Found 62 hits with Last Name = 'desvergne' and Initial = 'a'
Found 62 hits with Last Name = 'desvergne' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430967

(CHEMBL2337848)Show SMILES CC[C@H](C)C(=O)C(=O)N[C@H]1Cc2ccc(O)c(c2)-c2cccc3c2NC(=O)[C@@]3(O)[C@H](O)[C@H](NC(=O)[C@H](CC(N)=O)CC1=O)C(=O)N\C=C/C |r| Show InChI InChI=1S/C34H39N5O10/c1-4-11-36-31(46)27-29(44)34(49)21-8-6-7-19(26(21)39-33(34)48)20-12-17(9-10-23(20)40)13-22(37-32(47)28(43)16(3)5-2)24(41)14-18(15-25(35)42)30(45)38-27/h4,6-12,16,18,22,27,29,40,44,49H,5,13-15H2,1-3H3,(H2,35,42)(H,36,46)(H,37,47)(H,38,45)(H,39,48)/b11-4-/t16-,18-,22-,27-,29+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430968
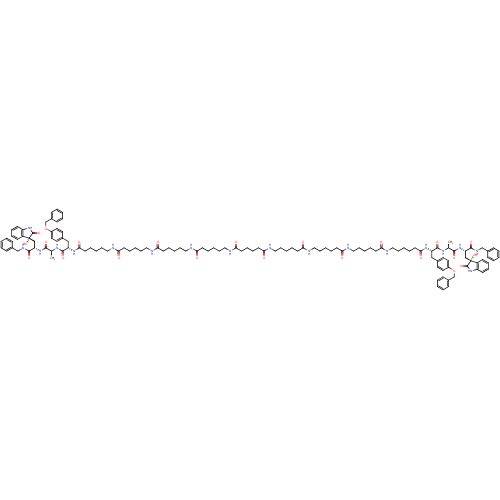
(CHEMBL2337847)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C128H172N18O22/c1-91(119(157)143-107(121(159)137-87-95-45-15-3-16-46-95)85-127(165)101-53-31-33-55-103(101)145-125(127)163)139-123(161)105(83-93-67-71-99(72-68-93)167-89-97-49-19-5-20-50-97)141-117(155)65-29-13-43-81-133-113(151)61-25-9-39-77-129-109(147)57-23-7-37-75-131-111(149)59-27-11-41-79-135-115(153)63-35-36-64-116(154)136-80-42-12-28-60-112(150)132-76-38-8-24-58-110(148)130-78-40-10-26-62-114(152)134-82-44-14-30-66-118(156)142-106(84-94-69-73-100(74-70-94)168-90-98-51-21-6-22-52-98)124(162)140-92(2)120(158)144-108(122(160)138-88-96-47-17-4-18-48-96)86-128(166)102-54-32-34-56-104(102)146-126(128)164/h3-6,15-22,31-34,45-56,67-74,91-92,105-108,165-166H,7-14,23-30,35-44,57-66,75-90H2,1-2H3,(H,129,147)(H,130,148)(H,131,149)(H,132,150)(H,133,151)(H,134,152)(H,135,153)(H,136,154)(H,137,159)(H,138,160)(H,139,161)(H,140,162)(H,141,155)(H,142,156)(H,143,157)(H,144,158)(H,145,163)(H,146,164)/t91-,92-,105-,106-,107-,108-,127+,128+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430962
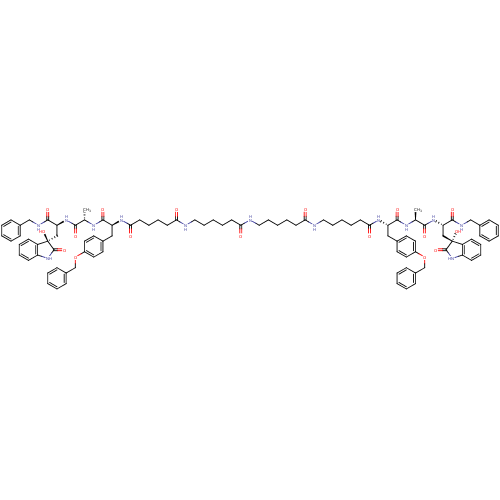
(CHEMBL2337843)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C98H117N13O17/c1-66(89(117)108-82(91(119)102-62-70-30-10-3-11-31-70)60-97(125)76-38-21-23-40-78(76)110-95(97)123)104-93(121)80(58-68-47-51-74(52-48-68)127-64-72-34-14-5-15-35-72)106-87(115)45-20-9-29-57-100-85(113)43-18-7-27-55-99-84(112)42-19-8-28-56-101-86(114)44-25-26-46-88(116)107-81(59-69-49-53-75(54-50-69)128-65-73-36-16-6-17-37-73)94(122)105-67(2)90(118)109-83(92(120)103-63-71-32-12-4-13-33-71)61-98(126)77-39-22-24-41-79(77)111-96(98)124/h3-6,10-17,21-24,30-41,47-54,66-67,80-83,125-126H,7-9,18-20,25-29,42-46,55-65H2,1-2H3,(H,99,112)(H,100,113)(H,101,114)(H,102,119)(H,103,120)(H,104,121)(H,105,122)(H,106,115)(H,107,116)(H,108,117)(H,109,118)(H,110,123)(H,111,124)/t66-,67-,80-,81-,82-,83-,97+,98+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430961

(CHEMBL2337844)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C104H128N14O18/c1-71(95(125)115-87(97(127)109-67-75-33-11-3-12-34-75)65-103(133)81-41-23-25-43-83(81)117-101(103)131)111-99(129)85(63-73-51-55-79(56-52-73)135-69-77-37-15-5-16-38-77)113-93(123)49-22-10-32-62-107-91(121)47-20-8-30-60-105-89(119)45-19-7-29-59-106-90(120)46-21-9-31-61-108-92(122)48-27-28-50-94(124)114-86(64-74-53-57-80(58-54-74)136-70-78-39-17-6-18-40-78)100(130)112-72(2)96(126)116-88(98(128)110-68-76-35-13-4-14-36-76)66-104(134)82-42-24-26-44-84(82)118-102(104)132/h3-6,11-18,23-26,33-44,51-58,71-72,85-88,133-134H,7-10,19-22,27-32,45-50,59-70H2,1-2H3,(H,105,119)(H,106,120)(H,107,121)(H,108,122)(H,109,127)(H,110,128)(H,111,129)(H,112,130)(H,113,123)(H,114,124)(H,115,125)(H,116,126)(H,117,131)(H,118,132)/t71-,72-,85-,86-,87-,88-,103+,104+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430969

(CHEMBL2337846)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C116H150N16O20/c1-81(107(141)129-97(109(143)123-77-85-39-13-3-14-40-85)75-115(149)91-47-27-29-49-93(91)131-113(115)147)125-111(145)95(73-83-59-63-89(64-60-83)151-79-87-43-17-5-18-44-87)127-105(139)57-25-11-37-71-119-101(135)53-21-7-33-67-117-99(133)51-23-9-35-69-121-103(137)55-31-32-56-104(138)122-70-36-10-24-52-100(134)118-68-34-8-22-54-102(136)120-72-38-12-26-58-106(140)128-96(74-84-61-65-90(66-62-84)152-80-88-45-19-6-20-46-88)112(146)126-82(2)108(142)130-98(110(144)124-78-86-41-15-4-16-42-86)76-116(150)92-48-28-30-50-94(92)132-114(116)148/h3-6,13-20,27-30,39-50,59-66,81-82,95-98,149-150H,7-12,21-26,31-38,51-58,67-80H2,1-2H3,(H,117,133)(H,118,134)(H,119,135)(H,120,136)(H,121,137)(H,122,138)(H,123,143)(H,124,144)(H,125,145)(H,126,146)(H,127,139)(H,128,140)(H,129,141)(H,130,142)(H,131,147)(H,132,148)/t81-,82-,95-,96-,97-,98-,115+,116+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430970

