 Found 591 hits with Last Name = 'ellinger' and Initial = 'b'
Found 591 hits with Last Name = 'ellinger' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50278487

(CHEMBL3585376)Show SMILES CN(C)CCOc1cc2ccc(OC3=CC(=O)c4cc5ccccc5cc4C3=O)cc2oc1=O |t:13| Show InChI InChI=1S/C27H21NO6/c1-28(2)9-10-32-25-13-18-7-8-19(14-23(18)34-27(25)31)33-24-15-22(29)20-11-16-5-3-4-6-17(16)12-21(20)26(24)30/h3-8,11-15H,9-10H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of 125-I echistatin from Vitronectin receptor (alpha v beta3) |
Eur J Med Chem 141: 138-148 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.005
BindingDB Entry DOI: 10.7270/Q2JD50B3 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50278488
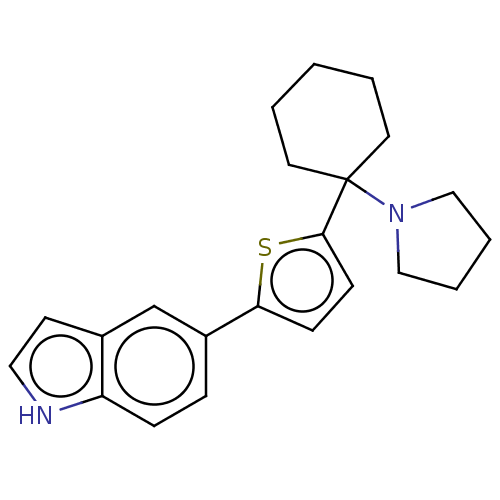
(CHEMBL4159241)Show SMILES C1CCN(C1)C1(CCCCC1)c1ccc(s1)-c1ccc2[nH]ccc2c1 Show InChI InChI=1S/C22H26N2S/c1-2-11-22(12-3-1,24-14-4-5-15-24)21-9-8-20(25-21)18-6-7-19-17(16-18)10-13-23-19/h6-10,13,16,23H,1-5,11-12,14-15H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothione disulfide as... |
Eur J Med Chem 141: 138-148 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.005
BindingDB Entry DOI: 10.7270/Q2JD50B3 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50278486
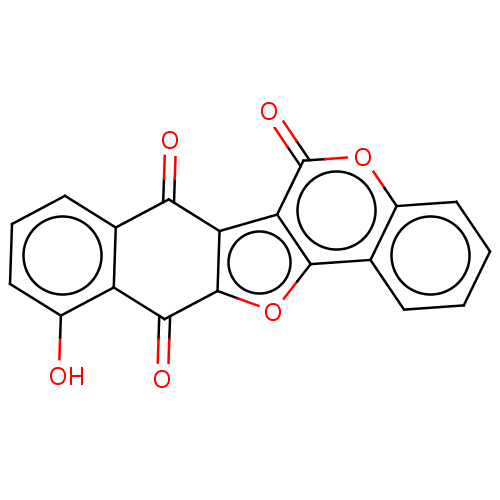
(CHEMBL4160100)Show SMILES Oc1cccc2C(=O)c3c(oc4c3c(=O)oc3ccccc43)C(=O)c12 Show InChI InChI=1S/C19H8O6/c20-10-6-3-5-9-12(10)16(22)18-13(15(9)21)14-17(25-18)8-4-1-2-7-11(8)24-19(14)23/h1-7,20H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothione d... |
Eur J Med Chem 141: 138-148 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.005
BindingDB Entry DOI: 10.7270/Q2JD50B3 |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50551183

