 Found 76 hits with Last Name = 'dupureur' and Initial = 'cm'
Found 76 hits with Last Name = 'dupureur' and Initial = 'cm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hormone-sensitive lipase
(Rattus norvegicus (Rat)) | BDBM50064422

(CHEMBL3401162)Show SMILES [H][C@@]12COC(=O)C1=C(C)O[P@](=O)(OCCCCCCCCCCCCCCCC)OC2 |r,t:7| Show InChI InChI=1S/C23H41O6P/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-27-30(25)28-19-21-18-26-23(24)22(21)20(2)29-30/h21H,3-19H2,1-2H3/t21-,30+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Irreversible inhibition of rat hormone sensitive lipase assessed as relative residual activity by kinetic analysis |
Bioorg Med Chem 23: 944-52 (2015)
Article DOI: 10.1016/j.bmc.2015.01.028
BindingDB Entry DOI: 10.7270/Q23X88BP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308520

(CHEMBL603622 | cyclophostin)Show SMILES CO[P@]1(=O)OC[C@H]2COC(=O)C2=C(C)O1 |r,t:12| Show InChI InChI=1S/C8H11O6P/c1-5-7-6(3-12-8(7)9)4-13-15(10,11-2)14-5/h6H,3-4H2,1-2H3/t6-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Irreversible inhibition of AChE (unknown origin) |
Bioorg Med Chem 23: 7529-34 (2015)
Article DOI: 10.1016/j.bmc.2015.10.044
BindingDB Entry DOI: 10.7270/Q2N58QCW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50022772

(CHEMBL1025 | Diisopropoxyphosphoryl fluoride | Dii...)Show InChI InChI=1S/C6H14FO3P/c1-5(2)9-11(7,8)10-6(3)4/h5-6H,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as equilibrium binding by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2265-74 (2010)
Article DOI: 10.1016/j.bmc.2010.01.063
BindingDB Entry DOI: 10.7270/Q2RV0NTP |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50241979
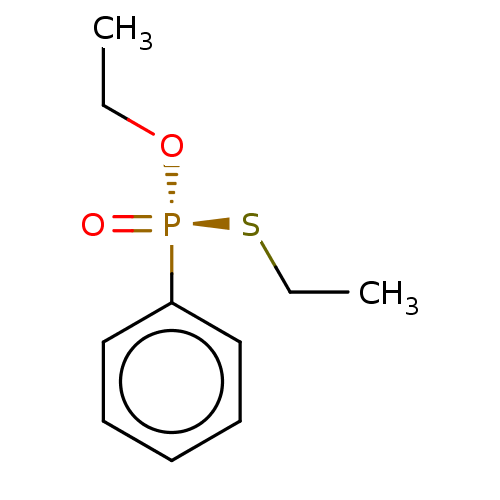
(CHEMBL4066365)Show InChI InChI=1S/C10H15O2PS/c1-3-12-13(11,14-4-2)10-8-6-5-7-9-10/h5-9H,3-4H2,1-2H3/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of equine BChE assessed as equilibrium constant using butyrylthiocholine iodide as substrate after 30 mins |
Bioorg Med Chem 25: 3053-3058 (2017)
Article DOI: 10.1016/j.bmc.2017.03.058
BindingDB Entry DOI: 10.7270/Q2H70J09 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50241979

(CHEMBL4066365)Show InChI InChI=1S/C10H15O2PS/c1-3-12-13(11,14-4-2)10-8-6-5-7-9-10/h5-9H,3-4H2,1-2H3/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as equilibrium constant using acetylthiocholine as substrate after 30 mins |
Bioorg Med Chem 25: 3053-3058 (2017)
Article DOI: 10.1016/j.bmc.2017.03.058
BindingDB Entry DOI: 10.7270/Q2H70J09 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308513
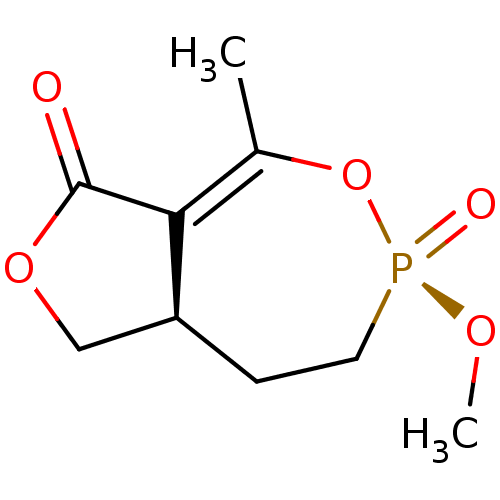
((3R,8aR)-3-Methoxy-5-methyl-3-oxo-8,8a-dihydro-1H-...)Show SMILES CO[P@@]1(=O)CC[C@H]2COC(=O)C2=C(C)O1 |r,t:12| Show InChI InChI=1S/C9H13O5P/c1-6-8-7(5-13-9(8)10)3-4-15(11,12-2)14-6/h7H,3-5H2,1-2H3/t7-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as equilibrium binding by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2265-74 (2010)
Article DOI: 10.1016/j.bmc.2010.01.063
BindingDB Entry DOI: 10.7270/Q2RV0NTP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308515

(2-Methoxy-7-methyl-2-oxo-2,3,4,5-tetrahydro-2lambd...)Show InChI InChI=1S/C9H15O5P/c1-7-8(9(10)12-2)5-4-6-15(11,13-3)14-7/h4-6H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as equilibrium binding by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2265-74 (2010)
Article DOI: 10.1016/j.bmc.2010.01.063
BindingDB Entry DOI: 10.7270/Q2RV0NTP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308513