(CHEMBL2337845)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C110H139N15O19/c1-76(101(133)122-92(103(135)116-72-80-36-12-3-13-37-80)70-109(141)86-44-25-27-46-88(86)124-107(109)139)118-105(137)90(68-78-55-59-84(60-56-78)143-74-82-40-16-5-17-41-82)120-99(131)53-24-11-35-67-114-97(129)51-22-9-33-65-112-95(127)49-20-7-31-63-111-94(126)48-21-8-32-64-113-96(128)50-23-10-34-66-115-98(130)52-29-30-54-100(132)121-91(69-79-57-61-85(62-58-79)144-75-83-42-18-6-19-43-83)106(138)119-77(2)102(134)123-93(104(136)117-73-81-38-14-4-15-39-81)71-110(142)87-45-26-28-47-89(87)125-108(110)140/h3-6,12-19,25-28,36-47,55-62,76-77,90-93,141-142H,7-11,20-24,29-35,48-54,63-75H2,1-2H3,(H,111,126)(H,112,127)(H,113,128)(H,114,129)(H,115,130)(H,116,135)(H,117,136)(H,118,137)(H,119,138)(H,120,131)(H,121,132)(H,122,133)(H,123,134)(H,124,139)(H,125,140)/t76-,77-,90-,91-,92-,93-,109+,110+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50485783
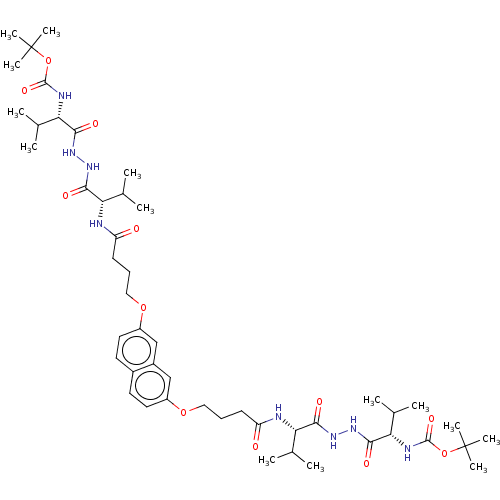
(CHEMBL2164408)Show SMILES CC(C)[C@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)NNC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C)cc2c1)C(=O)NNC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C |r| Show InChI InChI=1S/C48H76N8O12/c1-27(2)37(41(59)53-55-43(61)39(29(5)6)51-45(63)67-47(9,10)11)49-35(57)17-15-23-65-33-21-19-31-20-22-34(26-32(31)25-33)66-24-16-18-36(58)50-38(28(3)4)42(60)54-56-44(62)40(30(7)8)52-46(64)68-48(12,13)14/h19-22,25-30,37-40H,15-18,23-24H2,1-14H3,(H,49,57)(H,50,58)(H,51,63)(H,52,64)(H,53,59)(H,54,60)(H,55,61)(H,56,62)/t37-,38-,39-,40-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... |
J Med Chem 55: 6762-75 (2012)
Article DOI: 10.1021/jm300181j
BindingDB Entry DOI: 10.7270/Q2125WJF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430963
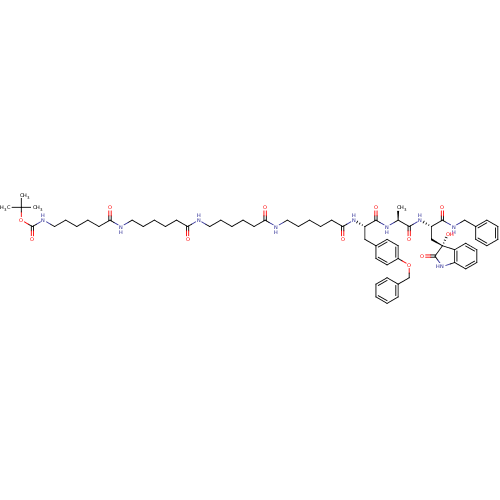
(CHEMBL2337842)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)OC(C)(C)C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C66H91N9O12/c1-47(60(80)74-55(61(81)71-45-49-25-11-5-12-26-49)44-66(85)52-29-19-20-30-53(52)75-63(66)83)72-62(82)54(43-48-35-37-51(38-36-48)86-46-50-27-13-6-14-28-50)73-59(79)34-18-10-23-41-69-57(77)32-16-8-21-39-67-56(76)31-15-7-22-40-68-58(78)33-17-9-24-42-70-64(84)87-65(2,3)4/h5-6,11-14,19-20,25-30,35-38,47,54-55,85H,7-10,15-18,21-24,31-34,39-46H2,1-4H3,(H,67,76)(H,68,78)(H,69,77)(H,70,84)(H,71,81)(H,72,82)(H,73,79)(H,74,80)(H,75,83)/t47-,54-,55-,66+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50485784
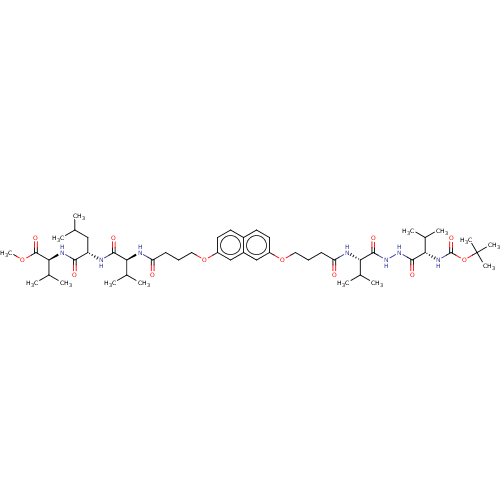
(CHEMBL2164407)Show SMILES COC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)NNC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C)cc2c1)C(C)C)C(C)C |r| Show InChI InChI=1S/C50H79N7O12/c1-28(2)25-37(44(60)54-43(32(9)10)48(64)66-14)51-45(61)40(29(3)4)52-38(58)17-15-23-67-35-21-19-33-20-22-36(27-34(33)26-35)68-24-16-18-39(59)53-41(30(5)6)46(62)56-57-47(63)42(31(7)8)55-49(65)69-50(11,12)13/h19-22,26-32,37,40-43H,15-18,23-25H2,1-14H3,(H,51,61)(H,52,58)(H,53,59)(H,54,60)(H,55,65)(H,56,62)(H,57,63)/t37-,40-,41-,42-,43-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... |
J Med Chem 55: 6762-75 (2012)
Article DOI: 10.1021/jm300181j
BindingDB Entry DOI: 10.7270/Q2125WJF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430964
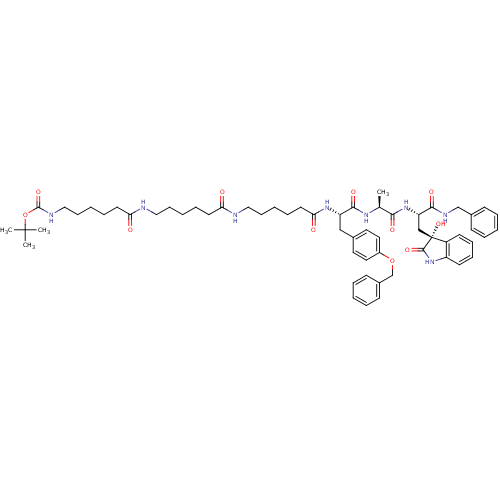
(CHEMBL2337841)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)OC(C)(C)C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C60H80N8O11/c1-42(54(72)67-50(55(73)64-40-44-22-10-5-11-23-44)39-60(77)47-26-17-18-27-48(47)68-57(60)75)65-56(74)49(38-43-31-33-46(34-32-43)78-41-45-24-12-6-13-25-45)66-53(71)30-16-9-20-36-62-51(69)28-14-7-19-35-61-52(70)29-15-8-21-37-63-58(76)79-59(2,3)4/h5-6,10-13,17-18,22-27,31-34,42,49-50,77H,7-9,14-16,19-21,28-30,35-41H2,1-4H3,(H,61,70)(H,62,69)(H,63,76)(H,64,73)(H,65,74)(H,66,71)(H,67,72)(H,68,75)/t42-,49-,50-,60+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50485785
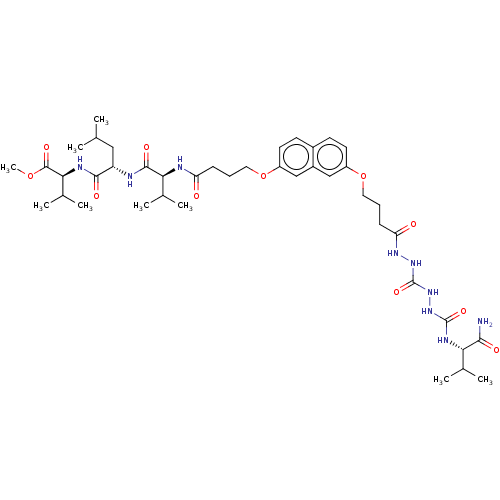
(CHEMBL2164409)Show SMILES COC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)NNC(=O)NNC(=O)N[C@@H](C(C)C)C(N)=O)cc2c1)C(C)C)C(C)C |r| Show InChI InChI=1S/C42H65N9O11/c1-23(2)20-31(38(55)46-36(26(7)8)40(57)60-9)44-39(56)35(25(5)6)45-32(52)12-10-18-61-29-16-14-27-15-17-30(22-28(27)21-29)62-19-11-13-33(53)48-50-42(59)51-49-41(58)47-34(24(3)4)37(43)54/h14-17,21-26,31,34-36H,10-13,18-20H2,1-9H3,(H2,43,54)(H,44,56)(H,45,52)(H,46,55)(H,48,53)(H2,47,49,58)(H2,50,51,59)/t31-,34-,35-,36-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... |
J Med Chem 55: 6762-75 (2012)
Article DOI: 10.1021/jm300181j
BindingDB Entry DOI: 10.7270/Q2125WJF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430963
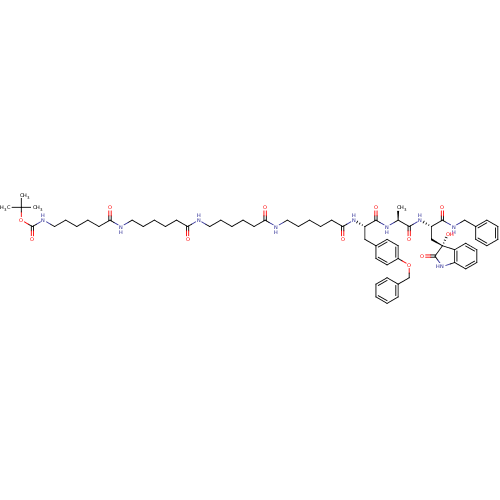
(CHEMBL2337842)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)OC(C)(C)C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C66H91N9O12/c1-47(60(80)74-55(61(81)71-45-49-25-11-5-12-26-49)44-66(85)52-29-19-20-30-53(52)75-63(66)83)72-62(82)54(43-48-35-37-51(38-36-48)86-46-50-27-13-6-14-28-50)73-59(79)34-18-10-23-41-69-57(77)32-16-8-21-39-67-56(76)31-15-7-22-40-68-58(78)33-17-9-24-42-70-64(84)87-65(2,3)4/h5-6,11-14,19-20,25-30,35-38,47,54-55,85H,7-10,15-18,21-24,31-34,39-46H2,1-4H3,(H,67,76)(H,68,78)(H,69,77)(H,70,84)(H,71,81)(H,72,82)(H,73,79)(H,74,80)(H,75,83)/t47-,54-,55-,66+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430964

