 Found 108 hits with Last Name = 'dooley' and Initial = 'r'
Found 108 hits with Last Name = 'dooley' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM20000
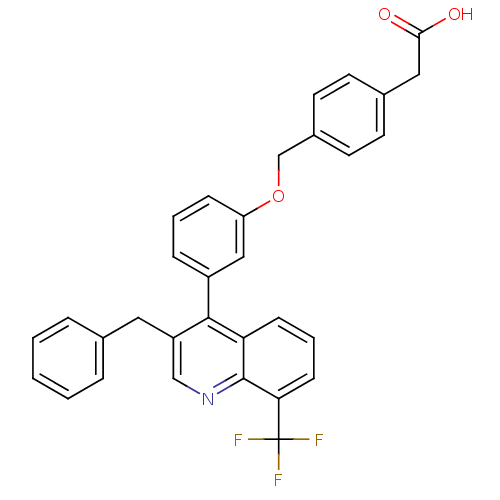
(2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES OC(=O)Cc1ccc(COc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C32H24F3NO3/c33-32(34,35)28-11-5-10-27-30(25(19-36-31(27)28)16-21-6-2-1-3-7-21)24-8-4-9-26(18-24)39-20-23-14-12-22(13-15-23)17-29(37)38/h1-15,18-19H,16-17,20H2,(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50227163

(2-(4-((3-(3-benzoyl-8-(trifluoromethyl)quinolin-5-...)Show SMILES CC#CCC(C(O)=O)c1ccc(COc2cccc(c2)-c2ccc(c3ncc(cc23)C(=O)c2ccccc2)C(F)(F)F)cc1 |w:4.3| Show InChI InChI=1S/C36H26F3NO4/c1-2-3-12-30(35(42)43)24-15-13-23(14-16-24)22-44-28-11-7-10-26(19-28)29-17-18-32(36(37,38)39)33-31(29)20-27(21-40-33)34(41)25-8-5-4-6-9-25/h4-11,13-21,30H,12,22H2,1H3,(H,42,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50227157
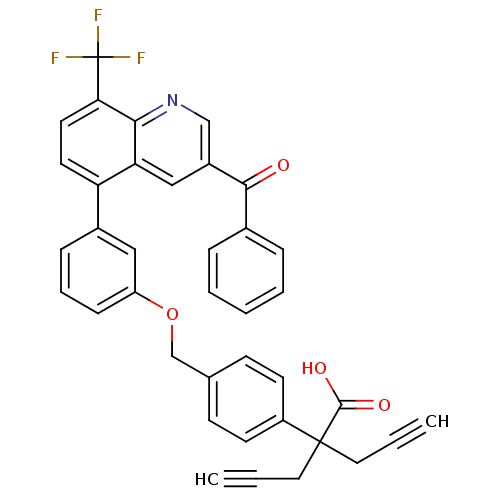
(2-(4-((3-(3-benzoyl-8-(trifluoromethyl)quinolin-5-...)Show SMILES OC(=O)C(CC#C)(CC#C)c1ccc(COc2cccc(c2)-c2ccc(c3ncc(cc23)C(=O)c2ccccc2)C(F)(F)F)cc1 Show InChI InChI=1S/C38H26F3NO4/c1-3-19-37(20-4-2,36(44)45)29-15-13-25(14-16-29)24-46-30-12-8-11-27(21-30)31-17-18-33(38(39,40)41)34-32(31)22-28(23-42-34)35(43)26-9-6-5-7-10-26/h1-2,5-18,21-23H,19-20,24H2,(H,44,45) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRalpha |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50227161

(2-(4-(3-(3-benzyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES OC(=O)Cc1ccc(NCc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)c2ccccc12 Show InChI InChI=1S/C36H27F3N2O2/c37-36(38,39)31-15-7-14-30-34(27(22-41-35(30)31)18-23-8-2-1-3-9-23)26-11-6-10-24(19-26)21-40-32-17-16-25(20-33(42)43)28-12-4-5-13-29(28)32/h1-17,19,22,40H,18,20-21H2,(H,42,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50227146

(2-(4-(3-(3-benzyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES Cc1cc(NCc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)c(C)cc1CC(O)=O Show InChI InChI=1S/C34H29F3N2O2/c1-21-15-30(22(2)14-26(21)18-31(40)41)38-19-24-10-6-11-25(17-24)32-27(16-23-8-4-3-5-9-23)20-39-33-28(32)12-7-13-29(33)34(35,36)37/h3-15,17,20,38H,16,18-19H2,1-2H3,(H,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50259277

((R,S/S,R)-syn-1-((4-methoxyphenyl)(methyl)amino)-3...)Show SMILES CNC[C@@H](O)[C@@H](N(C)c1ccc(OC)cc1)c1ccccc1 |r| Show InChI InChI=1S/C18H24N2O2/c1-19-13-17(21)18(14-7-5-4-6-8-14)20(2)15-9-11-16(22-3)12-10-15/h4-12,17-19,21H,13H2,1-3H3/t17-,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of serotonin uptake at human SERT expressed in human JAR cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM84745

(CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of serotonin uptake at human SERT expressed in human JAR cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50124566
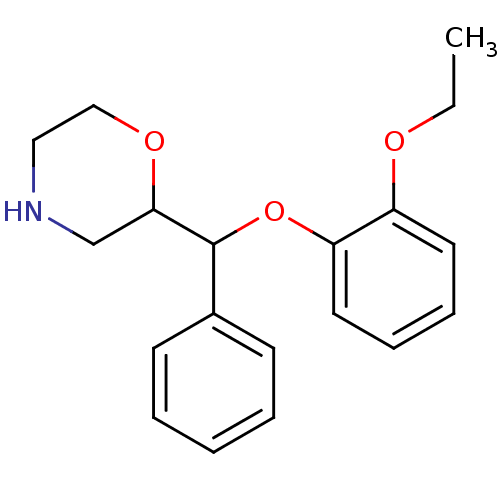
((R)-2-((R)-(2-ethoxyphenoxy)(phenyl)methyl)morphol...)Show InChI InChI=1S/C19H23NO3/c1-2-21-16-10-6-7-11-17(16)23-19(15-8-4-3-5-9-15)18-14-20-12-13-22-18/h3-11,18-20H,2,12-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK-Net6 cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50366567
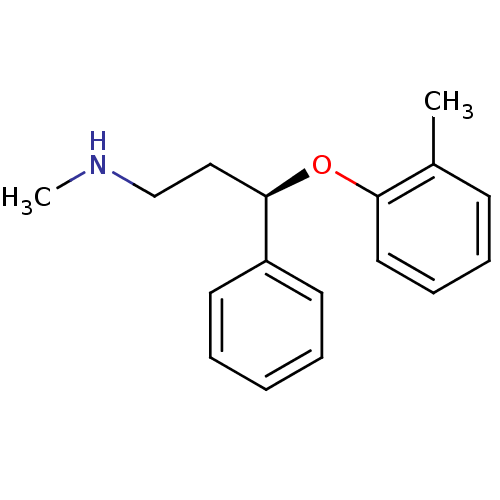
(ATOMOXETINE)Show InChI InChI=1S/C17H21NO/c1-14-8-6-7-11-16(14)19-17(12-13-18-2)15-9-4-3-5-10-15/h3-11,17-18H,12-13H2,1-2H3/t17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK-Net6 cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50227156
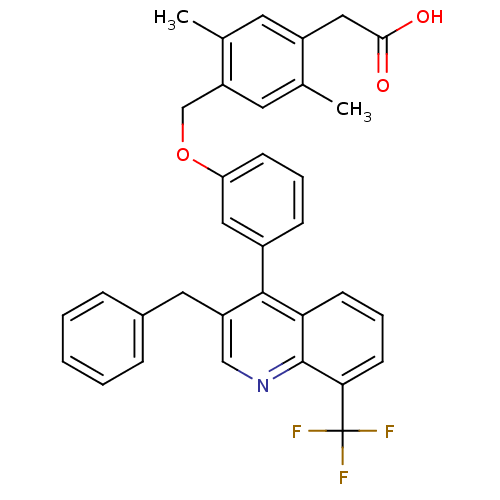
(2-(4-((3-(3-benzyl-8-(trifluoromethyl)quinolin-4-y...)Show SMILES Cc1cc(CC(O)=O)c(C)cc1COc1cccc(c1)-c1c(Cc2ccccc2)cnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C34H28F3NO3/c1-21-15-27(22(2)14-25(21)18-31(39)40)20-41-28-11-6-10-24(17-28)32-26(16-23-8-4-3-5-9-23)19-38-33-29(32)12-7-13-30(33)34(35,36)37/h3-15,17,19H,16,18,20H2,1-2H3,(H,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK-Net6 cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50227159
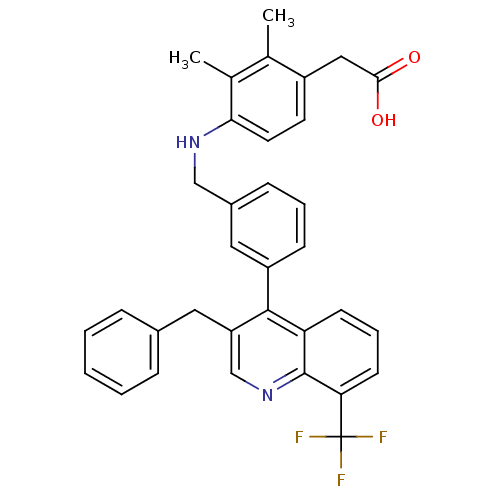
(2-(4-(3-(3-benzyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES Cc1c(C)c(NCc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)ccc1CC(O)=O Show InChI InChI=1S/C34H29F3N2O2/c1-21-22(2)30(15-14-25(21)18-31(40)41)38-19-24-10-6-11-26(17-24)32-27(16-23-8-4-3-5-9-23)20-39-33-28(32)12-7-13-29(33)34(35,36)37/h3-15,17,20,38H,16,18-19H2,1-2H3,(H,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50227158

