

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50226181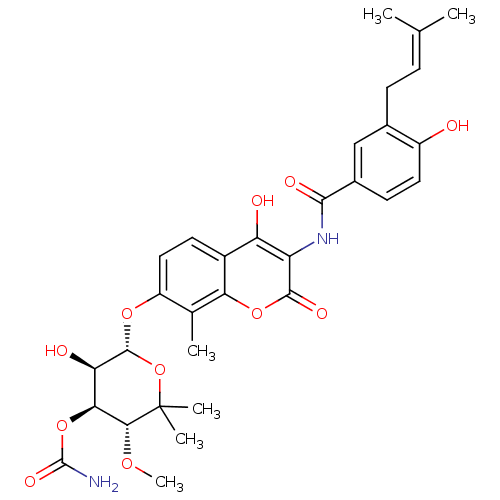 ((3R,4S,5R,6R)-5-hydroxy-6-(4-hydroxy-3-(4-hydroxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase-mediated supercoiling of relaxed DNA using pBR 322 as substrate by agarose gel electrophoresis analysi... | Bioorg Med Chem 22: 5970-87 (2014) Article DOI: 10.1016/j.bmc.2014.09.008 BindingDB Entry DOI: 10.7270/Q23X89MP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50226181 ((3R,4S,5R,6R)-5-hydroxy-6-(4-hydroxy-3-(4-hydroxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase B ATPase activity | Bioorg Med Chem 22: 5970-87 (2014) Article DOI: 10.1016/j.bmc.2014.09.008 BindingDB Entry DOI: 10.7270/Q23X89MP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147729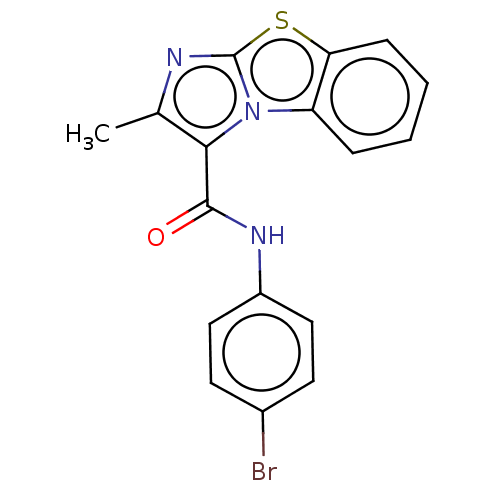 (CHEMBL3765483) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147691 (CHEMBL3764865) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147716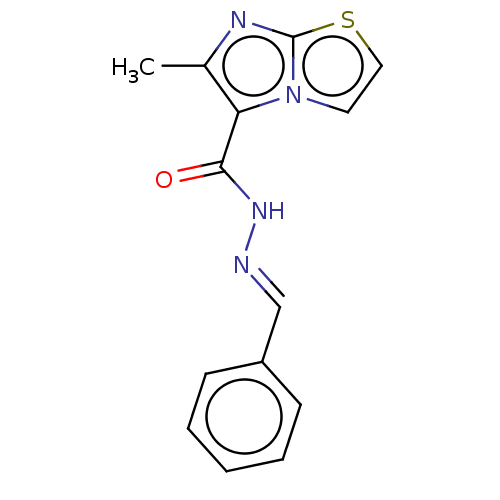 (CHEMBL3763241) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147695 (CHEMBL3765314) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147715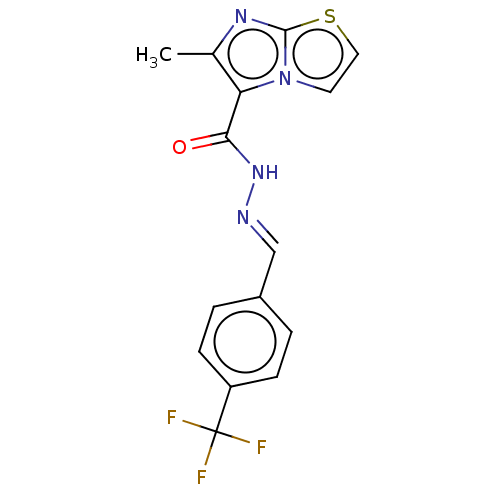 (CHEMBL3764614) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147724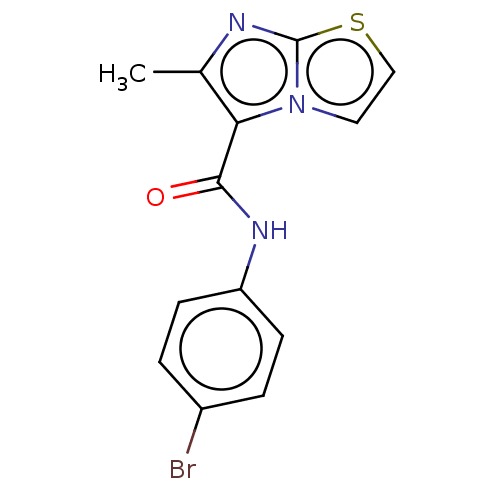 (CHEMBL3764280) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147725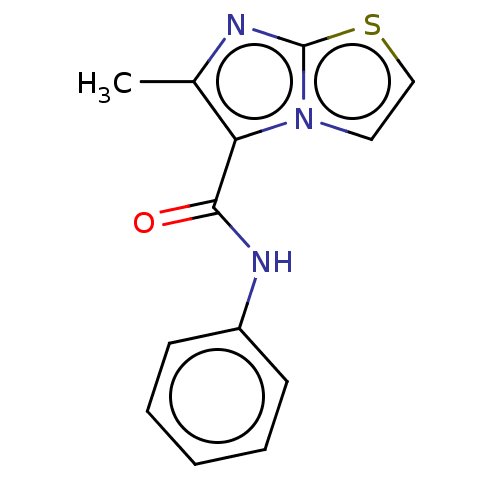 (CHEMBL3765736) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147732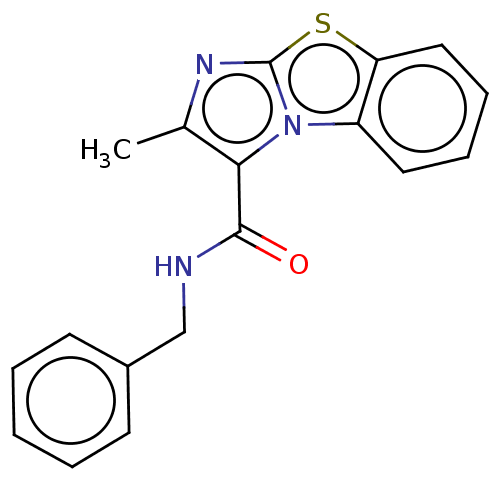 (CHEMBL3764694) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147733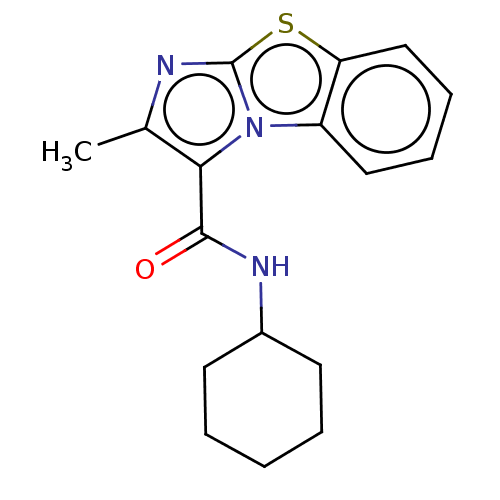 (CHEMBL3763165) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147719 (CHEMBL3763769) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147730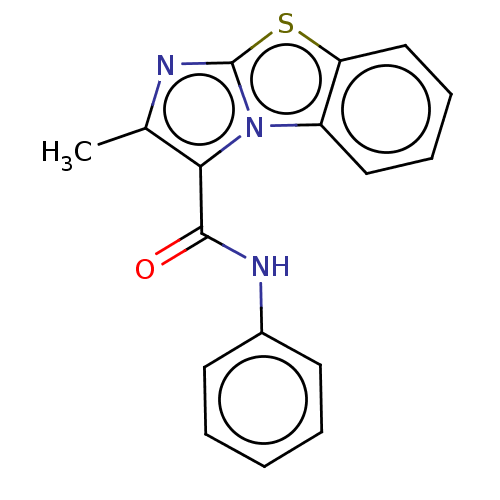 (CHEMBL3765612) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147727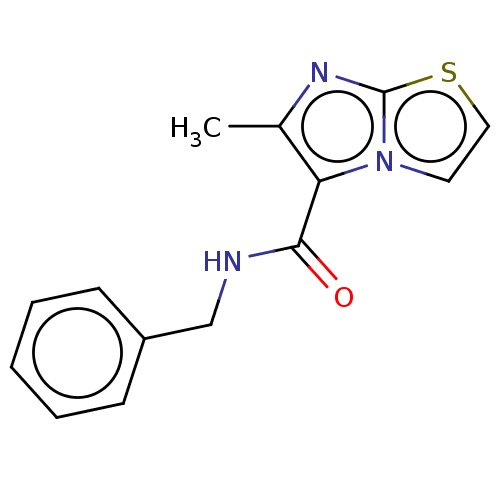 (CHEMBL3765386) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147693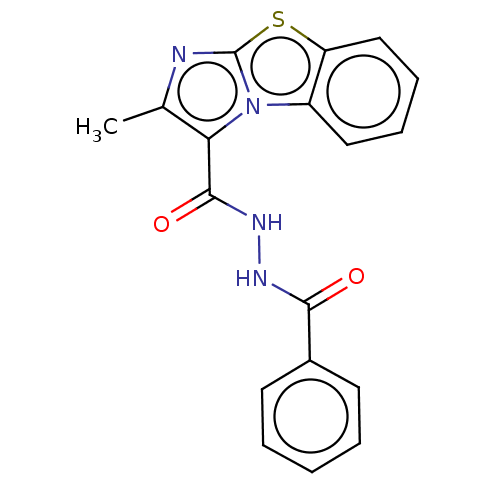 (CHEMBL3763613) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50497467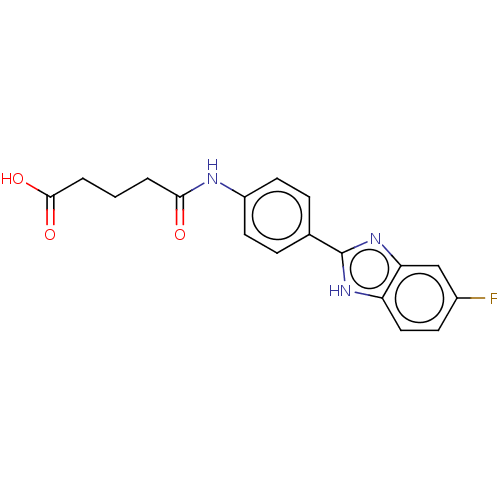 (CHEMBL3344011) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase-mediated supercoiling of relaxed DNA using pBR 322 as substrate by agarose gel electrophoresis analysi... | Bioorg Med Chem 22: 5970-87 (2014) Article DOI: 10.1016/j.bmc.2014.09.008 BindingDB Entry DOI: 10.7270/Q23X89MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147712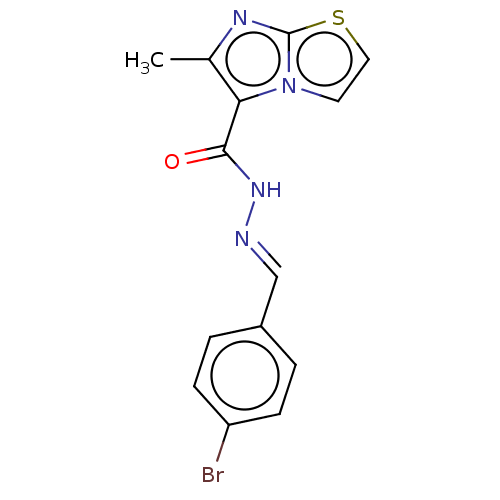 (CHEMBL3764292) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147696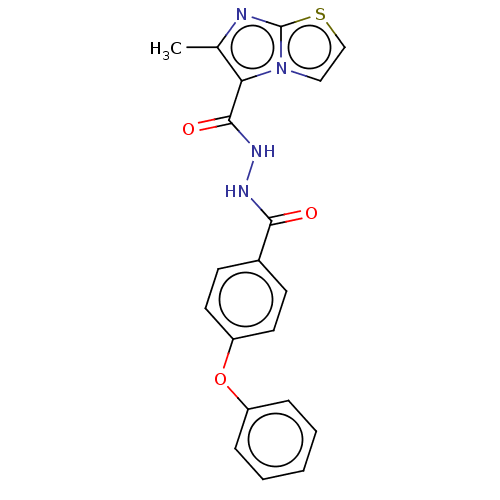 (CHEMBL3763438) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50497467 (CHEMBL3344011) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase B ATPase activity | Bioorg Med Chem 22: 5970-87 (2014) Article DOI: 10.1016/j.bmc.2014.09.008 BindingDB Entry DOI: 10.7270/Q23X89MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147690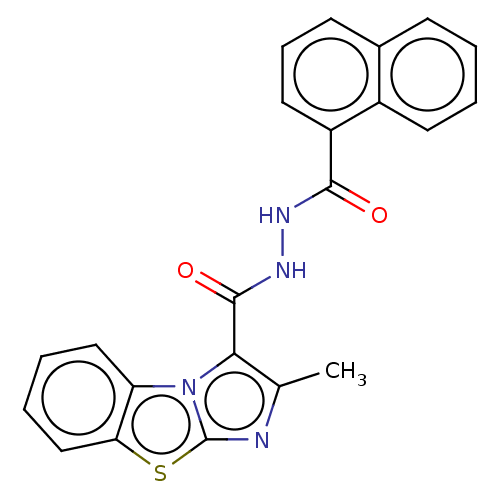 (CHEMBL3763254) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147723 (CHEMBL3764019) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50497516 (CHEMBL3344044) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase-mediated supercoiling of relaxed DNA using pBR 322 as substrate by agarose gel electrophoresis analysi... | Bioorg Med Chem 22: 5970-87 (2014) Article DOI: 10.1016/j.bmc.2014.09.008 BindingDB Entry DOI: 10.7270/Q23X89MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50497758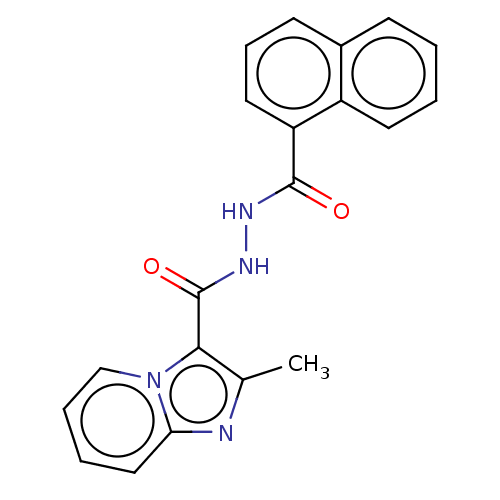 (CHEMBL3309662) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase expressed in Escherichia coli BL21 (DE3) by spectrophotometry | Bioorg Med Chem 22: 4223-32 (2014) Article DOI: 10.1016/j.bmc.2014.05.038 BindingDB Entry DOI: 10.7270/Q2D79FDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50497761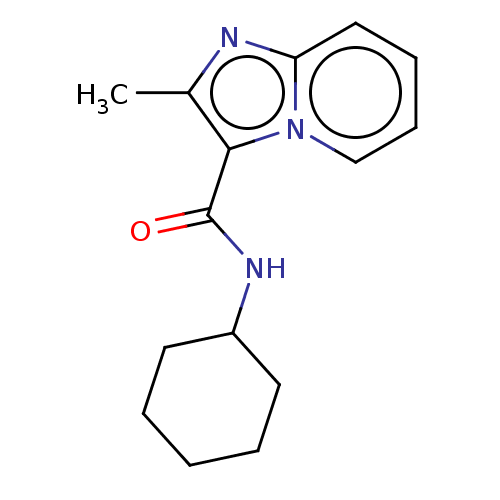 (CHEMBL1487172) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase expressed in Escherichia coli BL21 (DE3) by spectrophotometry | Bioorg Med Chem 22: 4223-32 (2014) Article DOI: 10.1016/j.bmc.2014.05.038 BindingDB Entry DOI: 10.7270/Q2D79FDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147728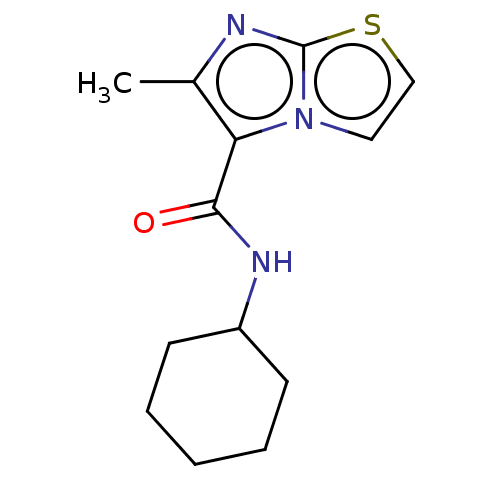 (CHEMBL3763965) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147722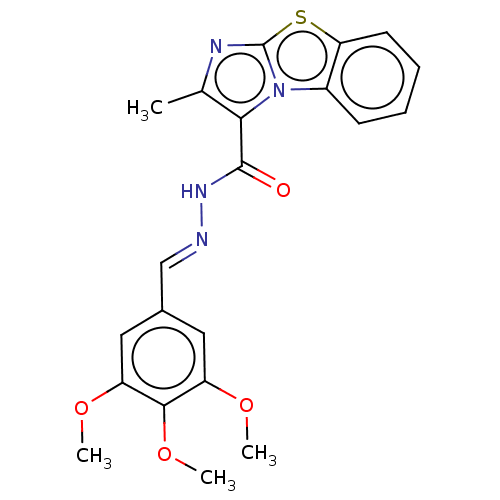 (CHEMBL3763344) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50497507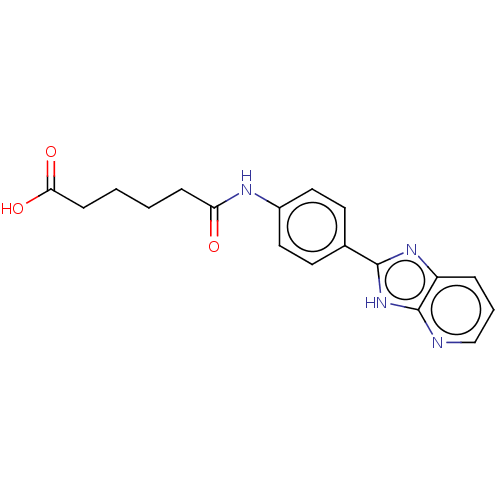 (CHEMBL3344033) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase-mediated supercoiling of relaxed DNA using pBR 322 as substrate by agarose gel electrophoresis analysi... | Bioorg