(CHEMBL2337841)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)OC(C)(C)C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C60H80N8O11/c1-42(54(72)67-50(55(73)64-40-44-22-10-5-11-23-44)39-60(77)47-26-17-18-27-48(47)68-57(60)75)65-56(74)49(38-43-31-33-46(34-32-43)78-41-45-24-12-6-13-25-45)66-53(71)30-16-9-20-36-62-51(69)28-14-7-19-35-61-52(70)29-15-8-21-37-63-58(76)79-59(2,3)4/h5-6,10-13,17-18,22-27,31-34,42,49-50,77H,7-9,14-16,19-21,28-30,35-41H2,1-4H3,(H,61,70)(H,62,69)(H,63,76)(H,64,73)(H,65,74)(H,66,71)(H,67,72)(H,68,75)/t42-,49-,50-,60+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50074687
(2-[2-(2-{4-[7-(3-{1-[1-(1-Methoxycarbonyl-2-methyl...)Show SMILES COC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCOc1ccc2ccc(OCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC)cc2c1)C(C)C)C(C)C Show InChI InChI=1S/C52H82N6O12/c1-29(2)25-39(47(61)57-45(33(9)10)51(65)67-13)53-49(63)43(31(5)6)55-41(59)17-15-23-69-37-21-19-35-20-22-38(28-36(35)27-37)70-24-16-18-42(60)56-44(32(7)8)50(64)54-40(26-30(3)4)48(62)58-46(34(11)12)52(66)68-14/h19-22,27-34,39-40,43-46H,15-18,23-26H2,1-14H3,(H,53,63)(H,54,64)(H,55,59)(H,56,60)(H,57,61)(H,58,62)/t39-,40-,43-,44-,45-,46-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... |
J Med Chem 55: 6762-75 (2012)
Article DOI: 10.1021/jm300181j
BindingDB Entry DOI: 10.7270/Q2125WJF |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50485786
(CHEMBL2164074)Show SMILES CC(C)[C@H](NC(=O)NNC(=O)NNC(=O)CCCOc1ccc2ccc(OCCCC(=O)NNC(=O)NNC(=O)N[C@@H](C(C)C)C(N)=O)cc2c1)C(N)=O |r| Show InChI InChI=1S/C32H48N12O10/c1-17(2)25(27(33)47)35-29(49)39-43-31(51)41-37-23(45)7-5-13-53-21-11-9-19-10-12-22(16-20(19)15-21)54-14-6-8-24(46)38-42-32(52)44-40-30(50)36-26(18(3)4)28(34)48/h9-12,15-18,25-26H,5-8,13-14H2,1-4H3,(H2,33,47)(H2,34,48)(H,37,45)(H,38,46)(H2,35,39,49)(H2,36,40,50)(H2,41,43,51)(H2,42,44,52)/t25-,26-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... |
J Med Chem 55: 6762-75 (2012)
Article DOI: 10.1021/jm300181j
BindingDB Entry DOI: 10.7270/Q2125WJF |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430966

(CHEMBL2337849)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C42H47N5O8/c1-27(36(48)45-35(37(49)43-25-29-13-7-5-8-14-29)24-42(53)32-17-11-12-18-33(32)46-39(42)51)44-38(50)34(47-40(52)55-41(2,3)4)23-28-19-21-31(22-20-28)54-26-30-15-9-6-10-16-30/h5-22,27,34-35,53H,23-26H2,1-4H3,(H,43,49)(H,44,50)(H,45,48)(H,46,51)(H,47,52)/t27-,34-,35-,42+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430966

(CHEMBL2337849)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C42H47N5O8/c1-27(36(48)45-35(37(49)43-25-29-13-7-5-8-14-29)24-42(53)32-17-11-12-18-33(32)46-39(42)51)44-38(50)34(47-40(52)55-41(2,3)4)23-28-19-21-31(22-20-28)54-26-30-15-9-6-10-16-30/h5-22,27,34-35,53H,23-26H2,1-4H3,(H,43,49)(H,44,50)(H,45,48)(H,46,51)(H,47,52)/t27-,34-,35-,42+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430965

(CHEMBL2337850)Show SMILES C[C@H](NC(=O)[C@@H](N)Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C37H39N5O6/c1-24(40-34(44)30(38)20-25-16-18-28(19-17-25)48-23-27-12-6-3-7-13-27)33(43)41-32(35(45)39-22-26-10-4-2-5-11-26)21-37(47)29-14-8-9-15-31(29)42-36(37)46/h2-19,24,30,32,47H,20-23,38H2,1H3,(H,39,45)(H,40,44)(H,41,43)(H,42,46)/t24-,30-,32-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed ... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430965

(CHEMBL2337850)Show SMILES C[C@H](NC(=O)[C@@H](N)Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C37H39N5O6/c1-24(40-34(44)30(38)20-25-16-18-28(19-17-25)48-23-27-12-6-3-7-13-27)33(43)41-32(35(45)39-22-26-10-4-2-5-11-26)21-37(47)29-14-8-9-15-31(29)42-36(37)46/h2-19,24,30,32,47H,20-23,38H2,1H3,(H,39,45)(H,40,44)(H,41,43)(H,42,46)/t24-,30-,32-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed ... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430967