((3R,8aR)-3-Methoxy-5-methyl-3-oxo-8,8a-dihydro-1H-...)Show SMILES CO[P@@]1(=O)CC[C@H]2COC(=O)C2=C(C)O1 |r,t:12| Show InChI InChI=1S/C9H13O5P/c1-6-8-7(5-13-9(8)10)3-4-15(11,12-2)14-6/h7H,3-5H2,1-2H3/t7-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as equilibrium binding by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2265-74 (2010)
Article DOI: 10.1016/j.bmc.2010.01.063
BindingDB Entry DOI: 10.7270/Q2RV0NTP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308517
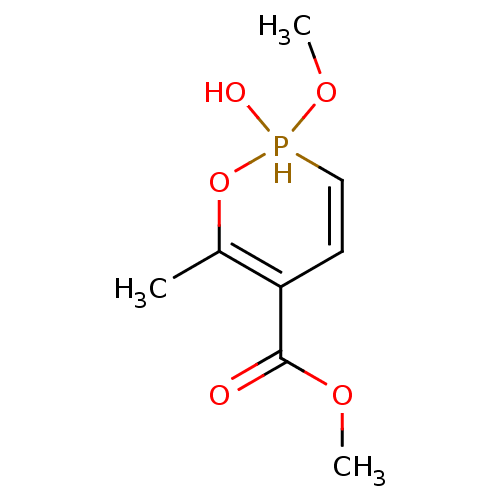
(2-Methoxy-6-methyl-2-oxo-3,4-dihydro-2H-2lambda*5*...)Show InChI InChI=1S/C8H13O5P/c1-6-7(8(9)11-2)4-5-14(10,12-3)13-6/h4-5,10,14H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE assessed as equilibrium binding by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2265-74 (2010)
Article DOI: 10.1016/j.bmc.2010.01.063
BindingDB Entry DOI: 10.7270/Q2RV0NTP |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Musca domestica) | BDBM50308520

(CHEMBL603622 | cyclophostin)Show SMILES CO[P@]1(=O)OC[C@H]2COC(=O)C2=C(C)O1 |r,t:12| Show InChI InChI=1S/C8H11O6P/c1-5-7-6(3-12-8(7)9)4-13-15(10,11-2)14-5/h6H,3-4H2,1-2H3/t6-,15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of housefly CSMA AChE |
Bioorg Med Chem 23: 7529-34 (2015)
Article DOI: 10.1016/j.bmc.2015.10.044
BindingDB Entry DOI: 10.7270/Q2N58QCW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308520

(CHEMBL603622 | cyclophostin)Show SMILES CO[P@]1(=O)OC[C@H]2COC(=O)C2=C(C)O1 |r,t:12| Show InChI InChI=1S/C8H11O6P/c1-5-7-6(3-12-8(7)9)4-13-15(10,11-2)14-5/h6H,3-4H2,1-2H3/t6-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2265-74 (2010)
Article DOI: 10.1016/j.bmc.2010.01.063
BindingDB Entry DOI: 10.7270/Q2RV0NTP |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50240416
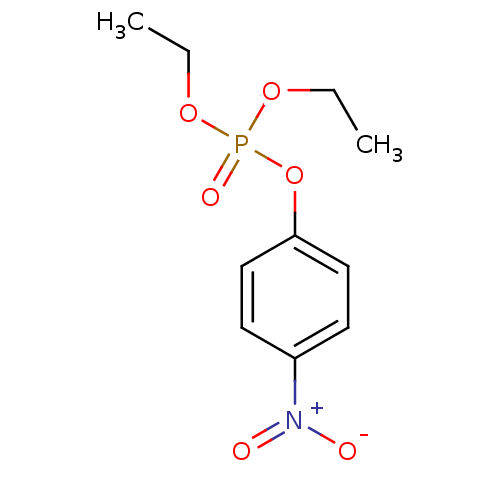
(CHEMBL23838 | O,O-diethyl O-p-nitrophenyl phosphat...)Show InChI InChI=1S/C10H14NO6P/c1-3-15-18(14,16-4-2)17-10-7-5-9(6-8-10)11(12)13/h5-8H,3-4H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human BChE using butyrylthiocholine as substrate measured for 30 secs by Ellman's method |
Bioorg Med Chem 25: 3053-3058 (2017)
Article DOI: 10.1016/j.bmc.2017.03.058
BindingDB Entry DOI: 10.7270/Q2H70J09 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50240416

(CHEMBL23838 | O,O-diethyl O-p-nitrophenyl phosphat...)Show InChI InChI=1S/C10H14NO6P/c1-3-15-18(14,16-4-2)17-10-7-5-9(6-8-10)11(12)13/h5-8H,3-4H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using p-nitrophenyl acetate as substrate measured for 30 secs by spectrophotometric analysis |
Bioorg Med Chem 25: 3053-3058 (2017)
Article DOI: 10.1016/j.bmc.2017.03.058
BindingDB Entry DOI: 10.7270/Q2H70J09 |
More data for this
Ligand-Target Pair | |
Hormone-sensitive lipase
(Rattus norvegicus (Rat)) | BDBM50064422

(CHEMBL3401162)Show SMILES [H][C@@]12COC(=O)C1=C(C)O[P@](=O)(OCCCCCCCCCCCCCCCC)OC2 |r,t:7| Show InChI InChI=1S/C23H41O6P/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-27-30(25)28-19-21-18-26-23(24)22(21)20(2)29-30/h21H,3-19H2,1-2H3/t21-,30+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of rat hormone sensitive lipase assessed as oleic acid release from [3H]triolein by scintillation counting |
Bioorg Med Chem 23: 944-52 (2015)
Article DOI: 10.1016/j.bmc.2015.01.028
BindingDB Entry DOI: 10.7270/Q23X88BP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50064426
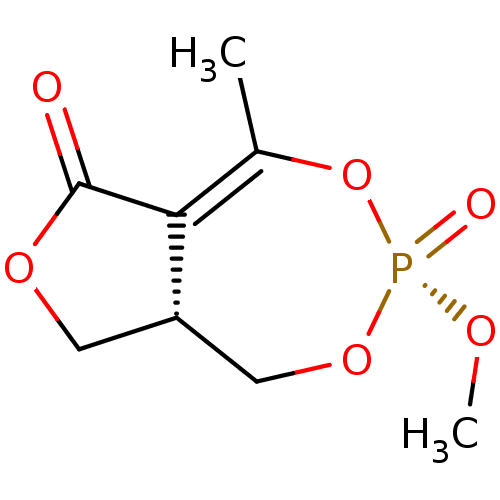
(CHEMBL3401159)Show SMILES [H][C@@]12COC(=O)C1=C(C)O[P@](=O)(OC)OC2 |r,t:7| Show InChI InChI=1S/C8H11O6P/c1-5-7-6(3-12-8(7)9)4-13-15(10,11-2)14-5/h6H,3-4H2,1-2H3/t6-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE at pH 8 |
Bioorg Med Chem 23: 7529-34 (2015)
Article DOI: 10.1016/j.bmc.2015.10.044
BindingDB Entry DOI: 10.7270/Q2N58QCW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308520

