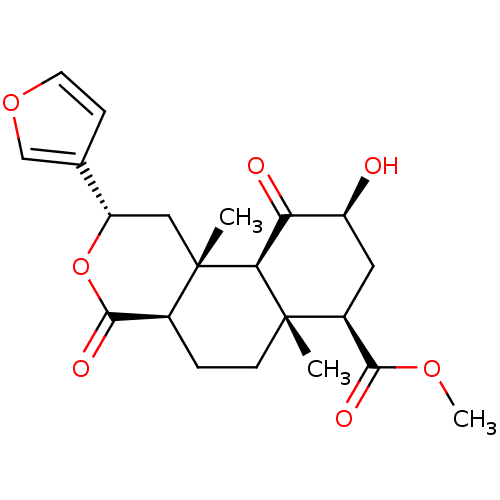 (CHEMBL424698 | Salvinorin B) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 25: 4689-92 (2015) Article DOI: 10.1016/j.bmcl.2015.06.092 BindingDB Entry DOI: 10.7270/Q2NV9M2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579543 (US11484525, Compound DD-144-ANTAGONIST-Covalent) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 5749-52 (2010) Article DOI: 10.1016/j.bmcl.2010.08.001 BindingDB Entry DOI: 10.7270/Q2S182QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50159165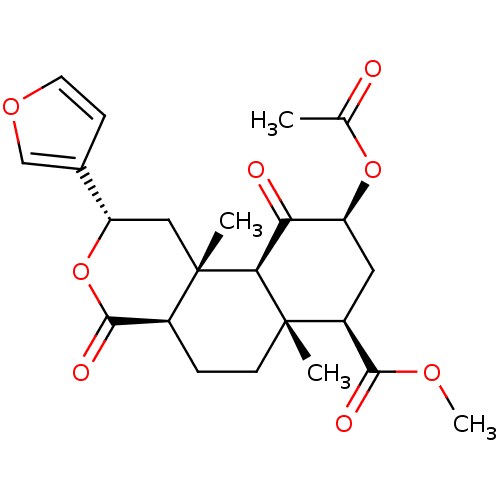 ((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(fura...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 25: 4689-92 (2015) Article DOI: 10.1016/j.bmcl.2015.06.092 BindingDB Entry DOI: 10.7270/Q2NV9M2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 25: 4689-92 (2015) Article DOI: 10.1016/j.bmcl.2015.06.092 BindingDB Entry DOI: 10.7270/Q2NV9M2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50123599 (ETORPHINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from FLAG-tagged mouse delta opioid receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 25: 4689-92 (2015) Article DOI: 10.1016/j.bmcl.2015.06.092 BindingDB Entry DOI: 10.7270/Q2NV9M2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579486 (US11484525, Compound Naloxone) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579540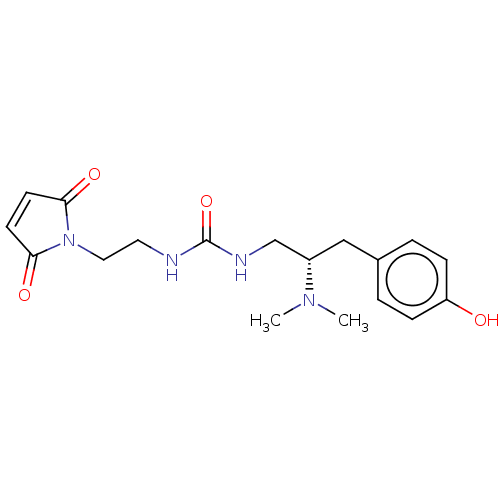 (US11484525, Compound DD-120-D1-covalent-compound) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579538 (US11484525, Compound BD-131LR) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579486 (US11484525, Compound Naloxone) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579515 (US11484525, Compound DD-88B-S) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50123598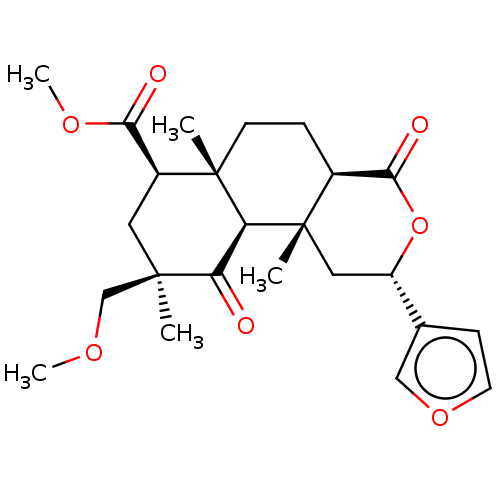 (CHEMBL3622713) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 25: 4689-92 (2015) Article DOI: 10.1016/j.bmcl.2015.06.092 BindingDB Entry DOI: 10.7270/Q2NV9M2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579541 (US11484525, Compound DD-138) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579544 (US11484525, Compound DD-158) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM462406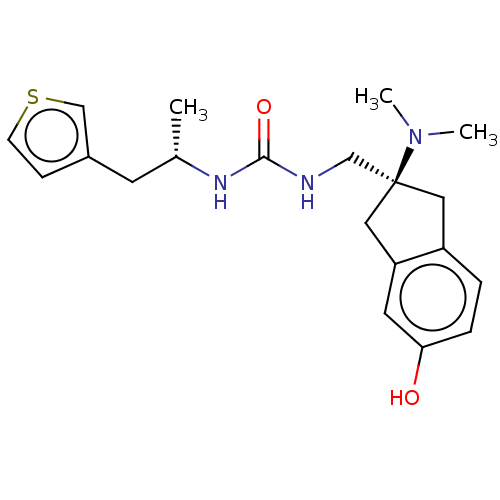 (US10780078, Compound DD 297A) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article US Patent | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description