(CHEMBL2337848)Show SMILES CC[C@H](C)C(=O)C(=O)N[C@H]1Cc2ccc(O)c(c2)-c2cccc3c2NC(=O)[C@@]3(O)[C@H](O)[C@H](NC(=O)[C@H](CC(N)=O)CC1=O)C(=O)N\C=C/C |r| Show InChI InChI=1S/C34H39N5O10/c1-4-11-36-31(46)27-29(44)34(49)21-8-6-7-19(26(21)39-33(34)48)20-12-17(9-10-23(20)40)13-22(37-32(47)28(43)16(3)5-2)24(41)14-18(15-25(35)42)30(45)38-27/h4,6-12,16,18,22,27,29,40,44,49H,5,13-15H2,1-3H3,(H2,35,42)(H,36,46)(H,37,47)(H,38,45)(H,39,48)/b11-4-/t16-,18-,22-,27-,29+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as remaining ac... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50069985
((S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C26H41N3O5/c1-17(2)12-21(15-30)27-24(31)22(13-18(3)4)28-25(32)23(14-19(5)6)29-26(33)34-16-20-10-8-7-9-11-20/h7-11,15,17-19,21-23H,12-14,16H2,1-6H3,(H,27,31)(H,28,32)(H,29,33)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of proteasome beta-5 subunit in HEK293 cells using Suc-LLVY-Glo as substrate incubated for 2 hrs prior to su... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50430961

(CHEMBL2337844)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C104H128N14O18/c1-71(95(125)115-87(97(127)109-67-75-33-11-3-12-34-75)65-103(133)81-41-23-25-43-83(81)117-101(103)131)111-99(129)85(63-73-51-55-79(56-52-73)135-69-77-37-15-5-16-38-77)113-93(123)49-22-10-32-62-107-91(121)47-20-8-30-60-105-89(119)45-19-7-29-59-106-90(120)46-21-9-31-61-108-92(122)48-27-28-50-94(124)114-86(64-74-53-57-80(58-54-74)136-70-78-39-17-6-18-40-78)100(130)112-72(2)96(126)116-88(98(128)110-68-76-35-13-4-14-36-76)66-104(134)82-42-24-26-44-84(82)118-102(104)132/h3-6,11-18,23-26,33-44,51-58,71-72,85-88,133-134H,7-10,19-22,27-32,45-50,59-70H2,1-2H3,(H,105,119)(H,106,120)(H,107,121)(H,108,122)(H,109,127)(H,110,128)(H,111,129)(H,112,130)(H,113,123)(H,114,124)(H,115,125)(H,116,126)(H,117,131)(H,118,132)/t71-,72-,85-,86-,87-,88-,103+,104+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of human constitutive 20s proteasome beta-2 subunit using Boc-LRR-AMC as substrate assessed as remaining activity... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50430968
(CHEMBL2337847)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C128H172N18O22/c1-91(119(157)143-107(121(159)137-87-95-45-15-3-16-46-95)85-127(165)101-53-31-33-55-103(101)145-125(127)163)139-123(161)105(83-93-67-71-99(72-68-93)167-89-97-49-19-5-20-50-97)141-117(155)65-29-13-43-81-133-113(151)61-25-9-39-77-129-109(147)57-23-7-37-75-131-111(149)59-27-11-41-79-135-115(153)63-35-36-64-116(154)136-80-42-12-28-60-112(150)132-76-38-8-24-58-110(148)130-78-40-10-26-62-114(152)134-82-44-14-30-66-118(156)142-106(84-94-69-73-100(74-70-94)168-90-98-51-21-6-22-52-98)124(162)140-92(2)120(158)144-108(122(160)138-88-96-47-17-4-18-48-96)86-128(166)102-54-32-34-56-104(102)146-126(128)164/h3-6,15-22,31-34,45-56,67-74,91-92,105-108,165-166H,7-14,23-30,35-44,57-66,75-90H2,1-2H3,(H,129,147)(H,130,148)(H,131,149)(H,132,150)(H,133,151)(H,134,152)(H,135,153)(H,136,154)(H,137,159)(H,138,160)(H,139,161)(H,140,162)(H,141,155)(H,142,156)(H,143,157)(H,144,158)(H,145,163)(H,146,164)/t91-,92-,105-,106-,107-,108-,127+,128+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of human constitutive 20s proteasome beta-2 subunit using Boc-LRR-AMC as substrate assessed as remaining activity... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50430969

(CHEMBL2337846)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C116H150N16O20/c1-81(107(141)129-97(109(143)123-77-85-39-13-3-14-40-85)75-115(149)91-47-27-29-49-93(91)131-113(115)147)125-111(145)95(73-83-59-63-89(64-60-83)151-79-87-43-17-5-18-44-87)127-105(139)57-25-11-37-71-119-101(135)53-21-7-33-67-117-99(133)51-23-9-35-69-121-103(137)55-31-32-56-104(138)122-70-36-10-24-52-100(134)118-68-34-8-22-54-102(136)120-72-38-12-26-58-106(140)128-96(74-84-61-65-90(66-62-84)152-80-88-45-19-6-20-46-88)112(146)126-82(2)108(142)130-98(110(144)124-78-86-41-15-4-16-42-86)76-116(150)92-48-28-30-50-94(92)132-114(116)148/h3-6,13-20,27-30,39-50,59-66,81-82,95-98,149-150H,7-12,21-26,31-38,51-58,67-80H2,1-2H3,(H,117,133)(H,118,134)(H,119,135)(H,120,136)(H,121,137)(H,122,138)(H,123,143)(H,124,144)(H,125,145)(H,126,146)(H,127,139)(H,128,140)(H,129,141)(H,130,142)(H,131,147)(H,132,148)/t81-,82-,95-,96-,97-,98-,115+,116+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of human constitutive 20s proteasome beta-2 subunit using Boc-LRR-AMC as substrate assessed as remaining activity... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50430970

(CHEMBL2337845)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C110H139N15O19/c1-76(101(133)122-92(103(135)116-72-80-36-12-3-13-37-80)70-109(141)86-44-25-27-46-88(86)124-107(109)139)118-105(137)90(68-78-55-59-84(60-56-78)143-74-82-40-16-5-17-41-82)120-99(131)53-24-11-35-67-114-97(129)51-22-9-33-65-112-95(127)49-20-7-31-63-111-94(126)48-21-8-32-64-113-96(128)50-23-10-34-66-115-98(130)52-29-30-54-100(132)121-91(69-79-57-61-85(62-58-79)144-75-83-42-18-6-19-43-83)106(138)119-77(2)102(134)123-93(104(136)117-73-81-38-14-4-15-39-81)71-110(142)87-45-26-28-47-89(87)125-108(110)140/h3-6,12-19,25-28,36-47,55-62,76-77,90-93,141-142H,7-11,20-24,29-35,48-54,63-75H2,1-2H3,(H,111,126)(H,112,127)(H,113,128)(H,114,129)(H,115,130)(H,116,135)(H,117,136)(H,118,137)(H,119,138)(H,120,131)(H,121,132)(H,122,133)(H,123,134)(H,124,139)(H,125,140)/t76-,77-,90-,91-,92-,93-,109+,110+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of human constitutive 20s proteasome beta-2 subunit using Boc-LRR-AMC as substrate assessed as remaining activity... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430969

(CHEMBL2337846)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C116H150N16O20/c1-81(107(141)129-97(109(143)123-77-85-39-13-3-14-40-85)75-115(149)91-47-27-29-49-93(91)131-113(115)147)125-111(145)95(73-83-59-63-89(64-60-83)151-79-87-43-17-5-18-44-87)127-105(139)57-25-11-37-71-119-101(135)53-21-7-33-67-117-99(133)51-23-9-35-69-121-103(137)55-31-32-56-104(138)122-70-36-10-24-52-100(134)118-68-34-8-22-54-102(136)120-72-38-12-26-58-106(140)128-96(74-84-61-65-90(66-62-84)152-80-88-45-19-6-20-46-88)112(146)126-82(2)108(142)130-98(110(144)124-78-86-41-15-4-16-42-86)76-116(150)92-48-28-30-50-94(92)132-114(116)148/h3-6,13-20,27-30,39-50,59-66,81-82,95-98,149-150H,7-12,21-26,31-38,51-58,67-80H2,1-2H3,(H,117,133)(H,118,134)(H,119,135)(H,120,136)(H,121,137)(H,122,138)(H,123,143)(H,124,144)(H,125,145)(H,126,146)(H,127,139)(H,128,140)(H,129,141)(H,130,142)(H,131,147)(H,132,148)/t81-,82-,95-,96-,97-,98-,115+,116+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as remaining ac... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50430962