(2-(4-(3-(3-benzyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES Cc1cc(OCc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)c(C)cc1CC(O)=O Show InChI InChI=1S/C34H28F3NO3/c1-21-15-30(22(2)14-26(21)18-31(39)40)41-20-24-10-6-11-25(17-24)32-27(16-23-8-4-3-5-9-23)19-38-33-28(32)12-7-13-29(33)34(35,36)37/h3-15,17,19H,16,18,20H2,1-2H3,(H,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM84745

(CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK-Net6 cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50227160

(2-(4-((3-(3-benzoyl-8-(trifluoromethyl)quinolin-5-...)Show SMILES OC(=O)C(CC=C)c1ccc(COc2cccc(c2)-c2ccc(c3ncc(cc23)C(=O)c2ccccc2)C(F)(F)F)cc1 |w:3.3| Show InChI InChI=1S/C35H26F3NO4/c1-2-7-29(34(41)42)23-14-12-22(13-15-23)21-43-27-11-6-10-25(18-27)28-16-17-31(35(36,37)38)32-30(28)19-26(20-39-32)33(40)24-8-4-3-5-9-24/h2-6,8-20,29H,1,7,21H2,(H,41,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50227151

(2-(5-(3-(3-benzyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES OC(=O)Cc1cccc2c(NCc3cccc(c3)-c3c(Cc4ccccc4)cnc4c(cccc34)C(F)(F)F)cccc12 Show InChI InChI=1S/C36H27F3N2O2/c37-36(38,39)31-16-6-15-30-34(27(22-41-35(30)31)18-23-8-2-1-3-9-23)26-12-4-10-24(19-26)21-40-32-17-7-13-28-25(20-33(42)43)11-5-14-29(28)32/h1-17,19,22,40H,18,20-21H2,(H,42,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50227162

(2-(4-((3-(3-benzoyl-8-(trifluoromethyl)quinolin-5-...)Show SMILES OC(=O)Cc1ccc(COc2cccc(c2)-c2ccc(c3ncc(cc23)C(=O)c2ccccc2)C(F)(F)F)cc1 Show InChI InChI=1S/C32H22F3NO4/c33-32(34,35)28-14-13-26(27-17-24(18-36-30(27)28)31(39)22-5-2-1-3-6-22)23-7-4-8-25(16-23)40-19-21-11-9-20(10-12-21)15-29(37)38/h1-14,16-18H,15,19H2,(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50227163

(2-(4-((3-(3-benzoyl-8-(trifluoromethyl)quinolin-5-...)Show SMILES CC#CCC(C(O)=O)c1ccc(COc2cccc(c2)-c2ccc(c3ncc(cc23)C(=O)c2ccccc2)C(F)(F)F)cc1 |w:4.3| Show InChI InChI=1S/C36H26F3NO4/c1-2-3-12-30(35(42)43)24-15-13-23(14-16-24)22-44-28-11-7-10-26(19-28)29-17-18-32(36(37,38)39)33-31(29)20-27(21-40-33)34(41)25-8-5-4-6-9-25/h4-11,13-21,30H,12,22H2,1H3,(H,42,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRalpha |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM20002
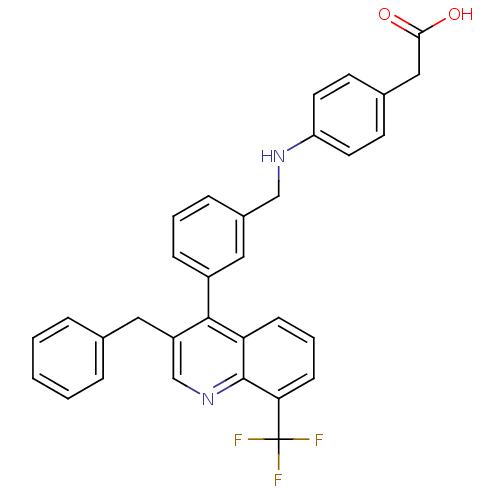
(2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...)Show SMILES OC(=O)Cc1ccc(NCc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C32H25F3N2O2/c33-32(34,35)28-11-5-10-27-30(25(20-37-31(27)28)16-21-6-2-1-3-7-21)24-9-4-8-23(17-24)19-36-26-14-12-22(13-15-26)18-29(38)39/h1-15,17,20,36H,16,18-19H2,(H,38,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM19993

(CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...)Show SMILES OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12F9NO3S/c18-14(19,20)10-27(31(29,30)13-4-2-1-3-5-13)12-8-6-11(7-9-12)15(28,16(21,22)23)17(24,25)26/h1-9,28H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM20000