(CHEMBL4747846)Show SMILES Oc1ccc(cc1)-c1ccc(-c2cc(O)cc(O)c2)c(c1)-c1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112047
BindingDB Entry DOI: 10.7270/Q2F47SSH |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM77970
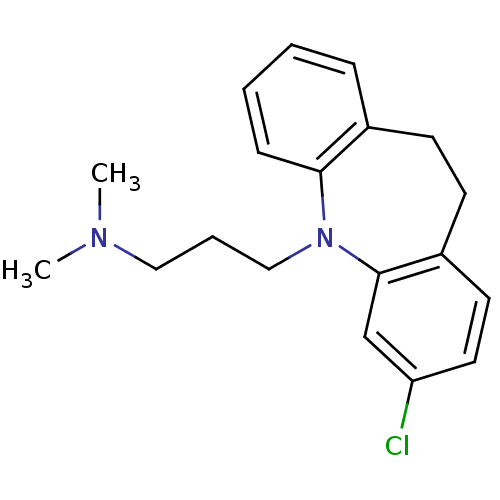
(3-(2-chloranyl-5,6-dihydrobenzo[b][1]benzazepin-11...)Show InChI InChI=1S/C19H23ClN2/c1-21(2)12-5-13-22-18-7-4-3-6-15(18)8-9-16-10-11-17(20)14-19(16)22/h3-4,6-7,10-11,14H,5,8-9,12-13H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothione disulfide as... |
Eur J Med Chem 141: 138-148 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.005
BindingDB Entry DOI: 10.7270/Q2JD50B3 |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50551184
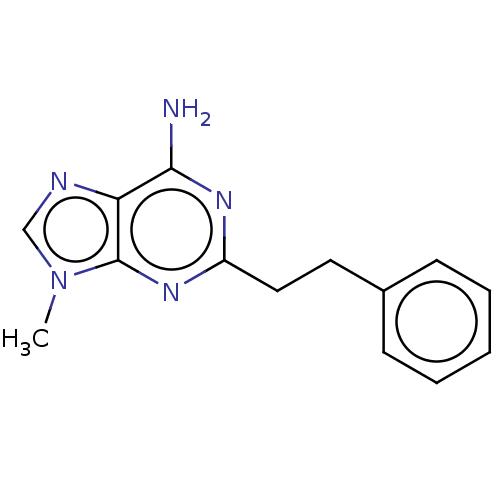
(CHEMBL4797185) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112047
BindingDB Entry DOI: 10.7270/Q2F47SSH |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50551180
(CHEMBL4760251)Show SMILES O=C1CCCCCNCc2ccc(Cc3ccc(CNCCCCCC(=O)N(Cc4ccccc4)CCCCCCCCN1Cc1ccccc1)cc3)cc2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112047
BindingDB Entry DOI: 10.7270/Q2F47SSH |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50551177

(CHEMBL4762279) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112047
BindingDB Entry DOI: 10.7270/Q2F47SSH |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50551178

(CHEMBL4785591)Show SMILES O=C1CCCCCNCc2ccc(cc2)-c2ccc(CNCCCCCC(=O)N(Cc3ccccc3)CCCCCCCCN1Cc1ccccc1)cc2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112047
BindingDB Entry DOI: 10.7270/Q2F47SSH |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50278489
(CHEMBL4170316)Show SMILES COc1cccc2C(=O)c3c(oc4c3c(=O)oc3ccccc43)C(=O)c12 Show InChI InChI=1S/C20H10O6/c1-24-12-8-4-6-10-13(12)17(22)19-14(16(10)21)15-18(26-19)9-5-2-3-7-11(9)25-20(15)23/h2-8H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothi... |
Eur J Med Chem 141: 138-148 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.005
BindingDB Entry DOI: 10.7270/Q2JD50B3 |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50551181
(CHEMBL54725)Show SMILES COc1ccccc1CNCc1ccc(CNCCCCCCCCCCCCNCc2ccc(CNCc3ccccc3OC)cc2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112047
BindingDB Entry DOI: 10.7270/Q2F47SSH |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50386758

(CHEMBL2046893)Show InChI InChI=1S/C23H24F2N2/c24-20-8-12-22(13-9-20)27(23-14-10-21(25)11-15-23)18-17-26-16-4-7-19-5-2-1-3-6-19/h1-3,5-6,8-15,26H,4,7,16-18H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112047
BindingDB Entry DOI: 10.7270/Q2F47SSH |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50551182

(CHEMBL4754292)Show SMILES [H][C@@]12CCN(CCCCCCN)[C@]1([H])c1ccccc1OC2 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112047
BindingDB Entry DOI: 10.7270/Q2F47SSH |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50551179
(CHEMBL4778279)Show SMILES O=C1CCCCCNCc2ccc(CNCCCCCC(=O)N(Cc3ccccc3)CCCCCCCCN1Cc1ccccc1)cc2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112047
BindingDB Entry DOI: 10.7270/Q2F47SSH |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50551175

(CHEMBL1993081) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112047
BindingDB Entry DOI: 10.7270/Q2F47SSH |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50551174

(CHEMBL158919) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112047
BindingDB Entry DOI: 10.7270/Q2F47SSH |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50551173