Med Chem 22: 5970-87 (2014) Article DOI: 10.1016/j.bmc.2014.09.008 BindingDB Entry DOI: 10.7270/Q23X89MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50497491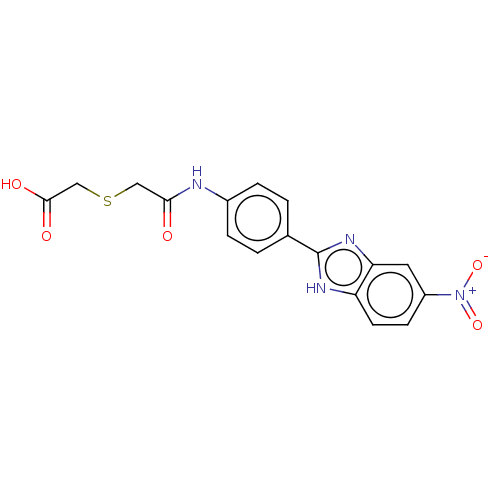 (CHEMBL3341781) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase-mediated supercoiling of relaxed DNA using pBR 322 as substrate by agarose gel electrophoresis analysi... | Bioorg Med Chem 22: 5970-87 (2014) Article DOI: 10.1016/j.bmc.2014.09.008 BindingDB Entry DOI: 10.7270/Q23X89MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147731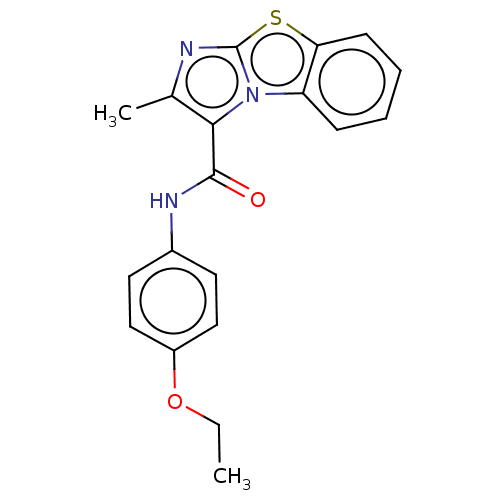 (CHEMBL3765655) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147721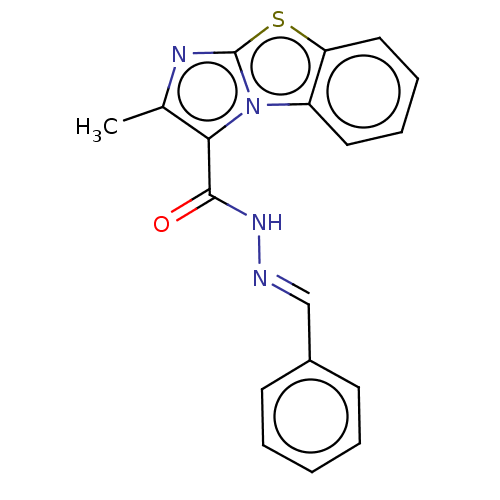 (CHEMBL3765594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50497480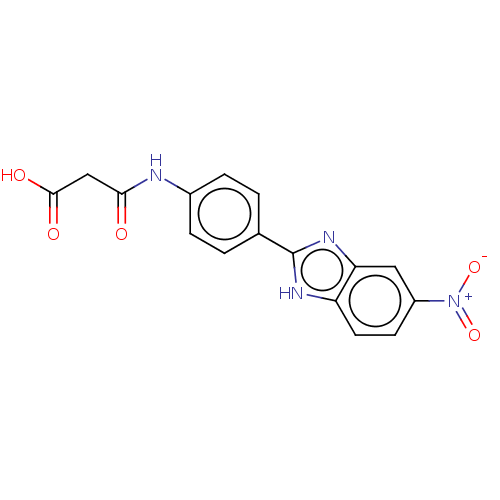 (CHEMBL3344019) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase-mediated supercoiling of relaxed DNA using pBR 322 as substrate by agarose gel electrophoresis analysi... | Bioorg Med Chem 22: 5970-87 (2014) Article DOI: 10.1016/j.bmc.2014.09.008 BindingDB Entry DOI: 10.7270/Q23X89MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50497516 (CHEMBL3344044) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase B ATPase activity | Bioorg Med Chem 22: 5970-87 (2014) Article DOI: 10.1016/j.bmc.2014.09.008 BindingDB Entry DOI: 10.7270/Q23X89MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147718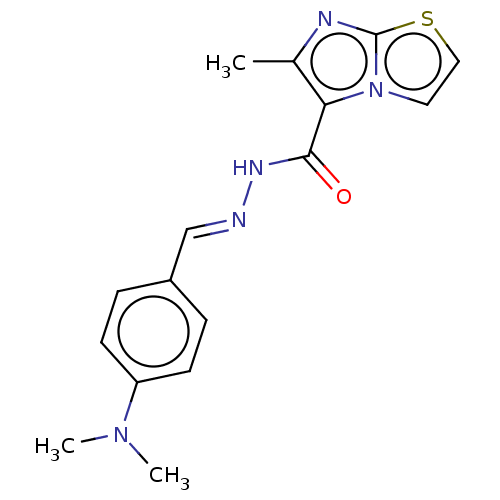 (CHEMBL3763895) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50497772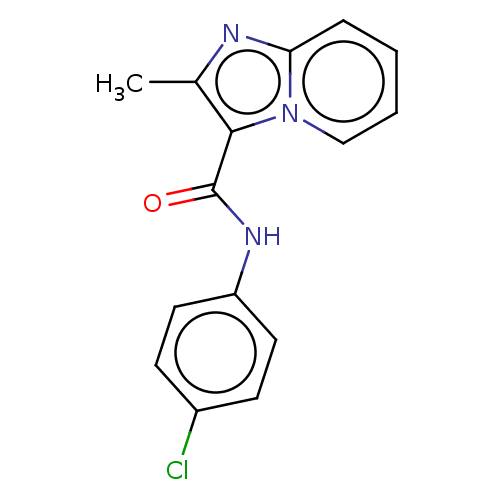 (CHEMBL1569394) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase expressed in Escherichia coli BL21 (DE3) by spectrophotometry | Bioorg Med Chem 22: 4223-32 (2014) Article DOI: 10.1016/j.bmc.2014.05.038 BindingDB Entry DOI: 10.7270/Q2D79FDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50497750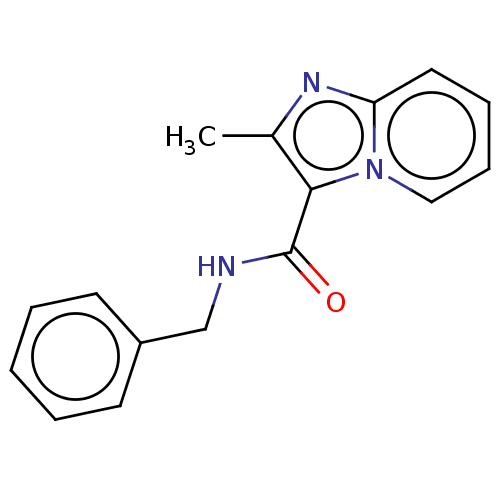 (CHEMBL2098049 | GSK957094A | TCMDC-142444) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase expressed in Escherichia coli BL21 (DE3) by spectrophotometry | Bioorg Med Chem 22: 4223-32 (2014) Article DOI: 10.1016/j.bmc.2014.05.038 BindingDB Entry DOI: 10.7270/Q2D79FDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50497767 (CHEMBL3310315) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase expressed in Escherichia coli BL21 (DE3) by spectrophotometry | Bioorg Med Chem 22: 4223-32 (2014) Article DOI: 10.1016/j.bmc.2014.05.038 BindingDB Entry DOI: 10.7270/Q2D79FDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50497488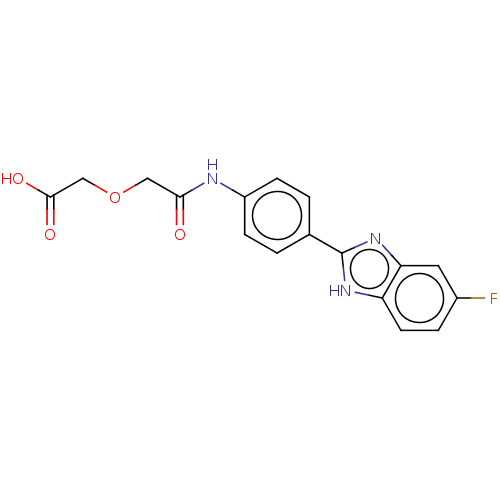 (CHEMBL3344036) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase-mediated supercoiling of relaxed DNA using pBR 322 as substrate by agarose gel electrophoresis analysi... | Bioorg Med Chem 22: 5970-87 (2014) Article DOI: 10.1016/j.bmc.2014.09.008 BindingDB Entry DOI: 10.7270/Q23X89MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50497507 (CHEMBL3344033) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase B ATPase activity | Bioorg Med Chem 22: 5970-87 (2014) Article DOI: 10.1016/j.bmc.2014.09.008 BindingDB