(CHEMBL603622 | cyclophostin)Show SMILES CO[P@]1(=O)OC[C@H]2COC(=O)C2=C(C)O1 |r,t:12| Show InChI InChI=1S/C8H11O6P/c1-5-7-6(3-12-8(7)9)4-13-15(10,11-2)14-5/h6H,3-4H2,1-2H3/t6-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE at pH 8 |
Bioorg Med Chem 23: 7529-34 (2015)
Article DOI: 10.1016/j.bmc.2015.10.044
BindingDB Entry DOI: 10.7270/Q2N58QCW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308520

(CHEMBL603622 | cyclophostin)Show SMILES CO[P@]1(=O)OC[C@H]2COC(=O)C2=C(C)O1 |r,t:12| Show InChI InChI=1S/C8H11O6P/c1-5-7-6(3-12-8(7)9)4-13-15(10,11-2)14-5/h6H,3-4H2,1-2H3/t6-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
Bioorg Med Chem 23: 7529-34 (2015)
Article DOI: 10.1016/j.bmc.2015.10.044
BindingDB Entry DOI: 10.7270/Q2N58QCW |
More data for this
Ligand-Target Pair | |
Hormone-sensitive lipase
(Rattus norvegicus (Rat)) | BDBM50064413
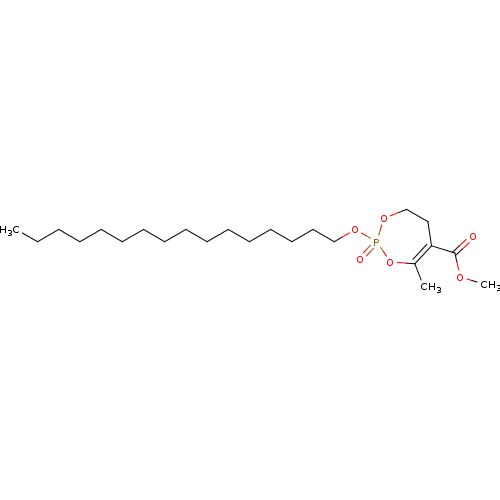
(CHEMBL3401168)Show SMILES CCCCCCCCCCCCCCCCOP1(=O)OCCC(C(=O)OC)=C(C)O1 |t:26| Show InChI InChI=1S/C23H43O6P/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-19-27-30(25)28-20-18-22(21(2)29-30)23(24)26-3/h4-20H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of rat hormone sensitive lipase assessed as oleic acid release from [3H]triolein by scintillation counting |
Bioorg Med Chem 23: 944-52 (2015)
Article DOI: 10.1016/j.bmc.2015.01.028
BindingDB Entry DOI: 10.7270/Q23X88BP |
More data for this
Ligand-Target Pair | |
Hormone-sensitive lipase
(Rattus norvegicus (Rat)) | BDBM50064423
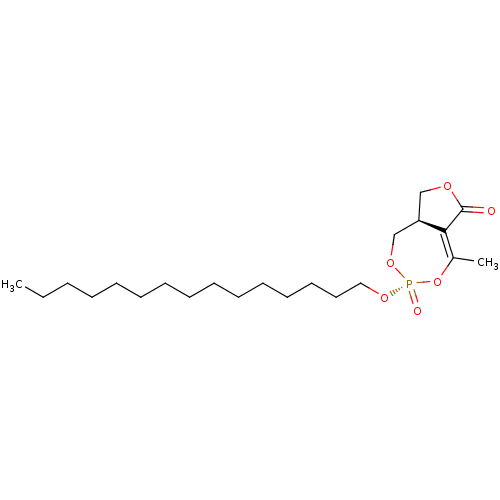
(CHEMBL3401161)Show SMILES [H][C@]12COC(=O)C1=C(C)O[P@](=O)(OCCCCCCCCCCCCCCC)OC2 |r,t:7| Show InChI InChI=1S/C22H39O6P/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-26-29(24)27-18-20-17-25-22(23)21(20)19(2)28-29/h20H,3-18H2,1-2H3/t20-,29-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of rat hormone sensitive lipase assessed as oleic acid release from [3H]triolein by scintillation counting |
Bioorg Med Chem 23: 944-52 (2015)
Article DOI: 10.1016/j.bmc.2015.01.028
BindingDB Entry DOI: 10.7270/Q23X88BP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50022772