(2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES OC(=O)Cc1ccc(COc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C32H24F3NO3/c33-32(34,35)28-11-5-10-27-30(25(19-36-31(27)28)16-21-6-2-1-3-7-21)24-8-4-9-26(18-24)39-20-23-14-12-22(13-15-23)17-29(37)38/h1-15,18-19H,16-17,20H2,(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRalpha |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50227154
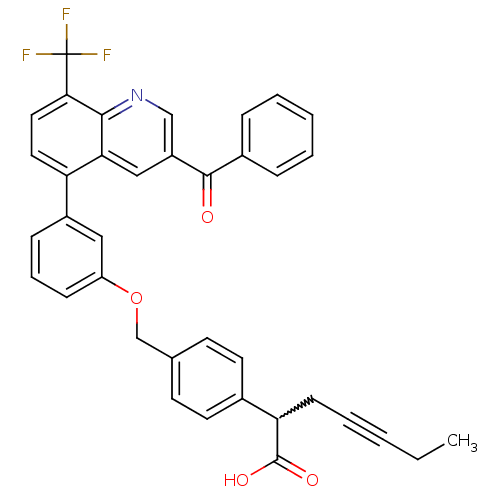
(2-(4-((3-(3-benzoyl-8-(trifluoromethyl)quinolin-5-...)Show SMILES CCC#CCC(C(O)=O)c1ccc(COc2cccc(c2)-c2ccc(c3ncc(cc23)C(=O)c2ccccc2)C(F)(F)F)cc1 |w:5.4| Show InChI InChI=1S/C37H28F3NO4/c1-2-3-5-13-31(36(43)44)25-16-14-24(15-17-25)23-45-29-12-8-11-27(20-29)30-18-19-33(37(38,39)40)34-32(30)21-28(22-41-34)35(42)26-9-6-4-7-10-26/h4,6-12,14-22,31H,2,13,23H2,1H3,(H,43,44) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50227145
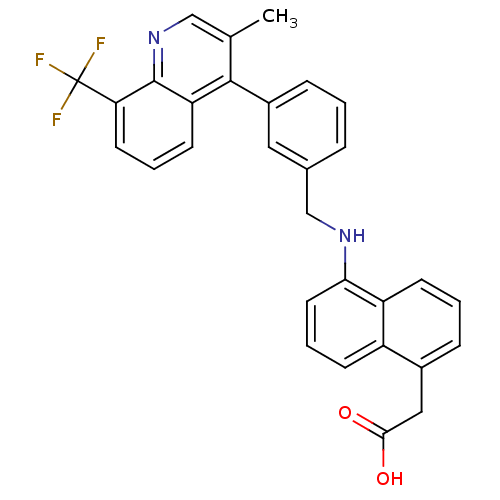
(2-(5-((3-(3-methyl-8-(trifluoromethyl)quinolin-4-y...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(CNc2cccc3c(CC(O)=O)cccc23)c1)C(F)(F)F Show InChI InChI=1S/C30H23F3N2O2/c1-18-16-35-29-24(11-4-12-25(29)30(31,32)33)28(18)21-8-2-6-19(14-21)17-34-26-13-5-9-22-20(15-27(36)37)7-3-10-23(22)26/h2-14,16,34H,15,17H2,1H3,(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM30130

(CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...)Show InChI InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of serotonin uptake at human SERT expressed in human JAR cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50227156

(2-(4-((3-(3-benzyl-8-(trifluoromethyl)quinolin-4-y...)Show SMILES Cc1cc(CC(O)=O)c(C)cc1COc1cccc(c1)-c1c(Cc2ccccc2)cnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C34H28F3NO3/c1-21-15-27(22(2)14-25(21)18-31(39)40)20-41-28-11-6-10-24(17-28)32-26(16-23-8-4-3-5-9-23)19-38-33-29(32)12-7-13-30(33)34(35,36)37/h3-15,17,19H,16,18,20H2,1-2H3,(H,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRalpha |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50227152
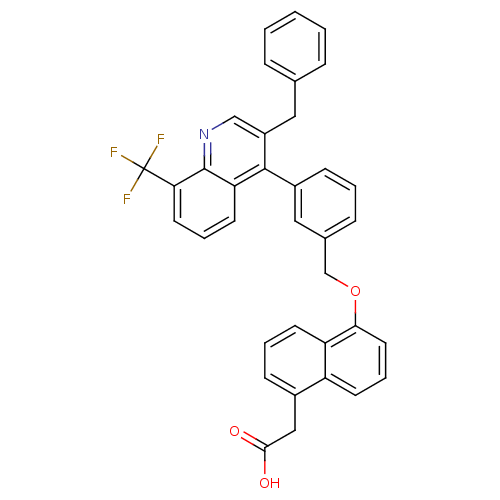
(2-(5-(3-(3-benzyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES OC(=O)Cc1cccc2c(OCc3cccc(c3)-c3c(Cc4ccccc4)cnc4c(cccc34)C(F)(F)F)cccc12 Show InChI InChI=1S/C36H26F3NO3/c37-36(38,39)31-16-6-15-30-34(27(21-40-35(30)31)18-23-8-2-1-3-9-23)26-12-4-10-24(19-26)22-43-32-17-7-13-28-25(20-33(41)42)11-5-14-29(28)32/h1-17,19,21H,18,20,22H2,(H,41,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50259340
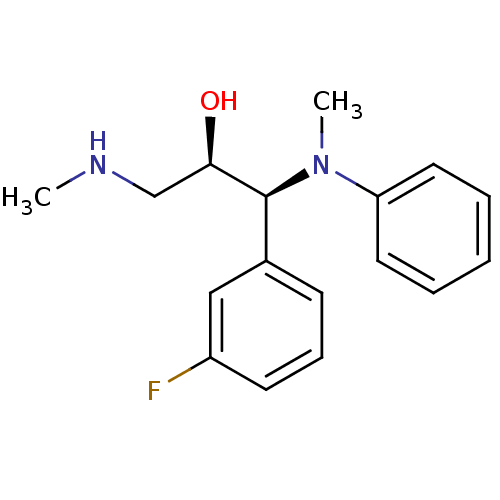
((1S,2R)-1-(3-fluorophenyl)-1-(methyl(phenyl)amino)...)Show SMILES CNC[C@@H](O)[C@@H](N(C)c1ccccc1)c1cccc(F)c1 |r| Show InChI InChI=1S/C17H21FN2O/c1-19-12-16(21)17(13-7-6-8-14(18)11-13)20(2)15-9-4-3-5-10-15/h3-11,16-17,19,21H,12H2,1-2H3/t16-,17+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK-Net6 cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM19993

(CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...)Show SMILES OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12F9NO3S/c18-14(19,20)10-27(31(29,30)13-4-2-1-3-5-13)12-8-6-11(7-9-12)15(28,16(21,22)23)17(24,25)26/h1-9,28H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRalpha |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50227147

(2-(5-((3-(8-(trifluoromethyl)quinolin-4-yl)phenyl)...)Show SMILES OC(=O)Cc1cccc2c(NCc3cccc(c3)-c3ccnc4c(cccc34)C(F)(F)F)cccc12 Show InChI InChI=1S/C29H21F3N2O2/c30-29(31,32)25-11-3-10-24-22(13-14-33-28(24)25)19-6-1-5-18(15-19)17-34-26-12-4-8-21-20(16-27(35)36)7-2-9-23(21)26/h1-15,34H,16-17H2,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50227160

(2-(4-((3-(3-benzoyl-8-(trifluoromethyl)quinolin-5-...)Show SMILES OC(=O)C(CC=C)c1ccc(COc2cccc(c2)-c2ccc(c3ncc(cc23)C(=O)c2ccccc2)C(F)(F)F)cc1 |w:3.3| Show InChI InChI=1S/C35H26F3NO4/c1-2-7-29(34(41)42)23-14-12-22(13-15-23)21-43-27-11-6-10-25(18-27)28-16-17-31(35(36,37)38)32-30(28)19-26(20-39-32)33(40)24-8-4-3-5-9-24/h2-6,8-20,29H,1,7,21H2,(H,41,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRalpha |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50259276
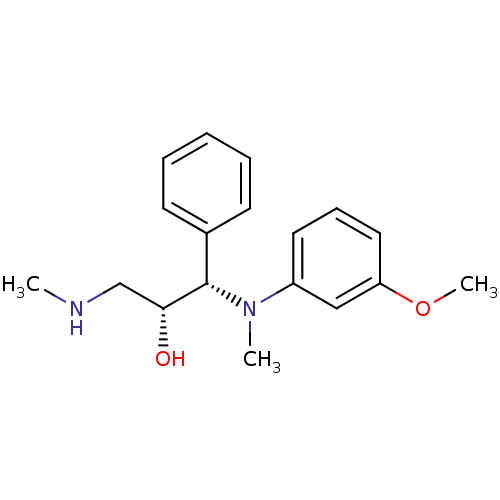
((R,S/S,R)-syn-1-((3-methoxyphenyl)(methyl)amino)-3...)Show SMILES CNC[C@@H](O)[C@@H](N(C)c1cccc(OC)c1)c1ccccc1 |r| Show InChI InChI=1S/C18H24N2O2/c1-19-13-17(21)18(14-8-5-4-6-9-14)20(2)15-10-7-11-16(12-15)22-3/h4-12,17-19,21H,13H2,1-3H3/t17-,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of serotonin uptake at human SERT expressed in human JAR cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50227162