(CHEMBL2337843)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C98H117N13O17/c1-66(89(117)108-82(91(119)102-62-70-30-10-3-11-31-70)60-97(125)76-38-21-23-40-78(76)110-95(97)123)104-93(121)80(58-68-47-51-74(52-48-68)127-64-72-34-14-5-15-35-72)106-87(115)45-20-9-29-57-100-85(113)43-18-7-27-55-99-84(112)42-19-8-28-56-101-86(114)44-25-26-46-88(116)107-81(59-69-49-53-75(54-50-69)128-65-73-36-16-6-17-37-73)94(122)105-67(2)90(118)109-83(92(120)103-63-71-32-12-4-13-33-71)61-98(126)77-39-22-24-41-79(77)111-96(98)124/h3-6,10-17,21-24,30-41,47-54,66-67,80-83,125-126H,7-9,18-20,25-29,42-46,55-65H2,1-2H3,(H,99,112)(H,100,113)(H,101,114)(H,102,119)(H,103,120)(H,104,121)(H,105,122)(H,106,115)(H,107,116)(H,108,117)(H,109,118)(H,110,123)(H,111,124)/t66-,67-,80-,81-,82-,83-,97+,98+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of human constitutive 20s proteasome beta-2 subunit using Boc-LRR-AMC as substrate assessed as remaining activity... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430968
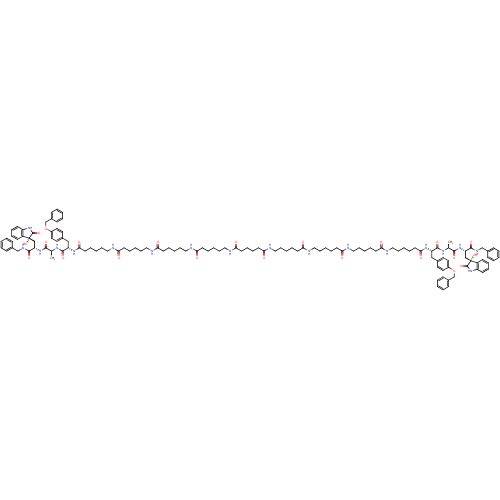
(CHEMBL2337847)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C128H172N18O22/c1-91(119(157)143-107(121(159)137-87-95-45-15-3-16-46-95)85-127(165)101-53-31-33-55-103(101)145-125(127)163)139-123(161)105(83-93-67-71-99(72-68-93)167-89-97-49-19-5-20-50-97)141-117(155)65-29-13-43-81-133-113(151)61-25-9-39-77-129-109(147)57-23-7-37-75-131-111(149)59-27-11-41-79-135-115(153)63-35-36-64-116(154)136-80-42-12-28-60-112(150)132-76-38-8-24-58-110(148)130-78-40-10-26-62-114(152)134-82-44-14-30-66-118(156)142-106(84-94-69-73-100(74-70-94)168-90-98-51-21-6-22-52-98)124(162)140-92(2)120(158)144-108(122(160)138-88-96-47-17-4-18-48-96)86-128(166)102-54-32-34-56-104(102)146-126(128)164/h3-6,15-22,31-34,45-56,67-74,91-92,105-108,165-166H,7-14,23-30,35-44,57-66,75-90H2,1-2H3,(H,129,147)(H,130,148)(H,131,149)(H,132,150)(H,133,151)(H,134,152)(H,135,153)(H,136,154)(H,137,159)(H,138,160)(H,139,161)(H,140,162)(H,141,155)(H,142,156)(H,143,157)(H,144,158)(H,145,163)(H,146,164)/t91-,92-,105-,106-,107-,108-,127+,128+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as remaining ac... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430970

(CHEMBL2337845)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C110H139N15O19/c1-76(101(133)122-92(103(135)116-72-80-36-12-3-13-37-80)70-109(141)86-44-25-27-46-88(86)124-107(109)139)118-105(137)90(68-78-55-59-84(60-56-78)143-74-82-40-16-5-17-41-82)120-99(131)53-24-11-35-67-114-97(129)51-22-9-33-65-112-95(127)49-20-7-31-63-111-94(126)48-21-8-32-64-113-96(128)50-23-10-34-66-115-98(130)52-29-30-54-100(132)121-91(69-79-57-61-85(62-58-79)144-75-83-42-18-6-19-43-83)106(138)119-77(2)102(134)123-93(104(136)117-73-81-38-14-4-15-39-81)71-110(142)87-45-26-28-47-89(87)125-108(110)140/h3-6,12-19,25-28,36-47,55-62,76-77,90-93,141-142H,7-11,20-24,29-35,48-54,63-75H2,1-2H3,(H,111,126)(H,112,127)(H,113,128)(H,114,129)(H,115,130)(H,116,135)(H,117,136)(H,118,137)(H,119,138)(H,120,131)(H,121,132)(H,122,133)(H,123,134)(H,124,139)(H,125,140)/t76-,77-,90-,91-,92-,93-,109+,110+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as remaining ac... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50370717
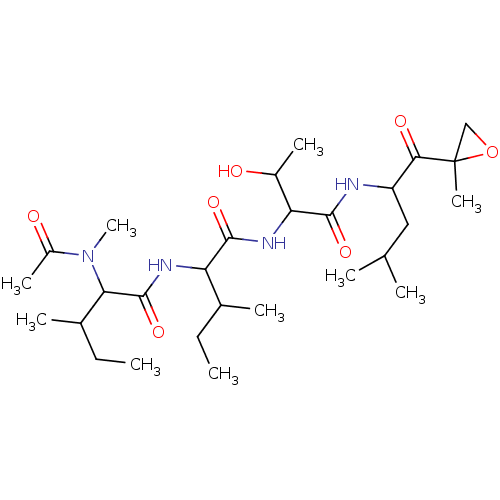
(EPOXOMYCIN | Epoxomicin)Show SMILES CCC(C)C(NC(=O)C(C(C)CC)N(C)C(C)=O)C(=O)NC(C(C)O)C(=O)NC(CC(C)C)C(=O)C1(C)CO1 Show InChI InChI=1S/C28H50N4O7/c1-11-16(5)21(30-27(38)23(17(6)12-2)32(10)19(8)34)25(36)31-22(18(7)33)26(37)29-20(13-15(3)4)24(35)28(9)14-39-28/h15-18,20-23,33H,11-14H2,1-10H3,(H,29,37)(H,30,38)(H,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of proteasome beta-5 subunit in HEK293 cells using Suc-LLVY-Glo as substrate incubated for 2 hrs prior to su... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430961

(CHEMBL2337844)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C104H128N14O18/c1-71(95(125)115-87(97(127)109-67-75-33-11-3-12-34-75)65-103(133)81-41-23-25-43-83(81)117-101(103)131)111-99(129)85(63-73-51-55-79(56-52-73)135-69-77-37-15-5-16-38-77)113-93(123)49-22-10-32-62-107-91(121)47-20-8-30-60-105-89(119)45-19-7-29-59-106-90(120)46-21-9-31-61-108-92(122)48-27-28-50-94(124)114-86(64-74-53-57-80(58-54-74)136-70-78-39-17-6-18-40-78)100(130)112-72(2)96(126)116-88(98(128)110-68-76-35-13-4-14-36-76)66-104(134)82-42-24-26-44-84(82)118-102(104)132/h3-6,11-18,23-26,33-44,51-58,71-72,85-88,133-134H,7-10,19-22,27-32,45-50,59-70H2,1-2H3,(H,105,119)(H,106,120)(H,107,121)(H,108,122)(H,109,127)(H,110,128)(H,111,129)(H,112,130)(H,113,123)(H,114,124)(H,115,125)(H,116,126)(H,117,131)(H,118,132)/t71-,72-,85-,86-,87-,88-,103+,104+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as remaining ac... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430962