(CHEMBL1025 | Diisopropoxyphosphoryl fluoride | Dii...)Show InChI InChI=1S/C6H14FO3P/c1-5(2)9-11(7,8)10-6(3)4/h5-6H,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2265-74 (2010)
Article DOI: 10.1016/j.bmc.2010.01.063
BindingDB Entry DOI: 10.7270/Q2RV0NTP |
More data for this
Ligand-Target Pair | |
Hormone-sensitive lipase
(Rattus norvegicus (Rat)) | BDBM50064414
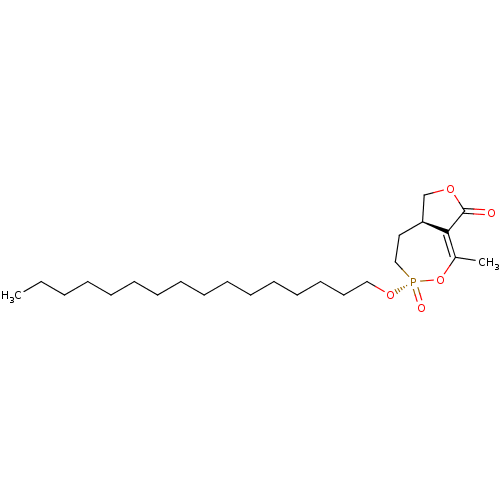
(CHEMBL3401167)Show SMILES [H][C@]12COC(=O)C1=C(C)O[P@@](=O)(CC2)OCCCCCCCCCCCCCCCC |r,t:7| Show InChI InChI=1S/C24H43O5P/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-18-28-30(26)19-17-22-20-27-24(25)23(22)21(2)29-30/h22H,3-20H2,1-2H3/t22-,30+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of rat hormone sensitive lipase assessed as oleic acid release from [3H]triolein by scintillation counting |
Bioorg Med Chem 23: 944-52 (2015)
Article DOI: 10.1016/j.bmc.2015.01.028
BindingDB Entry DOI: 10.7270/Q23X88BP |
More data for this
Ligand-Target Pair | |
Hormone-sensitive lipase
(Rattus norvegicus (Rat)) | BDBM50064421
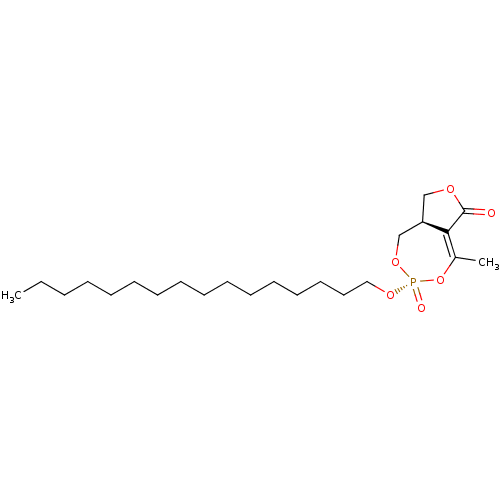
(CHEMBL3401163)Show SMILES [H][C@]12COC(=O)C1=C(C)O[P@](=O)(OCCCCCCCCCCCCCCCC)OC2 |r,t:7| Show InChI InChI=1S/C23H41O6P/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-27-30(25)28-19-21-18-26-23(24)22(21)20(2)29-30/h21H,3-19H2,1-2H3/t21-,30-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of rat hormone sensitive lipase assessed as oleic acid release from [3H]triolein by scintillation counting |
Bioorg Med Chem 23: 944-52 (2015)
Article DOI: 10.1016/j.bmc.2015.01.028
BindingDB Entry DOI: 10.7270/Q23X88BP |
More data for this
Ligand-Target Pair | |
Hormone-sensitive lipase
(Rattus norvegicus (Rat)) | BDBM50064418
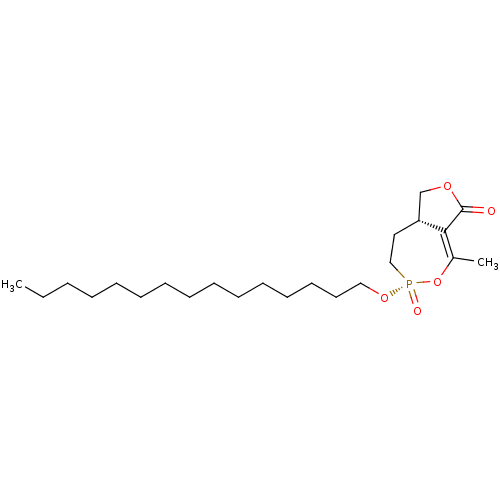
(CHEMBL3401164)Show SMILES [H][C@@]12COC(=O)C1=C(C)O[P@@](=O)(CC2)OCCCCCCCCCCCCCCC |r,t:7| Show InChI InChI=1S/C23H41O5P/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-17-27-29(25)18-16-21-19-26-23(24)22(21)20(2)28-29/h21H,3-19H2,1-2H3/t21-,29-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of rat hormone sensitive lipase assessed as oleic acid release from [3H]triolein by scintillation counting |
Bioorg Med Chem 23: 944-52 (2015)
Article DOI: 10.1016/j.bmc.2015.01.028
BindingDB Entry DOI: 10.7270/Q23X88BP |
More data for this
Ligand-Target Pair | |
Hormone-sensitive lipase
(Rattus norvegicus (Rat)) | BDBM50064416