Entry DOI: 10.7270/Q23X89MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50497494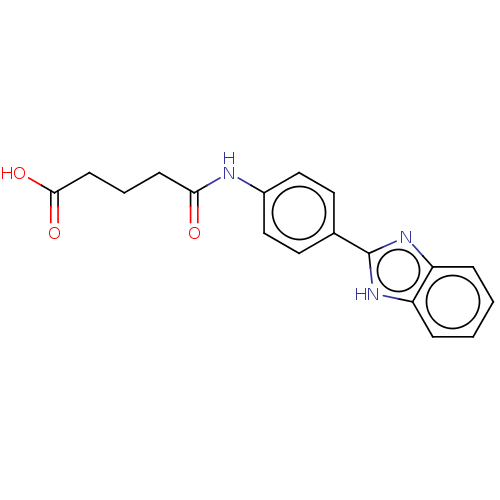 (CHEMBL3344000) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase-mediated supercoiling of relaxed DNA using pBR 322 as substrate by agarose gel electrophoresis analysi... | Bioorg Med Chem 22: 5970-87 (2014) Article DOI: 10.1016/j.bmc.2014.09.008 BindingDB Entry DOI: 10.7270/Q23X89MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50147699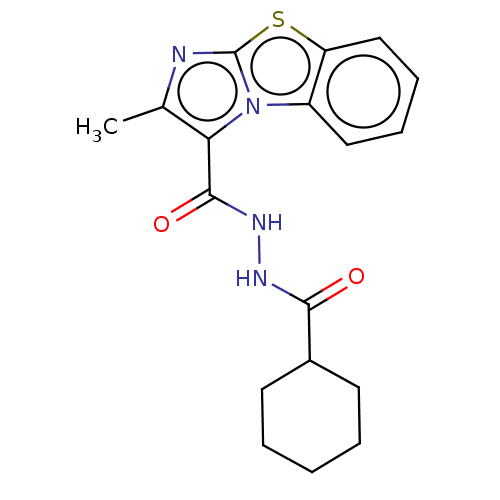 (CHEMBL3764636) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase using pantoic acid as substrate and beta-alanine as reactant assessed as NAD+ format... | Bioorg Med Chem 24: 1298-307 (2016) Article DOI: 10.1016/j.bmc.2016.01.059 BindingDB Entry DOI: 10.7270/Q2C82C5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50497511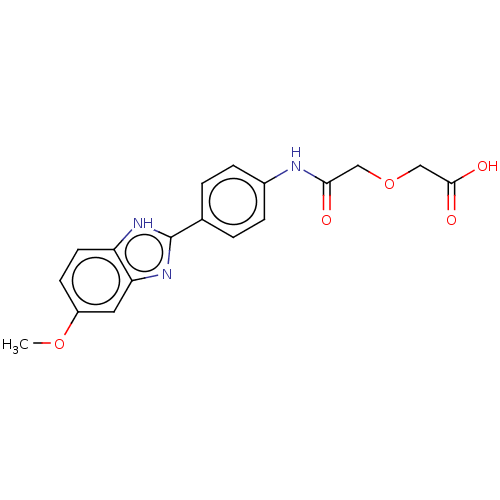 (CHEMBL3344041) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase-mediated supercoiling of relaxed DNA using pBR 322 as substrate by agarose gel electrophoresis analysi... | Bioorg Med Chem 22: 5970-87 (2014) Article DOI: 10.1016/j.bmc.2014.09.008 BindingDB Entry DOI: 10.7270/Q23X89MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50026388 (CHEMBL3331278) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science - Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant InhA expressed in Escherichia coli BL21 (DE3) using 2-trans-decenoyl substrate by spectrophotome... | Eur J Med Chem 86: 613-27 (2014) Article DOI: 10.1016/j.ejmech.2014.09.028 BindingDB Entry DOI: 