(2-(4-((3-(3-benzoyl-8-(trifluoromethyl)quinolin-5-...)Show SMILES OC(=O)Cc1ccc(COc2cccc(c2)-c2ccc(c3ncc(cc23)C(=O)c2ccccc2)C(F)(F)F)cc1 Show InChI InChI=1S/C32H22F3NO4/c33-32(34,35)28-14-13-26(27-17-24(18-36-30(27)28)31(39)22-5-2-1-3-6-22)23-7-4-8-25(16-23)40-19-21-11-9-20(10-12-21)15-29(37)38/h1-14,16-18H,15,19H2,(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRalpha |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50259273
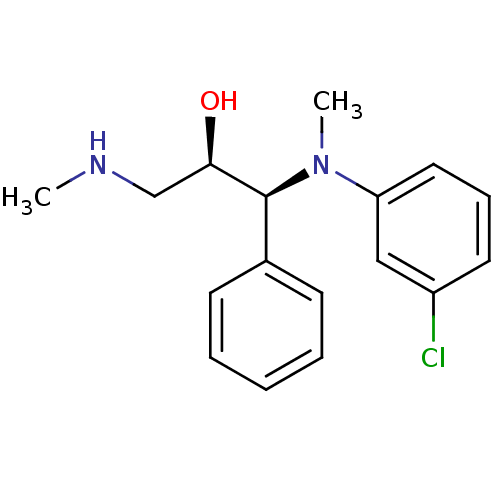
((R,S/S,R)-syn-1-((3-chlorophenyl)(methyl)amino)-3-...)Show SMILES CNC[C@@H](O)[C@@H](N(C)c1cccc(Cl)c1)c1ccccc1 |r| Show InChI InChI=1S/C17H21ClN2O/c1-19-12-16(21)17(13-7-4-3-5-8-13)20(2)15-10-6-9-14(18)11-15/h3-11,16-17,19,21H,12H2,1-2H3/t16-,17+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK-Net6 cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50227146

(2-(4-(3-(3-benzyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES Cc1cc(NCc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)c(C)cc1CC(O)=O Show InChI InChI=1S/C34H29F3N2O2/c1-21-15-30(22(2)14-26(21)18-31(40)41)38-19-24-10-6-11-25(17-24)32-27(16-23-8-4-3-5-9-23)20-39-33-28(32)12-7-13-29(33)34(35,36)37/h3-15,17,20,38H,16,18-19H2,1-2H3,(H,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRalpha |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50227154

(2-(4-((3-(3-benzoyl-8-(trifluoromethyl)quinolin-5-...)Show SMILES CCC#CCC(C(O)=O)c1ccc(COc2cccc(c2)-c2ccc(c3ncc(cc23)C(=O)c2ccccc2)C(F)(F)F)cc1 |w:5.4| Show InChI InChI=1S/C37H28F3NO4/c1-2-3-5-13-31(36(43)44)25-16-14-24(15-17-25)23-45-29-12-8-11-27(20-29)30-18-19-33(37(38,39)40)34-32(30)21-28(22-41-34)35(42)26-9-6-4-7-10-26/h4,6-12,14-22,31H,2,13,23H2,1H3,(H,43,44) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRalpha |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50227161

(2-(4-(3-(3-benzyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES OC(=O)Cc1ccc(NCc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)c2ccccc12 Show InChI InChI=1S/C36H27F3N2O2/c37-36(38,39)31-15-7-14-30-34(27(22-41-35(30)31)18-23-8-2-1-3-9-23)26-11-6-10-24(19-26)21-40-32-17-16-25(20-33(42)43)28-12-4-5-13-29(28)32/h1-17,19,22,40H,18,20-21H2,(H,42,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRalpha |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50259274
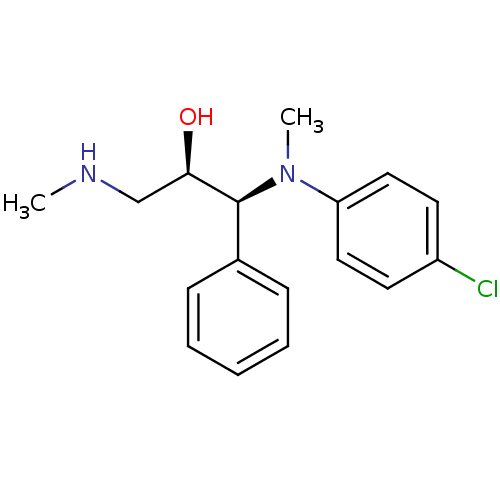
((R,S/S,R)-syn-1-((4-chlorophenyl)(methyl)amino)-3-...)Show SMILES CNC[C@@H](O)[C@@H](N(C)c1ccc(Cl)cc1)c1ccccc1 |r| Show InChI InChI=1S/C17H21ClN2O/c1-19-12-16(21)17(13-6-4-3-5-7-13)20(2)15-10-8-14(18)9-11-15/h3-11,16-17,19,21H,12H2,1-2H3/t16-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of serotonin uptake at human SERT expressed in human JAR cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50005536
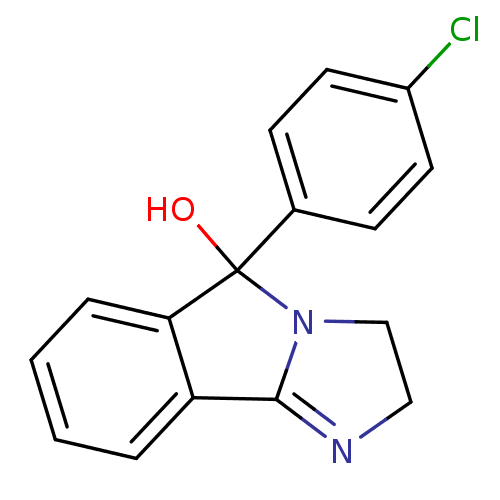
(42-548 | 5-(4-Chloro-phenyl)-2,5-dihydro-3H-imidaz...)Show InChI InChI=1S/C16H13ClN2O/c17-12-7-5-11(6-8-12)16(20)14-4-2-1-3-13(14)15-18-9-10-19(15)16/h1-8,20H,9-10H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of [3H]WIN-35428 binding to human recombinant DAT expressed in CHO cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50259274