(CHEMBL3401165)Show SMILES [H][C@]12COC(=O)C1=C(C)O[P@@](=O)(CC2)OCCCCCCCCCCCCCCC |r,t:7| Show InChI InChI=1S/C23H41O5P/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-17-27-29(25)18-16-21-19-26-23(24)22(21)20(2)28-29/h21H,3-19H2,1-2H3/t21-,29+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of rat hormone sensitive lipase assessed as oleic acid release from [3H]triolein by scintillation counting |
Bioorg Med Chem 23: 944-52 (2015)
Article DOI: 10.1016/j.bmc.2015.01.028
BindingDB Entry DOI: 10.7270/Q23X88BP |
More data for this
Ligand-Target Pair | |
Hormone-sensitive lipase
(Rattus norvegicus (Rat)) | BDBM50064425
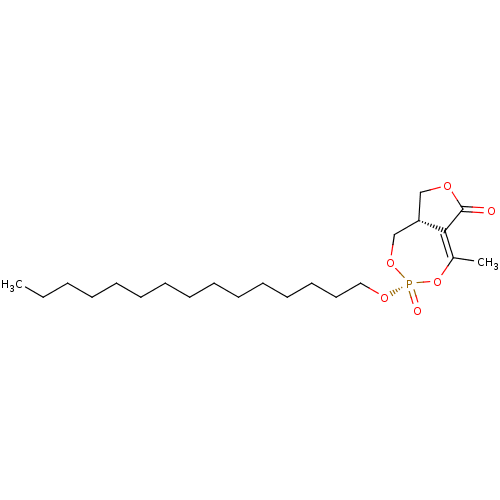
(CHEMBL3401160)Show SMILES [H][C@@]12COC(=O)C1=C(C)O[P@](=O)(OCCCCCCCCCCCCCCC)OC2 |r,t:7| Show InChI InChI=1S/C22H39O6P/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-26-29(24)27-18-20-17-25-22(23)21(20)19(2)28-29/h20H,3-18H2,1-2H3/t20-,29+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of rat hormone sensitive lipase assessed as oleic acid release from [3H]triolein by scintillation counting |
Bioorg Med Chem 23: 944-52 (2015)
Article DOI: 10.1016/j.bmc.2015.01.028
BindingDB Entry DOI: 10.7270/Q23X88BP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50499370
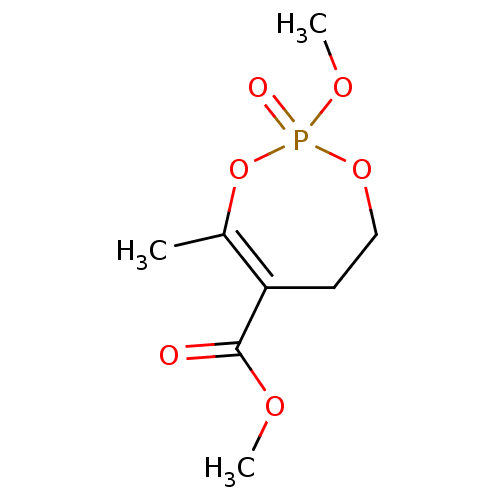
(CHEMBL3736339)Show InChI InChI=1S/C8H13O6P/c1-6-7(8(9)11-2)4-5-13-15(10,12-3)14-6/h4-5H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE at pH 8 |
Bioorg Med Chem 23: 7529-34 (2015)
Article DOI: 10.1016/j.bmc.2015.10.044
BindingDB Entry DOI: 10.7270/Q2N58QCW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50499370

(CHEMBL3736339)Show InChI InChI=1S/C8H13O6P/c1-6-7(8(9)11-2)4-5-13-15(10,12-3)14-6/h4-5H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE at pH 6 |
Bioorg Med Chem 23: 7529-34 (2015)
Article DOI: 10.1016/j.bmc.2015.10.044
BindingDB Entry DOI: 10.7270/Q2N58QCW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308513

((3R,8aR)-3-Methoxy-5-methyl-3-oxo-8,8a-dihydro-1H-...)Show SMILES CO[P@@]1(=O)CC[C@H]2COC(=O)C2=C(C)O1 |r,t:12| Show InChI InChI=1S/C9H13O5P/c1-6-8-7(5-13-9(8)10)3-4-15(11,12-2)14-6/h7H,3-5H2,1-2H3/t7-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2265-74 (2010)
Article DOI: 10.1016/j.bmc.2010.01.063
BindingDB Entry DOI: 10.7270/Q2RV0NTP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50241989
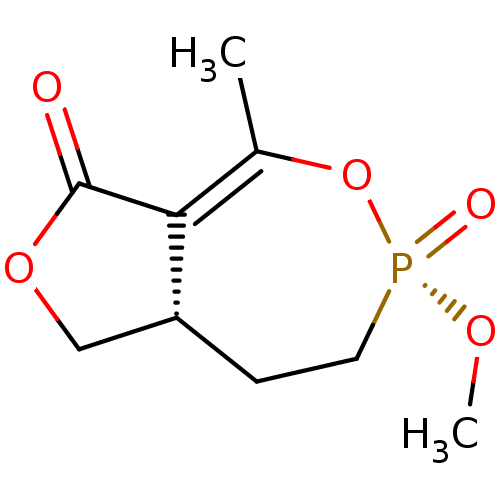
(CHEMBL2179375)Show SMILES [H][C@@]12COC(=O)C1=C(C)O[P@@](=O)(CC2)OC |r,t:7| Show InChI InChI=1S/C9H13O5P/c1-6-8-7(5-13-9(8)10)3-4-15(11,12-2)14-6/h7H,3-5H2,1-2H3/t7-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 30 min followed by substrate addition measured ever... |
Bioorg Med Chem 25: 3053-3058 (2017)
Article DOI: 10.1016/j.bmc.2017.03.058
BindingDB Entry DOI: 10.7270/Q2H70J09 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50241989

(CHEMBL2179375)Show SMILES [H][C@@]12COC(=O)C1=C(C)O[P@@](=O)(CC2)OC |r,t:7| Show InChI InChI=1S/C9H13O5P/c1-6-8-7(5-13-9(8)10)3-4-15(11,12-2)14-6/h7H,3-5H2,1-2H3/t7-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE at pH 8 |
Bioorg Med Chem 23: 7529-34 (2015)
Article DOI: 10.1016/j.bmc.2015.10.044
BindingDB Entry DOI: 10.7270/Q2N58QCW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308517