10.7270/Q2SJ1N7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50026396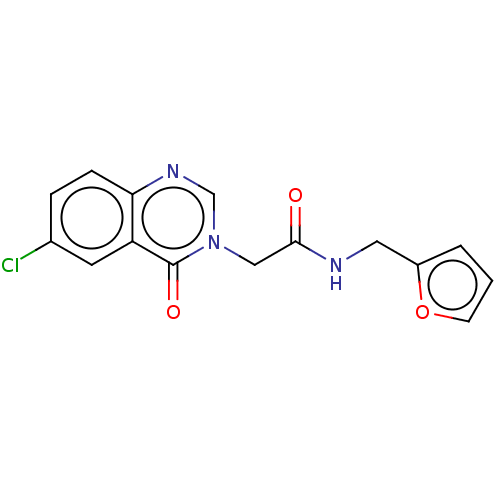 (CHEMBL3331275) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology& Science - Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant InhA expressed in Escherichia coli BL21 (DE3) using 2-trans-decenoyl substrate by spectrophotome... | Eur J Med Chem 86: 613-27 (2014) Article DOI: 10.1016/j.ejmech.2014.09.028 BindingDB Entry DOI: 10.7270/Q2SJ1N7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM166646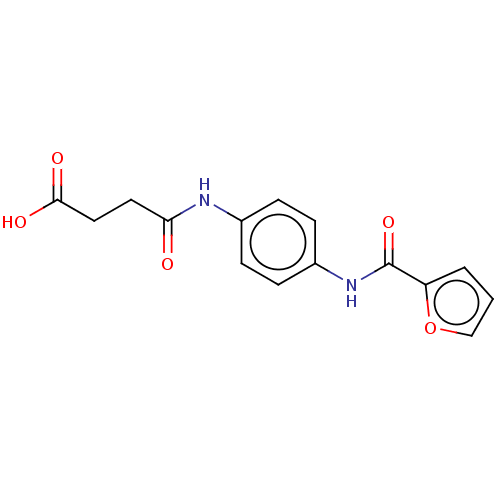 (4-((4-(furan-2-carboxamido)phenyl)amino)-4-oxobuta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase-mediated supercoiling of relaxed DNA using pBR 322 as substrate by agarose gel electrophoresis analysi... | Bioorg Med Chem 22: 5970-87 (2014) Article DOI: 10.1016/j.bmc.2014.09.008 BindingDB Entry DOI: 10.7270/Q23X89MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50497500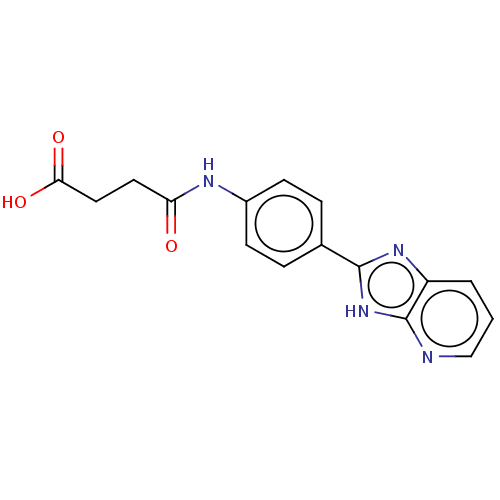 (CHEMBL3344030) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase-mediated supercoiling of relaxed DNA using pBR 322 as substrate by agarose gel electrophoresis analysi... | Bioorg Med Chem 22: 5970-87 (2014) Article DOI: 10.1016/j.bmc.2014.09.008 BindingDB Entry DOI: 10.7270/Q23X89MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50497493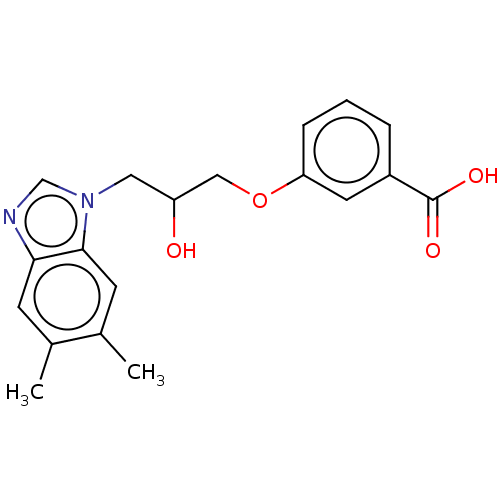 (CHEMBL3343999) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase-mediated supercoiling of relaxed DNA using pBR 322 as substrate by agarose gel electrophoresis analysi... | Bioorg