(CHEMBL4753331) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112047
BindingDB Entry DOI: 10.7270/Q2F47SSH |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50062821
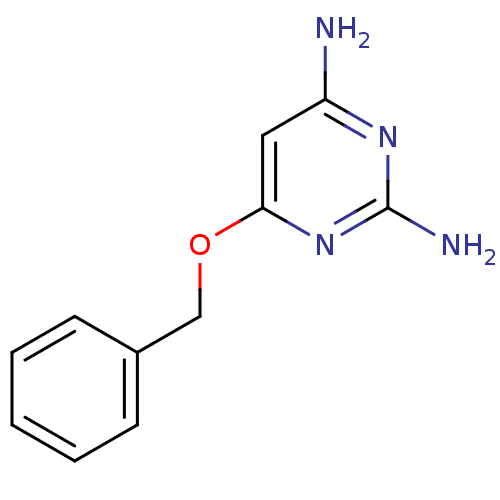
(6-Benzyloxy-pyrimidine-2,4-diamine | CHEMBL121445)Show InChI InChI=1S/C11H12N4O/c12-9-6-10(15-11(13)14-9)16-7-8-4-2-1-3-5-8/h1-6H,7H2,(H4,12,13,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112047
BindingDB Entry DOI: 10.7270/Q2F47SSH |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50551176

(CHEMBL4748094) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112047
BindingDB Entry DOI: 10.7270/Q2F47SSH |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600683
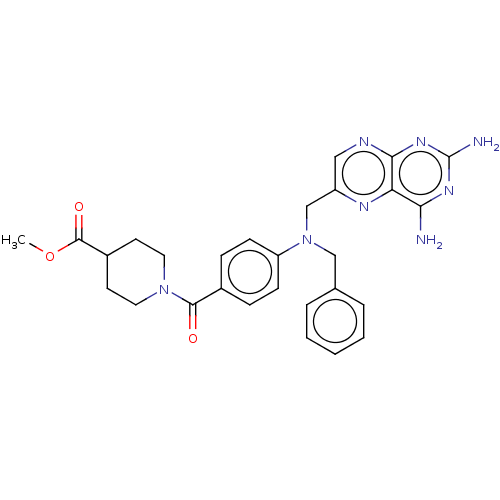
(CHEMBL5184936)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(Cc1ccccc1)Cc1cnc2nc(N)nc(N)c2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600703
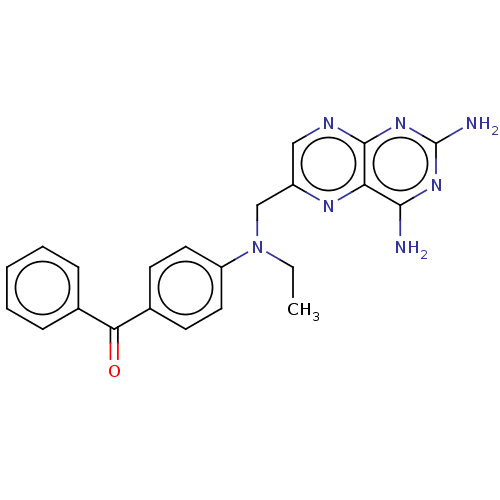
(CHEMBL5201183)Show SMILES CCN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)c1ccccc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600694

(CHEMBL5204239)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NCCc3ccccc3)cnc2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600702
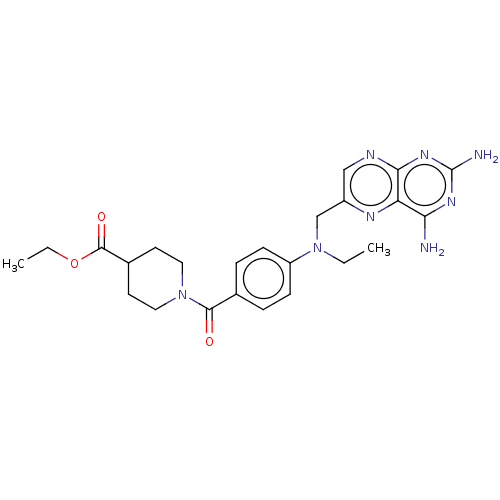
(CHEMBL5199171)Show SMILES CCOC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(CC)Cc1cnc2nc(N)nc(N)c2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600693

(CHEMBL5175012)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)c3ccccc3)cnc2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600684