(2-Methoxy-6-methyl-2-oxo-3,4-dihydro-2H-2lambda*5*...)Show InChI InChI=1S/C8H13O5P/c1-6-7(8(9)11-2)4-5-14(10,12-3)13-6/h4-5,10,14H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2265-74 (2010)
Article DOI: 10.1016/j.bmc.2010.01.063
BindingDB Entry DOI: 10.7270/Q2RV0NTP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50499368
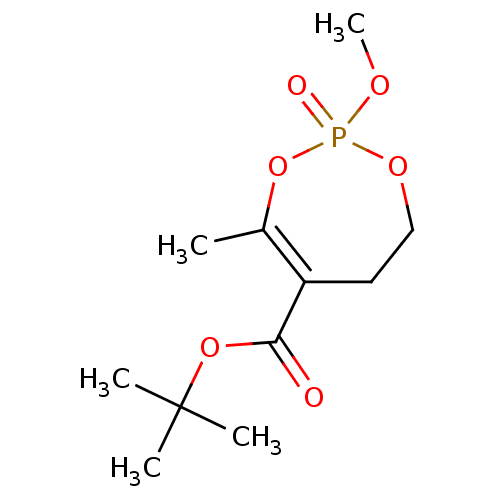
(CHEMBL3735606)Show InChI InChI=1S/C11H19O6P/c1-8-9(10(12)16-11(2,3)4)6-7-15-18(13,14-5)17-8/h6-7H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE at pH 8 |
Bioorg Med Chem 23: 7529-34 (2015)
Article DOI: 10.1016/j.bmc.2015.10.044
BindingDB Entry DOI: 10.7270/Q2N58QCW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308514
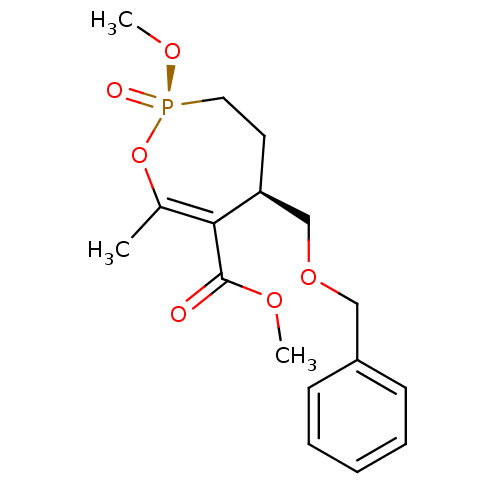
((2R,6R)-6-Benzyloxymethyl-2-methoxy-4-methyl-2-oxo...)Show SMILES COC(=O)C1=C(C)O[P@](=O)(CC[C@H]1COCc1ccccc1)OC |r,c:4| Show InChI InChI=1S/C17H23O6P/c1-13-16(17(18)20-2)15(9-10-24(19,21-3)23-13)12-22-11-14-7-5-4-6-8-14/h4-8,15H,9-12H2,1-3H3/t15-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2265-74 (2010)
Article DOI: 10.1016/j.bmc.2010.01.063
BindingDB Entry DOI: 10.7270/Q2RV0NTP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50499368

(CHEMBL3735606)Show InChI InChI=1S/C11H19O6P/c1-8-9(10(12)16-11(2,3)4)6-7-15-18(13,14-5)17-8/h6-7H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE at pH 6 |
Bioorg Med Chem 23: 7529-34 (2015)
Article DOI: 10.1016/j.bmc.2015.10.044
BindingDB Entry DOI: 10.7270/Q2N58QCW |
More data for this
Ligand-Target Pair | |
Hormone-sensitive lipase
(Rattus norvegicus (Rat)) | BDBM50064415
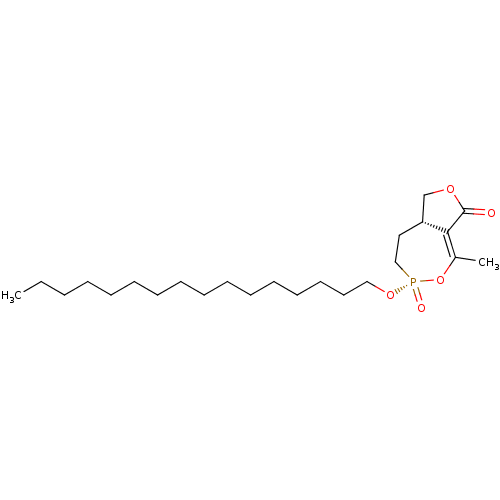
(CHEMBL3401166)Show SMILES [H][C@@]12COC(=O)C1=C(C)O[P@@](=O)(CC2)OCCCCCCCCCCCCCCCC |r,t:7| Show InChI InChI=1S/C24H43O5P/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-18-28-30(26)19-17-22-20-27-24(25)23(22)21(2)29-30/h22H,3-20H2,1-2H3/t22-,30-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of rat hormone sensitive lipase assessed as oleic acid release from [3H]triolein by scintillation counting |
Bioorg Med Chem 23: 944-52 (2015)
Article DOI: 10.1016/j.bmc.2015.01.028
BindingDB Entry DOI: 10.7270/Q23X88BP |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50241979

(CHEMBL4066365)Show InChI InChI=1S/C10H15O2PS/c1-3-12-13(11,14-4-2)10-8-6-5-7-9-10/h5-9H,3-4H2,1-2H3/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of horse BChE using butyrylthiocholine iodide as substrate measured for 30 secs by Ellman's method |
Bioorg Med Chem 25: 3053-3058 (2017)
Article DOI: 10.1016/j.bmc.2017.03.058
BindingDB Entry DOI: 10.7270/Q2H70J09 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308515