Med Chem 22: 5970-87 (2014) Article DOI: 10.1016/j.bmc.2014.09.008 BindingDB Entry DOI: 10.7270/Q23X89MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Staphylococcus aureus) | BDBM50497491 (CHEMBL3341781) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase B ATPase activity | Bioorg Med Chem 22: 5970-87 (2014) Article DOI: 10.1016/j.bmc.2014.09.008 BindingDB Entry DOI: 10.7270/Q23X89MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50497756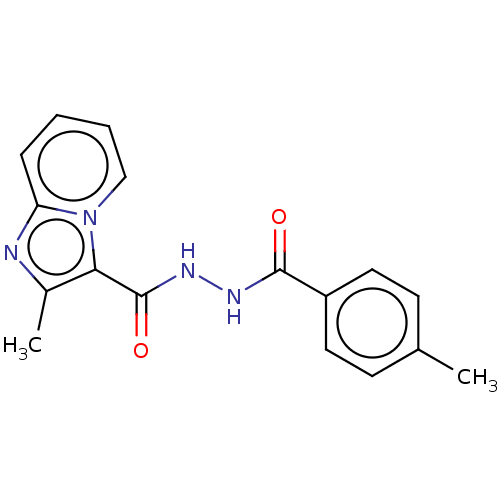 (CHEMBL3310302) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase expressed in Escherichia coli BL21 (DE3) by spectrophotometry | Bioorg Med Chem 22: 4223-32 (2014) Article DOI: 10.1016/j.bmc.2014.05.038 BindingDB Entry DOI: 10.7270/Q2D79FDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50497759 (CHEMBL3309661) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase expressed in Escherichia coli BL21 (DE3) by spectrophotometry | Bioorg Med Chem 22: 4223-32 (2014) Article DOI: 10.1016/j.bmc.2014.05.038 BindingDB Entry DOI: 10.7270/Q2D79FDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50497775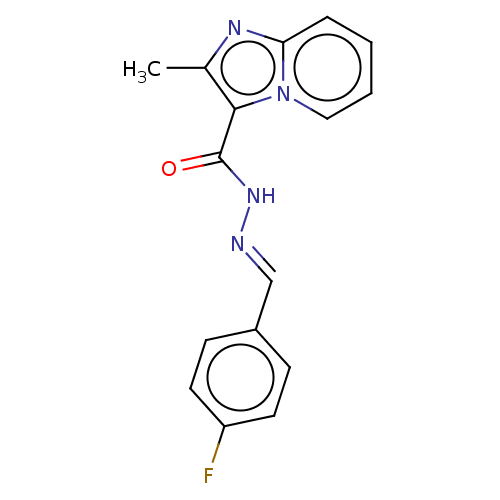 (CHEMBL3207709) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology & Science-Pilani Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis pantothenate synthetase expressed in Escherichia coli BL21 (DE3) by spectrophotometry | Bioorg Med Chem 22: 4223-32 (2014) Article DOI: 10.1016/j.bmc.2014.05.038 BindingDB Entry DOI: 10.7270/Q2D79FDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 232 total ) | Next | Last >> |