This study supports a structure-based approach for GPCR ligand discovery. These new chemotypes may stabilize receptor conformations not explored prev... | Bioorg Med Chem Lett 5: 2707-12 (1995) Article DOI: 10.1016/0960-894X(95)00461-2 BindingDB Entry DOI: 10.7270/Q2XW4GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579542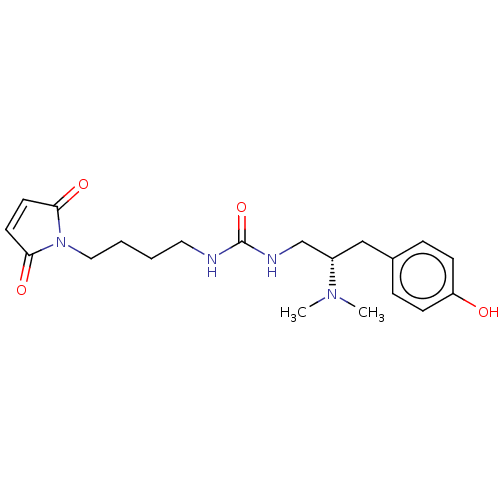 (US11484525, Compound DD-139) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579537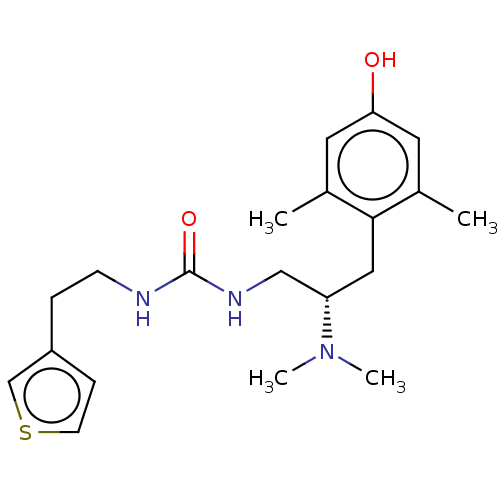 (US11484525, Compound DD-34L-ANTAGONIST) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM462406 (US10780078, Compound DD 297A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article US Patent | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description This study supports a structure-based approach for GPCR ligand discovery. These new chemotypes may stabilize receptor conformations not explored prev... | Bioorg Med Chem Lett 5: 2707-12 (1995) Article DOI: 10.1016/0960-894X(95)00461-2 BindingDB Entry DOI: 10.7270/Q2XW4GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579539 (US11484525, Compound BD-131LS) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579527 (US11484525, Compound DD-57L) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579522 (US11484525, Compound DD-63L-LB-S) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579539 (US11484525, Compound BD-131LS) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579538 (US11484525, Compound BD-131LR) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579520 (US11484525, Compound DD-63L-LA-R | US11484525, Com...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM579538 (US11484525, Compound BD-131LR) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50518036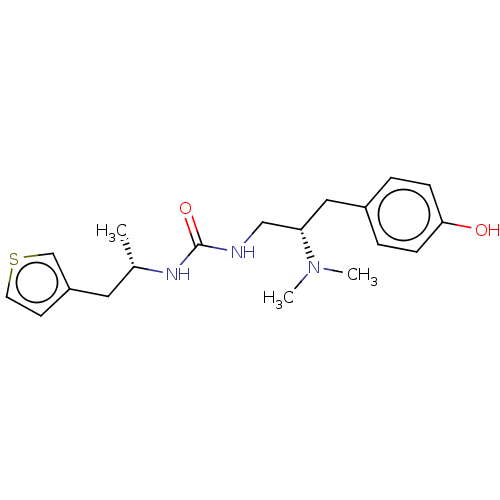 (CHEMBL4467777 | US11484525, Compound BD-122LS-PZM2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579543 (US11484525, Compound DD-144-ANTAGONIST-Covalent) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579520 (US11484525, Compound DD-63L-LA-R | US11484525, Com...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50123597 (CHEMBL3622712) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 25: 4689-92 (2015) Article DOI: 10.1016/j.bmcl.2015.06.092 BindingDB Entry DOI: 10.7270/Q2NV9M2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579522 (US11484525, Compound DD-63L-LB-S) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579557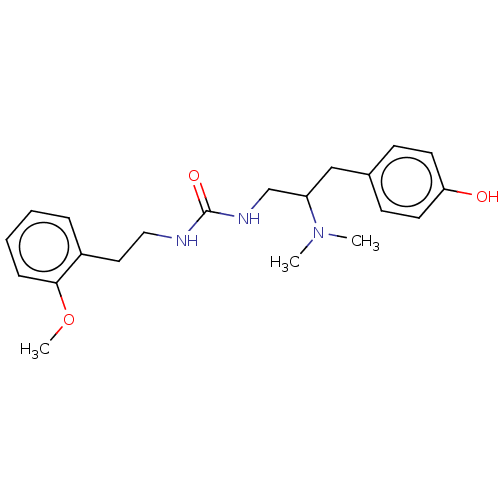 (US11484525, Compound DD198) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579486 (US11484525, Compound Naloxone) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50327511 ((3S,4aR,4bS,8R,8aR,10aR)-3-Furan-3-yl-4a,8a-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 5749-52 (2010) Article DOI: 10.1016/j.bmcl.2010.08.001 BindingDB Entry DOI: 10.7270/Q2S182QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | US Patent | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579533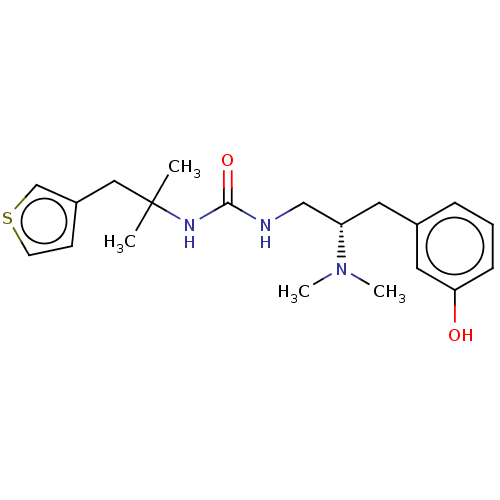 (US11484525, Compound DD-46L) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM50493818 (Oliceridine | TRV-130 | US11124523, Comparative Co...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB US Patent | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579562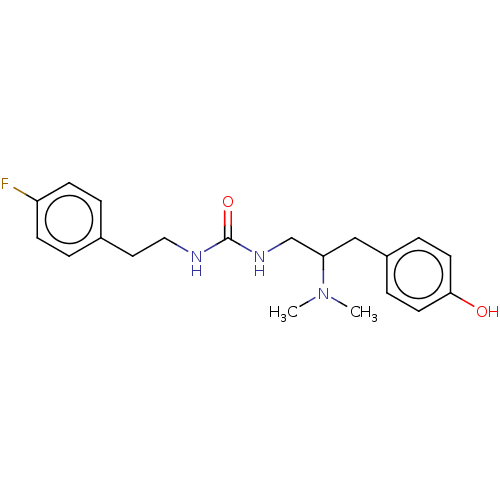 (US11484525, Compound DDLM-15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579537 (US11484525, Compound DD-34L-ANTAGONIST) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579503 (US11484525, Compound DD-81) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579533 (US11484525, Compound DD-46L) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579485 (US11484525, Compound Morphine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579532 (US11484525, Compound DD-47LS) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579546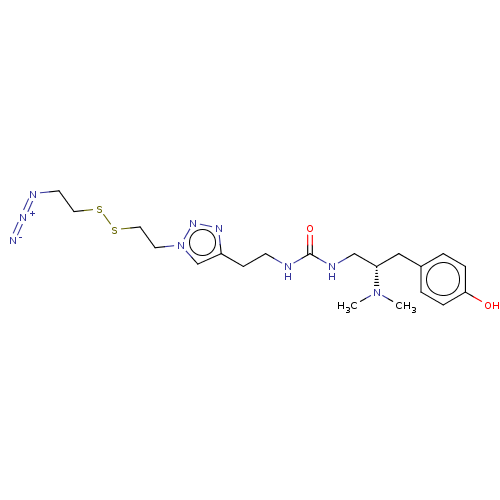 (US11484525, Compound DD-159) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579523 (US11484525, Compound DD-53-LS) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM462395 (US10780078, Compound DD 272B) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article US Patent | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description This study supports a structure-based approach for GPCR ligand discovery. These new chemotypes may stabilize receptor conformations not explored prev... | Bioorg Med Chem Lett 5: 2707-12 (1995) Article DOI: 10.1016/0960-894X(95)00461-2 BindingDB Entry DOI: 10.7270/Q2XW4GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579561 (US11484525, Compound DDLM-10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579519 (US11484525, Compound DD-61L-B-S) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM50518036 (CHEMBL4467777 | US11484525, Compound BD-122LS-PZM2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 822 total ) | Next | Last >> |