(2-Methoxy-7-methyl-2-oxo-2,3,4,5-tetrahydro-2lambd...)Show InChI InChI=1S/C9H15O5P/c1-7-8(9(10)12-2)5-4-6-15(11,13-3)14-7/h4-6H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2265-74 (2010)
Article DOI: 10.1016/j.bmc.2010.01.063
BindingDB Entry DOI: 10.7270/Q2RV0NTP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50499367
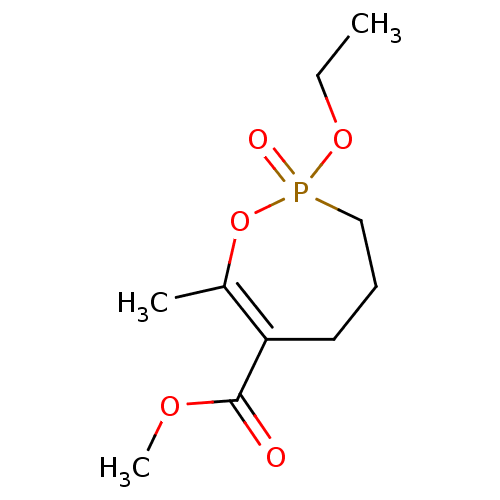
(CHEMBL3735042)Show InChI InChI=1S/C10H17O5P/c1-4-14-16(12)7-5-6-9(8(2)15-16)10(11)13-3/h4-7H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE at pH 8 |
Bioorg Med Chem 23: 7529-34 (2015)
Article DOI: 10.1016/j.bmc.2015.10.044
BindingDB Entry DOI: 10.7270/Q2N58QCW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50241979

(CHEMBL4066365)Show InChI InChI=1S/C10H15O2PS/c1-3-12-13(11,14-4-2)10-8-6-5-7-9-10/h5-9H,3-4H2,1-2H3/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 30 secs by Ellman's method |
Bioorg Med Chem 25: 3053-3058 (2017)
Article DOI: 10.1016/j.bmc.2017.03.058
BindingDB Entry DOI: 10.7270/Q2H70J09 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50499367

(CHEMBL3735042)Show InChI InChI=1S/C10H17O5P/c1-4-14-16(12)7-5-6-9(8(2)15-16)10(11)13-3/h4-7H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE at pH 6 |
Bioorg Med Chem 23: 7529-34 (2015)
Article DOI: 10.1016/j.bmc.2015.10.044
BindingDB Entry DOI: 10.7270/Q2N58QCW |
More data for this
Ligand-Target Pair | |
Hormone-sensitive lipase
(Rattus norvegicus (Rat)) | BDBM50064408
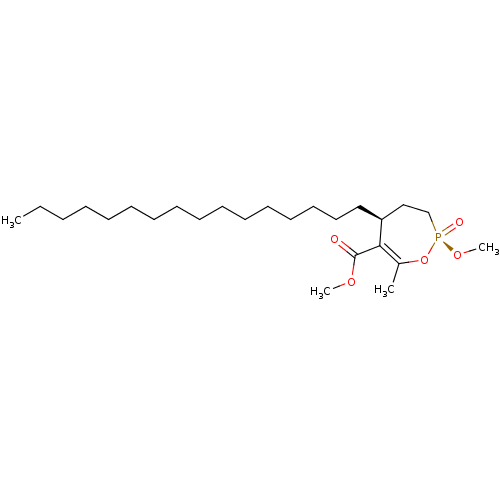
(CHEMBL3401171)Show SMILES CCCCCCCCCCCCCCCC[C@H]1CC[P@@](=O)(OC)OC(C)=C1C(=O)OC |r,c:25| Show InChI InChI=1S/C25H47O5P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-23-20-21-31(27,29-4)30-22(2)24(23)25(26)28-3/h23H,5-21H2,1-4H3/t23-,31+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of rat hormone sensitive lipase assessed as oleic acid release from [3H]triolein by scintillation counting |
Bioorg Med Chem 23: 944-52 (2015)
Article DOI: 10.1016/j.bmc.2015.01.028
BindingDB Entry DOI: 10.7270/Q23X88BP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308515

(2-Methoxy-7-methyl-2-oxo-2,3,4,5-tetrahydro-2lambd...)Show InChI InChI=1S/C9H15O5P/c1-7-8(9(10)12-2)5-4-6-15(11,13-3)14-7/h4-6H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE at pH 8 |
Bioorg Med Chem 23: 7529-34 (2015)
Article DOI: 10.1016/j.bmc.2015.10.044
BindingDB Entry DOI: 10.7270/Q2N58QCW |
More data for this
Ligand-Target Pair | |
Hormone-sensitive lipase
(Rattus norvegicus (Rat)) | BDBM50064410
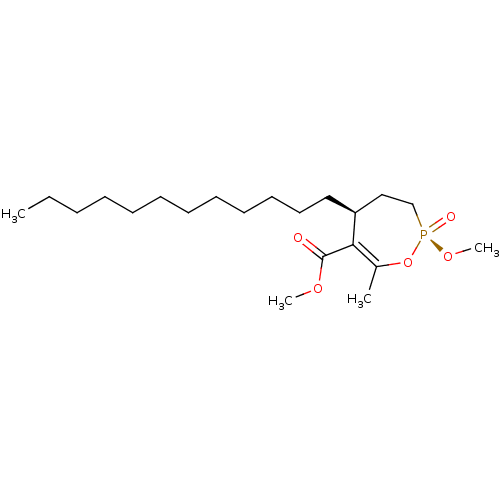
(CHEMBL3401170)Show SMILES CCCCCCCCCCCC[C@H]1CC[P@@](=O)(OC)OC(C)=C1C(=O)OC |r,c:21| Show InChI InChI=1S/C21H39O5P/c1-5-6-7-8-9-10-11-12-13-14-15-19-16-17-27(23,25-4)26-18(2)20(19)21(22)24-3/h19H,5-17H2,1-4H3/t19-,27+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of rat hormone sensitive lipase assessed as oleic acid release from [3H]triolein by scintillation counting |
Bioorg Med Chem 23: 944-52 (2015)
Article DOI: 10.1016/j.bmc.2015.01.028
BindingDB Entry DOI: 10.7270/Q23X88BP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308515

(2-Methoxy-7-methyl-2-oxo-2,3,4,5-tetrahydro-2lambd...)Show InChI InChI=1S/C9H15O5P/c1-7-8(9(10)12-2)5-4-6-15(11,13-3)14-7/h4-6H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE at pH 6 |
Bioorg Med Chem 23: 7529-34 (2015)
Article DOI: 10.1016/j.bmc.2015.10.044
BindingDB Entry DOI: 10.7270/Q2N58QCW |
More data for this
Ligand-Target Pair | |
Hormone-sensitive lipase
(Rattus norvegicus (Rat)) | BDBM50064427