(CHEMBL2337843)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C98H117N13O17/c1-66(89(117)108-82(91(119)102-62-70-30-10-3-11-31-70)60-97(125)76-38-21-23-40-78(76)110-95(97)123)104-93(121)80(58-68-47-51-74(52-48-68)127-64-72-34-14-5-15-35-72)106-87(115)45-20-9-29-57-100-85(113)43-18-7-27-55-99-84(112)42-19-8-28-56-101-86(114)44-25-26-46-88(116)107-81(59-69-49-53-75(54-50-69)128-65-73-36-16-6-17-37-73)94(122)105-67(2)90(118)109-83(92(120)103-63-71-32-12-4-13-33-71)61-98(126)77-39-22-24-41-79(77)111-96(98)124/h3-6,10-17,21-24,30-41,47-54,66-67,80-83,125-126H,7-9,18-20,25-29,42-46,55-65H2,1-2H3,(H,99,112)(H,100,113)(H,101,114)(H,102,119)(H,103,120)(H,104,121)(H,105,122)(H,106,115)(H,107,116)(H,108,117)(H,109,118)(H,110,123)(H,111,124)/t66-,67-,80-,81-,82-,83-,97+,98+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as remaining ac... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50430967

(CHEMBL2337848)Show SMILES CC[C@H](C)C(=O)C(=O)N[C@H]1Cc2ccc(O)c(c2)-c2cccc3c2NC(=O)[C@@]3(O)[C@H](O)[C@H](NC(=O)[C@H](CC(N)=O)CC1=O)C(=O)N\C=C/C |r| Show InChI InChI=1S/C34H39N5O10/c1-4-11-36-31(46)27-29(44)34(49)21-8-6-7-19(26(21)39-33(34)48)20-12-17(9-10-23(20)40)13-22(37-32(47)28(43)16(3)5-2)24(41)14-18(15-25(35)42)30(45)38-27/h4,6-12,16,18,22,27,29,40,44,49H,5,13-15H2,1-3H3,(H2,35,42)(H,36,46)(H,37,47)(H,38,45)(H,39,48)/b11-4-/t16-,18-,22-,27-,29+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of postacid activity of human constitutive 20s proteasome beta-1 subunit using Z-LLE-betaNA as substrate assessed as remaining activity in... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50430967

(CHEMBL2337848)Show SMILES CC[C@H](C)C(=O)C(=O)N[C@H]1Cc2ccc(O)c(c2)-c2cccc3c2NC(=O)[C@@]3(O)[C@H](O)[C@H](NC(=O)[C@H](CC(N)=O)CC1=O)C(=O)N\C=C/C |r| Show InChI InChI=1S/C34H39N5O10/c1-4-11-36-31(46)27-29(44)34(49)21-8-6-7-19(26(21)39-33(34)48)20-12-17(9-10-23(20)40)13-22(37-32(47)28(43)16(3)5-2)24(41)14-18(15-25(35)42)30(45)38-27/h4,6-12,16,18,22,27,29,40,44,49H,5,13-15H2,1-3H3,(H2,35,42)(H,36,46)(H,37,47)(H,38,45)(H,39,48)/b11-4-/t16-,18-,22-,27-,29+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of human constitutive 20s proteasome beta-2 subunit using Boc-LRR-AMC as substrate assessed as remaining activity... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430964

(CHEMBL2337841)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)OC(C)(C)C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C60H80N8O11/c1-42(54(72)67-50(55(73)64-40-44-22-10-5-11-23-44)39-60(77)47-26-17-18-27-48(47)68-57(60)75)65-56(74)49(38-43-31-33-46(34-32-43)78-41-45-24-12-6-13-25-45)66-53(71)30-16-9-20-36-62-51(69)28-14-7-19-35-61-52(70)29-15-8-21-37-63-58(76)79-59(2,3)4/h5-6,10-13,17-18,22-27,31-34,42,49-50,77H,7-9,14-16,19-21,28-30,35-41H2,1-4H3,(H,61,70)(H,62,69)(H,63,76)(H,64,73)(H,65,74)(H,66,71)(H,67,72)(H,68,75)/t42-,49-,50-,60+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 252 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of proteasome beta-5 subunit in HEK293 cells using Suc-LLVY-Glo as substrate incubated for 2 hrs prior to su... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50370717

(EPOXOMYCIN | Epoxomicin)Show SMILES CCC(C)C(NC(=O)C(C(C)CC)N(C)C(C)=O)C(=O)NC(C(C)O)C(=O)NC(CC(C)C)C(=O)C1(C)CO1 Show InChI InChI=1S/C28H50N4O7/c1-11-16(5)21(30-27(38)23(17(6)12-2)32(10)19(8)34)25(36)31-22(18(7)33)26(37)29-20(13-15(3)4)24(35)28(9)14-39-28/h15-18,20-23,33H,11-14H2,1-10H3,(H,29,37)(H,30,38)(H,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of postacid activity of 20s proteasome beta-1 subunit in HEK293 cells using Z-nLPnLD-Glo as substrate incubated for 2 hrs prior to substra... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50430968
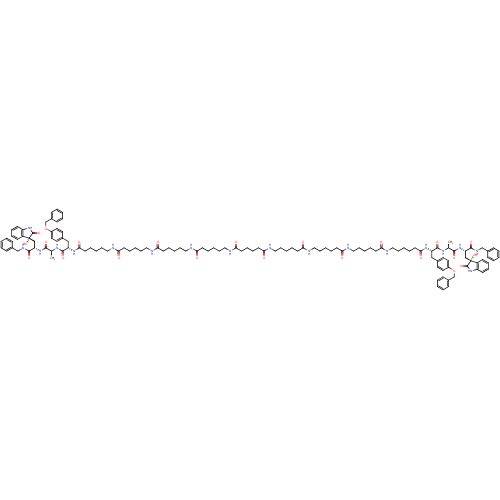
(CHEMBL2337847)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C128H172N18O22/c1-91(119(157)143-107(121(159)137-87-95-45-15-3-16-46-95)85-127(165)101-53-31-33-55-103(101)145-125(127)163)139-123(161)105(83-93-67-71-99(72-68-93)167-89-97-49-19-5-20-50-97)141-117(155)65-29-13-43-81-133-113(151)61-25-9-39-77-129-109(147)57-23-7-37-75-131-111(149)59-27-11-41-79-135-115(153)63-35-36-64-116(154)136-80-42-12-28-60-112(150)132-76-38-8-24-58-110(148)130-78-40-10-26-62-114(152)134-82-44-14-30-66-118(156)142-106(84-94-69-73-100(74-70-94)168-90-98-51-21-6-22-52-98)124(162)140-92(2)120(158)144-108(122(160)138-88-96-47-17-4-18-48-96)86-128(166)102-54-32-34-56-104(102)146-126(128)164/h3-6,15-22,31-34,45-56,67-74,91-92,105-108,165-166H,7-14,23-30,35-44,57-66,75-90H2,1-2H3,(H,129,147)(H,130,148)(H,131,149)(H,132,150)(H,133,151)(H,134,152)(H,135,153)(H,136,154)(H,137,159)(H,138,160)(H,139,161)(H,140,162)(H,141,155)(H,142,156)(H,143,157)(H,144,158)(H,145,163)(H,146,164)/t91-,92-,105-,106-,107-,108-,127+,128+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 336 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of postacid activity of human constitutive 20s proteasome beta-1 subunit using Z-LLE-betaNA as substrate assessed as remaining activity in... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50430969