((R,S/S,R)-syn-1-((4-chlorophenyl)(methyl)amino)-3-...)Show SMILES CNC[C@@H](O)[C@@H](N(C)c1ccc(Cl)cc1)c1ccccc1 |r| Show InChI InChI=1S/C17H21ClN2O/c1-19-12-16(21)17(13-6-4-3-5-7-13)20(2)15-10-8-14(18)9-11-15/h3-11,16-17,19,21H,12H2,1-2H3/t16-,17+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK-Net6 cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50227157

(2-(4-((3-(3-benzoyl-8-(trifluoromethyl)quinolin-5-...)Show SMILES OC(=O)C(CC#C)(CC#C)c1ccc(COc2cccc(c2)-c2ccc(c3ncc(cc23)C(=O)c2ccccc2)C(F)(F)F)cc1 Show InChI InChI=1S/C38H26F3NO4/c1-3-19-37(20-4-2,36(44)45)29-15-13-25(14-16-29)24-46-30-12-8-11-27(21-30)31-17-18-33(38(39,40)41)34-32(31)22-28(23-42-34)35(43)26-9-6-5-7-10-26/h1-2,5-18,21-23H,19-20,24H2,(H,44,45) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50259230
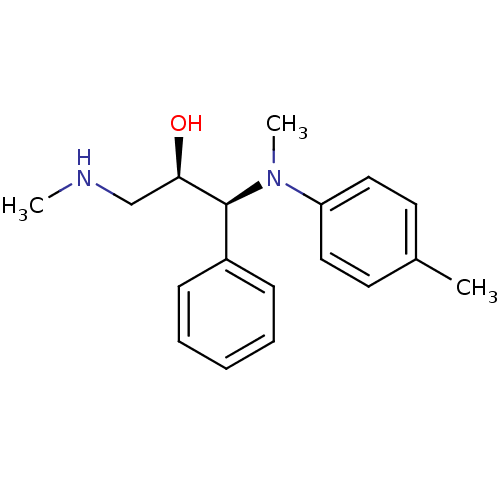
((R,S/S,R)-syn-1-(methyl(p-tolyl)amino)-3-(methylam...)Show SMILES CNC[C@@H](O)[C@@H](N(C)c1ccc(C)cc1)c1ccccc1 |r| Show InChI InChI=1S/C18H24N2O/c1-14-9-11-16(12-10-14)20(3)18(17(21)13-19-2)15-7-5-4-6-8-15/h4-12,17-19,21H,13H2,1-3H3/t17-,18+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK-Net6 cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50259227
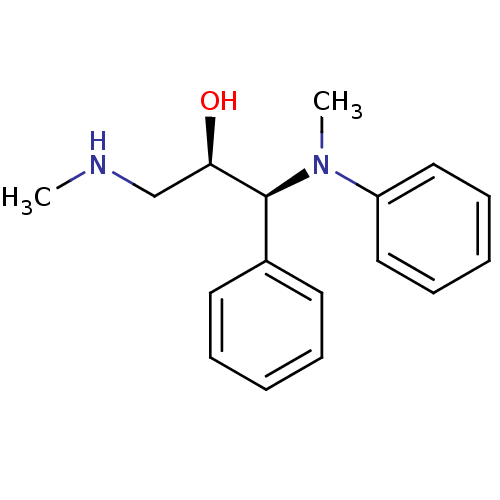
((R,S/S,R)-syn-1-(methyl(phenyl)amino)-3-(methylami...)Show SMILES CNC[C@@H](O)[C@@H](N(C)c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C17H22N2O/c1-18-13-16(20)17(14-9-5-3-6-10-14)19(2)15-11-7-4-8-12-15/h3-12,16-18,20H,13H2,1-2H3/t16-,17+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK-Net6 cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50227158