(CHEMBL3401172)Show SMILES CCCCCCCCCCCCCCCCCC[C@H]1CC[P@@](=O)(OC)OC(C)=C1C(=O)OC |r,c:27| Show InChI InChI=1S/C27H51O5P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-25-22-23-33(29,31-4)32-24(2)26(25)27(28)30-3/h25H,5-23H2,1-4H3/t25-,33+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of rat hormone sensitive lipase assessed as oleic acid release from [3H]triolein by scintillation counting |
Bioorg Med Chem 23: 944-52 (2015)
Article DOI: 10.1016/j.bmc.2015.01.028
BindingDB Entry DOI: 10.7270/Q23X88BP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50241988
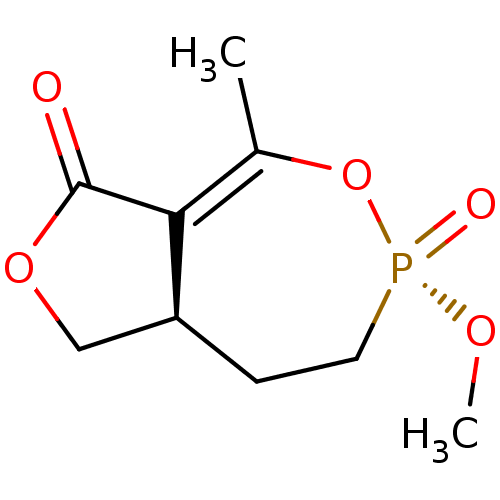
(CHEMBL592433)Show SMILES [H][C@]12COC(=O)C1=C(C)O[P@@](=O)(CC2)OC |r,t:7| Show InChI InChI=1S/C9H13O5P/c1-6-8-7(5-13-9(8)10)3-4-15(11,12-2)14-6/h7H,3-5H2,1-2H3/t7-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE at pH 8 |
Bioorg Med Chem 23: 7529-34 (2015)
Article DOI: 10.1016/j.bmc.2015.10.044
BindingDB Entry DOI: 10.7270/Q2N58QCW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50241988

(CHEMBL592433)Show SMILES [H][C@]12COC(=O)C1=C(C)O[P@@](=O)(CC2)OC |r,t:7| Show InChI InChI=1S/C9H13O5P/c1-6-8-7(5-13-9(8)10)3-4-15(11,12-2)14-6/h7H,3-5H2,1-2H3/t7-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 30 min followed by substrate addition measured ever... |
Bioorg Med Chem 25: 3053-3058 (2017)
Article DOI: 10.1016/j.bmc.2017.03.058
BindingDB Entry DOI: 10.7270/Q2H70J09 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308513

((3R,8aR)-3-Methoxy-5-methyl-3-oxo-8,8a-dihydro-1H-...)Show SMILES CO[P@@]1(=O)CC[C@H]2COC(=O)C2=C(C)O1 |r,t:12| Show InChI InChI=1S/C9H13O5P/c1-6-8-7(5-13-9(8)10)3-4-15(11,12-2)14-6/h7H,3-5H2,1-2H3/t7-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2265-74 (2010)
Article DOI: 10.1016/j.bmc.2010.01.063
BindingDB Entry DOI: 10.7270/Q2RV0NTP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308514

((2R,6R)-6-Benzyloxymethyl-2-methoxy-4-methyl-2-oxo...)Show SMILES COC(=O)C1=C(C)O[P@](=O)(CC[C@H]1COCc1ccccc1)OC |r,c:4| Show InChI InChI=1S/C17H23O6P/c1-13-16(17(18)20-2)15(9-10-24(19,21-3)23-13)12-22-11-14-7-5-4-6-8-14/h4-8,15H,9-12H2,1-3H3/t15-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2265-74 (2010)
Article DOI: 10.1016/j.bmc.2010.01.063
BindingDB Entry DOI: 10.7270/Q2RV0NTP |
More data for this
Ligand-Target Pair | |
Hormone-sensitive lipase
(Rattus norvegicus (Rat)) | BDBM50064411
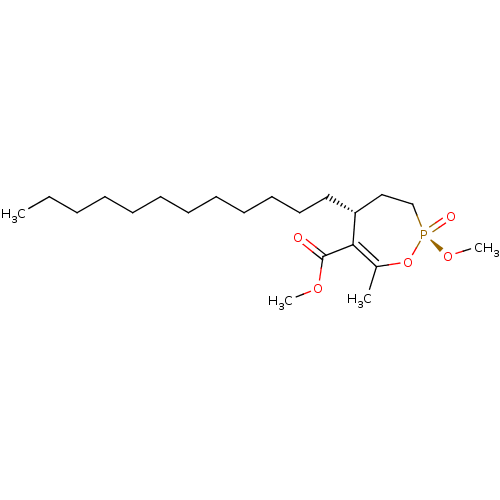
(CHEMBL2179369)Show SMILES CCCCCCCCCCCC[C@@H]1CC[P@@](=O)(OC)OC(C)=C1C(=O)OC |r,c:21| Show InChI InChI=1S/C21H39O5P/c1-5-6-7-8-9-10-11-12-13-14-15-19-16-17-27(23,25-4)26-18(2)20(19)21(22)24-3/h19H,5-17H2,1-4H3/t19-,27-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri St. Louis
Curated by ChEMBL
| Assay Description
Inhibition of rat hormone sensitive lipase assessed as oleic acid release from [3H]triolein by scintillation counting |
Bioorg Med Chem 23: 944-52 (2015)
Article DOI: 10.1016/j.bmc.2015.01.028
BindingDB Entry DOI: 10.7270/Q23X88BP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data