(CHEMBL2337846)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C116H150N16O20/c1-81(107(141)129-97(109(143)123-77-85-39-13-3-14-40-85)75-115(149)91-47-27-29-49-93(91)131-113(115)147)125-111(145)95(73-83-59-63-89(64-60-83)151-79-87-43-17-5-18-44-87)127-105(139)57-25-11-37-71-119-101(135)53-21-7-33-67-117-99(133)51-23-9-35-69-121-103(137)55-31-32-56-104(138)122-70-36-10-24-52-100(134)118-68-34-8-22-54-102(136)120-72-38-12-26-58-106(140)128-96(74-84-61-65-90(66-62-84)152-80-88-45-19-6-20-46-88)112(146)126-82(2)108(142)130-98(110(144)124-78-86-41-15-4-16-42-86)76-116(150)92-48-28-30-50-94(92)132-114(116)148/h3-6,13-20,27-30,39-50,59-66,81-82,95-98,149-150H,7-12,21-26,31-38,51-58,67-80H2,1-2H3,(H,117,133)(H,118,134)(H,119,135)(H,120,136)(H,121,137)(H,122,138)(H,123,143)(H,124,144)(H,125,145)(H,126,146)(H,127,139)(H,128,140)(H,129,141)(H,130,142)(H,131,147)(H,132,148)/t81-,82-,95-,96-,97-,98-,115+,116+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 346 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of postacid activity of human constitutive 20s proteasome beta-1 subunit using Z-LLE-betaNA as substrate assessed as remaining activity in... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50430961

(CHEMBL2337844)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C104H128N14O18/c1-71(95(125)115-87(97(127)109-67-75-33-11-3-12-34-75)65-103(133)81-41-23-25-43-83(81)117-101(103)131)111-99(129)85(63-73-51-55-79(56-52-73)135-69-77-37-15-5-16-38-77)113-93(123)49-22-10-32-62-107-91(121)47-20-8-30-60-105-89(119)45-19-7-29-59-106-90(120)46-21-9-31-61-108-92(122)48-27-28-50-94(124)114-86(64-74-53-57-80(58-54-74)136-70-78-39-17-6-18-40-78)100(130)112-72(2)96(126)116-88(98(128)110-68-76-35-13-4-14-36-76)66-104(134)82-42-24-26-44-84(82)118-102(104)132/h3-6,11-18,23-26,33-44,51-58,71-72,85-88,133-134H,7-10,19-22,27-32,45-50,59-70H2,1-2H3,(H,105,119)(H,106,120)(H,107,121)(H,108,122)(H,109,127)(H,110,128)(H,111,129)(H,112,130)(H,113,123)(H,114,124)(H,115,125)(H,116,126)(H,117,131)(H,118,132)/t71-,72-,85-,86-,87-,88-,103+,104+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of postacid activity of human constitutive 20s proteasome beta-1 subunit using Z-LLE-betaNA as substrate assessed as remaining activity in... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50430970

(CHEMBL2337845)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C110H139N15O19/c1-76(101(133)122-92(103(135)116-72-80-36-12-3-13-37-80)70-109(141)86-44-25-27-46-88(86)124-107(109)139)118-105(137)90(68-78-55-59-84(60-56-78)143-74-82-40-16-5-17-41-82)120-99(131)53-24-11-35-67-114-97(129)51-22-9-33-65-112-95(127)49-20-7-31-63-111-94(126)48-21-8-32-64-113-96(128)50-23-10-34-66-115-98(130)52-29-30-54-100(132)121-91(69-79-57-61-85(62-58-79)144-75-83-42-18-6-19-43-83)106(138)119-77(2)102(134)123-93(104(136)117-73-81-38-14-4-15-39-81)71-110(142)87-45-26-28-47-89(87)125-108(110)140/h3-6,12-19,25-28,36-47,55-62,76-77,90-93,141-142H,7-11,20-24,29-35,48-54,63-75H2,1-2H3,(H,111,126)(H,112,127)(H,113,128)(H,114,129)(H,115,130)(H,116,135)(H,117,136)(H,118,137)(H,119,138)(H,120,131)(H,121,132)(H,122,133)(H,123,134)(H,124,139)(H,125,140)/t76-,77-,90-,91-,92-,93-,109+,110+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 448 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of postacid activity of human constitutive 20s proteasome beta-1 subunit using Z-LLE-betaNA as substrate assessed as remaining activity in... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430964

(CHEMBL2337841)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)OC(C)(C)C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C60H80N8O11/c1-42(54(72)67-50(55(73)64-40-44-22-10-5-11-23-44)39-60(77)47-26-17-18-27-48(47)68-57(60)75)65-56(74)49(38-43-31-33-46(34-32-43)78-41-45-24-12-6-13-25-45)66-53(71)30-16-9-20-36-62-51(69)28-14-7-19-35-61-52(70)29-15-8-21-37-63-58(76)79-59(2,3)4/h5-6,10-13,17-18,22-27,31-34,42,49-50,77H,7-9,14-16,19-21,28-30,35-41H2,1-4H3,(H,61,70)(H,62,69)(H,63,76)(H,64,73)(H,65,74)(H,66,71)(H,67,72)(H,68,75)/t42-,49-,50-,60+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as remaining ac... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430970

(CHEMBL2337845)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C110H139N15O19/c1-76(101(133)122-92(103(135)116-72-80-36-12-3-13-37-80)70-109(141)86-44-25-27-46-88(86)124-107(109)139)118-105(137)90(68-78-55-59-84(60-56-78)143-74-82-40-16-5-17-41-82)120-99(131)53-24-11-35-67-114-97(129)51-22-9-33-65-112-95(127)49-20-7-31-63-111-94(126)48-21-8-32-64-113-96(128)50-23-10-34-66-115-98(130)52-29-30-54-100(132)121-91(69-79-57-61-85(62-58-79)144-75-83-42-18-6-19-43-83)106(138)119-77(2)102(134)123-93(104(136)117-73-81-38-14-4-15-39-81)71-110(142)87-45-26-28-47-89(87)125-108(110)140/h3-6,12-19,25-28,36-47,55-62,76-77,90-93,141-142H,7-11,20-24,29-35,48-54,63-75H2,1-2H3,(H,111,126)(H,112,127)(H,113,128)(H,114,129)(H,115,130)(H,116,135)(H,117,136)(H,118,137)(H,119,138)(H,120,131)(H,121,132)(H,122,133)(H,123,134)(H,124,139)(H,125,140)/t76-,77-,90-,91-,92-,93-,109+,110+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 472 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of proteasome beta-5 subunit in HEK293 cells using Suc-LLVY-Glo as substrate incubated for 2 hrs prior to su... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430969

(CHEMBL2337846)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C116H150N16O20/c1-81(107(141)129-97(109(143)123-77-85-39-13-3-14-40-85)75-115(149)91-47-27-29-49-93(91)131-113(115)147)125-111(145)95(73-83-59-63-89(64-60-83)151-79-87-43-17-5-18-44-87)127-105(139)57-25-11-37-71-119-101(135)53-21-7-33-67-117-99(133)51-23-9-35-69-121-103(137)55-31-32-56-104(138)122-70-36-10-24-52-100(134)118-68-34-8-22-54-102(136)120-72-38-12-26-58-106(140)128-96(74-84-61-65-90(66-62-84)152-80-88-45-19-6-20-46-88)112(146)126-82(2)108(142)130-98(110(144)124-78-86-41-15-4-16-42-86)76-116(150)92-48-28-30-50-94(92)132-114(116)148/h3-6,13-20,27-30,39-50,59-66,81-82,95-98,149-150H,7-12,21-26,31-38,51-58,67-80H2,1-2H3,(H,117,133)(H,118,134)(H,119,135)(H,120,136)(H,121,137)(H,122,138)(H,123,143)(H,124,144)(H,125,145)(H,126,146)(H,127,139)(H,128,140)(H,129,141)(H,130,142)(H,131,147)(H,132,148)/t81-,82-,95-,96-,97-,98-,115+,116+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 476 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of proteasome beta-5 subunit in HEK293 cells using Suc-LLVY-Glo as substrate incubated for 2 hrs prior to su... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430962

(CHEMBL2337843)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C98H117N13O17/c1-66(89(117)108-82(91(119)102-62-70-30-10-3-11-31-70)60-97(125)76-38-21-23-40-78(76)110-95(97)123)104-93(121)80(58-68-47-51-74(52-48-68)127-64-72-34-14-5-15-35-72)106-87(115)45-20-9-29-57-100-85(113)43-18-7-27-55-99-84(112)42-19-8-28-56-101-86(114)44-25-26-46-88(116)107-81(59-69-49-53-75(54-50-69)128-65-73-36-16-6-17-37-73)94(122)105-67(2)90(118)109-83(92(120)103-63-71-32-12-4-13-33-71)61-98(126)77-39-22-24-41-79(77)111-96(98)124/h3-6,10-17,21-24,30-41,47-54,66-67,80-83,125-126H,7-9,18-20,25-29,42-46,55-65H2,1-2H3,(H,99,112)(H,100,113)(H,101,114)(H,102,119)(H,103,120)(H,104,121)(H,105,122)(H,106,115)(H,107,116)(H,108,117)(H,109,118)(H,110,123)(H,111,124)/t66-,67-,80-,81-,82-,83-,97+,98+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 541 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of proteasome beta-5 subunit in HEK293 cells using Suc-LLVY-Glo as substrate incubated for 2 hrs prior to su... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50430962