(2-(4-(3-(3-benzyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES Cc1cc(OCc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)c(C)cc1CC(O)=O Show InChI InChI=1S/C34H28F3NO3/c1-21-15-30(22(2)14-26(21)18-31(39)40)41-20-24-10-6-11-25(17-24)32-27(16-23-8-4-3-5-9-23)19-38-33-28(32)12-7-13-29(33)34(35,36)37/h3-15,17,19H,16,18,20H2,1-2H3,(H,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRalpha |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50259229
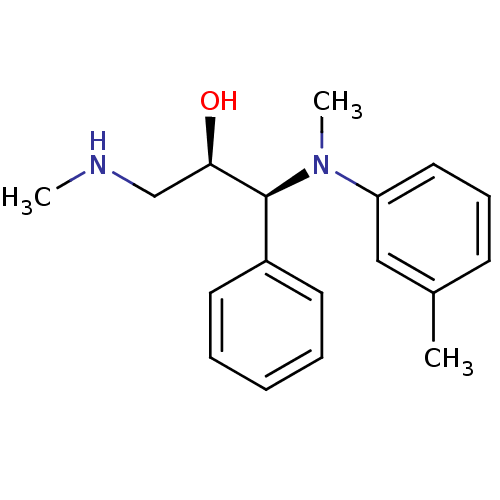
((R,S/S,R)-syn-1-(methyl(m-tolyl)amino)-3-(methylam...)Show SMILES CNC[C@@H](O)[C@@H](N(C)c1cccc(C)c1)c1ccccc1 |r| Show InChI InChI=1S/C18H24N2O/c1-14-8-7-11-16(12-14)20(3)18(17(21)13-19-2)15-9-5-4-6-10-15/h4-12,17-19,21H,13H2,1-3H3/t17-,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of serotonin uptake at human SERT expressed in human JAR cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50259231

((R,S/S,R)-syn-1-((4-fluorophenyl)(methyl)amino)-3-...)Show SMILES CNC[C@@H](O)[C@@H](N(C)c1ccc(F)cc1)c1ccccc1 |r| Show InChI InChI=1S/C17H21FN2O/c1-19-12-16(21)17(13-6-4-3-5-7-13)20(2)15-10-8-14(18)9-11-15/h3-11,16-17,19,21H,12H2,1-2H3/t16-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of serotonin uptake at human SERT expressed in human JAR cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50259343
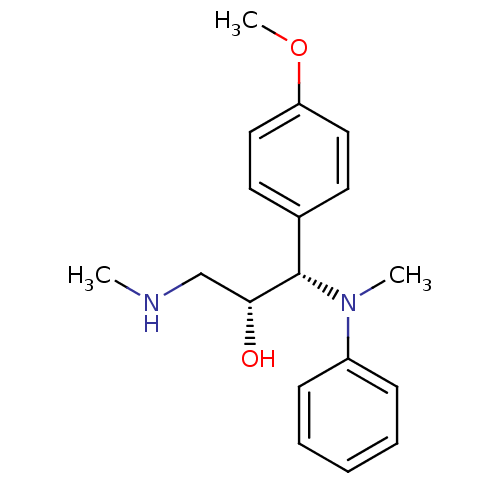
((R,S/S,R)-syn-1-(4-methoxyphenyl)-1-(methyl(phenyl...)Show SMILES CNC[C@@H](O)[C@@H](N(C)c1ccccc1)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C18H24N2O2/c1-19-13-17(21)18(14-9-11-16(22-3)12-10-14)20(2)15-7-5-4-6-8-15/h4-12,17-19,21H,13H2,1-3H3/t17-,18+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine uptake at human NET expressed in MDCK-Net6 cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50227148

(5-(3-(3-benzyl-8-(trifluoromethyl)quinolin-4-yl)be...)Show SMILES OC(=O)c1cccc2c(NCc3cccc(c3)-c3c(Cc4ccccc4)cnc4c(cccc34)C(F)(F)F)cccc12 Show InChI InChI=1S/C35H25F3N2O2/c36-35(37,38)30-16-6-15-29-32(25(21-40-33(29)30)18-22-8-2-1-3-9-22)24-11-4-10-23(19-24)20-39-31-17-7-12-26-27(31)13-5-14-28(26)34(41)42/h1-17,19,21,39H,18,20H2,(H,41,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRbeta |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50259273

((R,S/S,R)-syn-1-((3-chlorophenyl)(methyl)amino)-3-...)Show SMILES CNC[C@@H](O)[C@@H](N(C)c1cccc(Cl)c1)c1ccccc1 |r| Show InChI InChI=1S/C17H21ClN2O/c1-19-12-16(21)17(13-7-4-3-5-8-13)20(2)15-10-6-9-14(18)11-15/h3-11,16-17,19,21H,12H2,1-2H3/t16-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of serotonin uptake at human SERT expressed in human JAR cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM20002

(2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...)Show SMILES OC(=O)Cc1ccc(NCc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C32H25F3N2O2/c33-32(34,35)28-11-5-10-27-30(25(20-37-31(27)28)16-21-6-2-1-3-7-21)24-9-4-8-23(17-24)19-36-26-14-12-22(13-15-26)18-29(38)39/h1-15,17,20,36H,16,18-19H2,(H,38,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity at human LXRalpha |
Bioorg Med Chem Lett 18: 54-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.013
BindingDB Entry DOI: 10.7270/Q2N879JT |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50366567

(ATOMOXETINE)Show InChI InChI=1S/C17H21NO/c1-14-8-6-7-11-16(14)19-17(12-13-18-2)15-9-4-3-5-10-15/h3-11,17-18H,12-13H2,1-2H3/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of serotonin uptake at human SERT expressed in human JAR cells |
Bioorg Med Chem Lett 19: 2464-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.054
BindingDB Entry DOI: 10.7270/Q2T43V1C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data