(CHEMBL2337843)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C98H117N13O17/c1-66(89(117)108-82(91(119)102-62-70-30-10-3-11-31-70)60-97(125)76-38-21-23-40-78(76)110-95(97)123)104-93(121)80(58-68-47-51-74(52-48-68)127-64-72-34-14-5-15-35-72)106-87(115)45-20-9-29-57-100-85(113)43-18-7-27-55-99-84(112)42-19-8-28-56-101-86(114)44-25-26-46-88(116)107-81(59-69-49-53-75(54-50-69)128-65-73-36-16-6-17-37-73)94(122)105-67(2)90(118)109-83(92(120)103-63-71-32-12-4-13-33-71)61-98(126)77-39-22-24-41-79(77)111-96(98)124/h3-6,10-17,21-24,30-41,47-54,66-67,80-83,125-126H,7-9,18-20,25-29,42-46,55-65H2,1-2H3,(H,99,112)(H,100,113)(H,101,114)(H,102,119)(H,103,120)(H,104,121)(H,105,122)(H,106,115)(H,107,116)(H,108,117)(H,109,118)(H,110,123)(H,111,124)/t66-,67-,80-,81-,82-,83-,97+,98+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of postacid activity of human constitutive 20s proteasome beta-1 subunit using Z-LLE-betaNA as substrate assessed as remaining activity in... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50069985

((S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C26H41N3O5/c1-17(2)12-21(15-30)27-24(31)22(13-18(3)4)28-25(32)23(14-19(5)6)29-26(33)34-16-20-10-8-7-9-11-20/h7-11,15,17-19,21-23H,12-14,16H2,1-6H3,(H,27,31)(H,28,32)(H,29,33)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of postacid activity of 20s proteasome beta-1 subunit in HEK293 cells using Z-nLPnLD-Glo as substrate incubated for 2 hrs prior to substra... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50430963
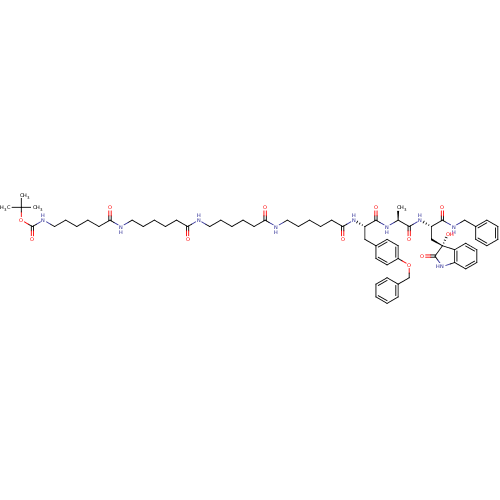
(CHEMBL2337842)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)OC(C)(C)C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C66H91N9O12/c1-47(60(80)74-55(61(81)71-45-49-25-11-5-12-26-49)44-66(85)52-29-19-20-30-53(52)75-63(66)83)72-62(82)54(43-48-35-37-51(38-36-48)86-46-50-27-13-6-14-28-50)73-59(79)34-18-10-23-41-69-57(77)32-16-8-21-39-67-56(76)31-15-7-22-40-68-58(78)33-17-9-24-42-70-64(84)87-65(2,3)4/h5-6,11-14,19-20,25-30,35-38,47,54-55,85H,7-10,15-18,21-24,31-34,39-46H2,1-4H3,(H,67,76)(H,68,78)(H,69,77)(H,70,84)(H,71,81)(H,72,82)(H,73,79)(H,74,80)(H,75,83)/t47-,54-,55-,66+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of human constitutive 20s proteasome beta-2 subunit using Boc-LRR-AMC as substrate assessed as remaining activity... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430963
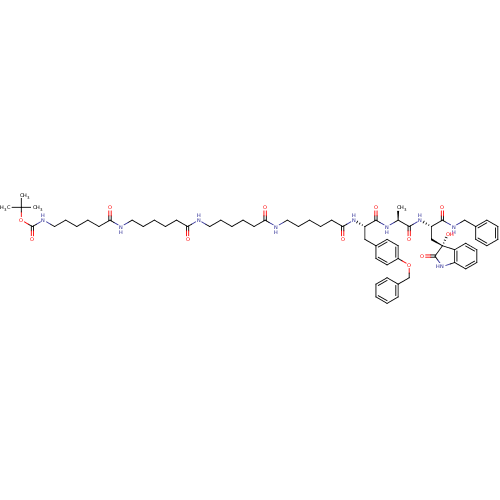
(CHEMBL2337842)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)OC(C)(C)C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C66H91N9O12/c1-47(60(80)74-55(61(81)71-45-49-25-11-5-12-26-49)44-66(85)52-29-19-20-30-53(52)75-63(66)83)72-62(82)54(43-48-35-37-51(38-36-48)86-46-50-27-13-6-14-28-50)73-59(79)34-18-10-23-41-69-57(77)32-16-8-21-39-67-56(76)31-15-7-22-40-68-58(78)33-17-9-24-42-70-64(84)87-65(2,3)4/h5-6,11-14,19-20,25-30,35-38,47,54-55,85H,7-10,15-18,21-24,31-34,39-46H2,1-4H3,(H,67,76)(H,68,78)(H,69,77)(H,70,84)(H,71,81)(H,72,82)(H,73,79)(H,74,80)(H,75,83)/t47-,54-,55-,66+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 707 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as remaining ac... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430961

(CHEMBL2337844)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C104H128N14O18/c1-71(95(125)115-87(97(127)109-67-75-33-11-3-12-34-75)65-103(133)81-41-23-25-43-83(81)117-101(103)131)111-99(129)85(63-73-51-55-79(56-52-73)135-69-77-37-15-5-16-38-77)113-93(123)49-22-10-32-62-107-91(121)47-20-8-30-60-105-89(119)45-19-7-29-59-106-90(120)46-21-9-31-61-108-92(122)48-27-28-50-94(124)114-86(64-74-53-57-80(58-54-74)136-70-78-39-17-6-18-40-78)100(130)112-72(2)96(126)116-88(98(128)110-68-76-35-13-4-14-36-76)66-104(134)82-42-24-26-44-84(82)118-102(104)132/h3-6,11-18,23-26,33-44,51-58,71-72,85-88,133-134H,7-10,19-22,27-32,45-50,59-70H2,1-2H3,(H,105,119)(H,106,120)(H,107,121)(H,108,122)(H,109,127)(H,110,128)(H,111,129)(H,112,130)(H,113,123)(H,114,124)(H,115,125)(H,116,126)(H,117,131)(H,118,132)/t71-,72-,85-,86-,87-,88-,103+,104+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 756 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of proteasome beta-5 subunit in HEK293 cells using Suc-LLVY-Glo as substrate incubated for 2 hrs prior to su... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50430964

(CHEMBL2337841)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)OC(C)(C)C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C60H80N8O11/c1-42(54(72)67-50(55(73)64-40-44-22-10-5-11-23-44)39-60(77)47-26-17-18-27-48(47)68-57(60)75)65-56(74)49(38-43-31-33-46(34-32-43)78-41-45-24-12-6-13-25-45)66-53(71)30-16-9-20-36-62-51(69)28-14-7-19-35-61-52(70)29-15-8-21-37-63-58(76)79-59(2,3)4/h5-6,10-13,17-18,22-27,31-34,42,49-50,77H,7-9,14-16,19-21,28-30,35-41H2,1-4H3,(H,61,70)(H,62,69)(H,63,76)(H,64,73)(H,65,74)(H,66,71)(H,67,72)(H,68,75)/t42-,49-,50-,60+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 867 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of human constitutive 20s proteasome beta-2 subunit using Boc-LRR-AMC as substrate assessed as remaining activity... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data