(CHEMBL5182125)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(SCc2cnc3nc(N)nc(N)c3n2)nc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600701
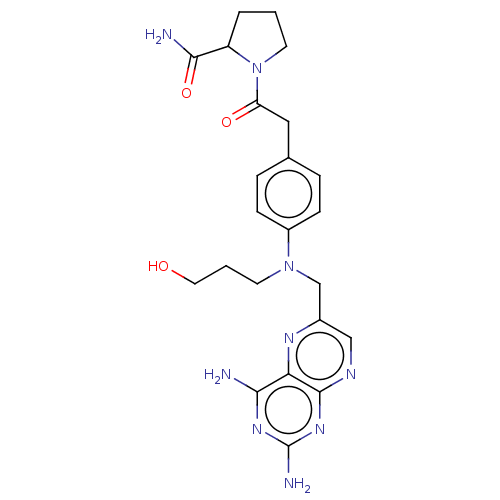
(CHEMBL5200612)Show SMILES NC(=O)C1CCCN1C(=O)Cc1ccc(cc1)N(CCCO)Cc1cnc2nc(N)nc(N)c2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600679

(CHEMBL426 | Methotrexate | TCMDC-123832 | TCMDC-12...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM228619
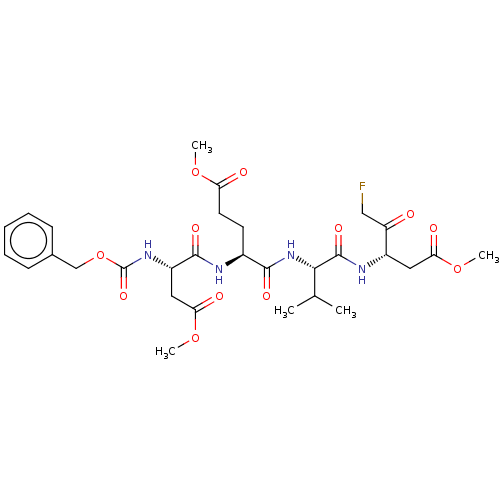
(US9345789, Z-DEVD-FMK)Show SMILES COC(=O)CC[C@H](NC(=O)[C@H](CC(=O)OC)NC(=O)OCc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(=O)OC)C(=O)CF Show InChI InChI=1S/C30H41FN4O12/c1-17(2)26(29(42)33-20(22(36)15-31)13-24(38)45-4)35-27(40)19(11-12-23(37)44-3)32-28(41)21(14-25(39)46-5)34-30(43)47-16-18-9-7-6-8-10-18/h6-10,17,19-21,26H,11-16H2,1-5H3,(H,32,41)(H,33,42)(H,34,43)(H,35,40)/t19-,20-,21-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD)
| Assay Description
Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... |
bioRxiv 2020: (2020)
Article DOI: 10.1101/2020.12.16.422677
BindingDB Entry DOI: 10.7270/Q26M39VR |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM228619

(US9345789, Z-DEVD-FMK)Show SMILES COC(=O)CC[C@H](NC(=O)[C@H](CC(=O)OC)NC(=O)OCc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(=O)OC)C(=O)CF Show InChI InChI=1S/C30H41FN4O12/c1-17(2)26(29(42)33-20(22(36)15-31)13-24(38)45-4)35-27(40)19(11-12-23(37)44-3)32-28(41)21(14-25(39)46-5)34-30(43)47-16-18-9-7-6-8-10-18/h6-10,17,19-21,26H,11-16H2,1-5H3,(H,32,41)(H,33,42)(H,34,43)(H,35,40)/t19-,20-,21-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD)
| Assay Description
Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... |
bioRxiv 2020: (2020)
Article DOI: 10.1101/2020.12.16.422677
BindingDB Entry DOI: 10.7270/Q26M39VR |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM46060
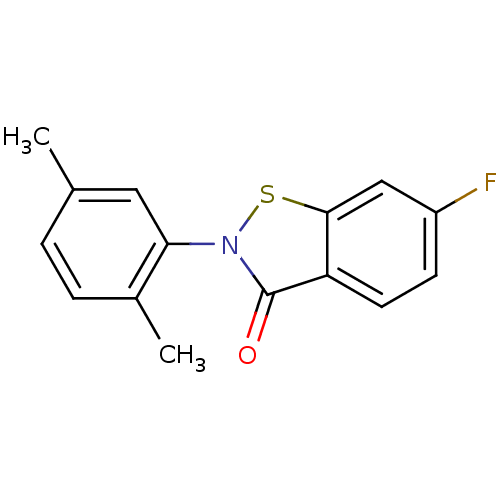
(2-(2,5-dimethylphenyl)-6-fluoranyl-1,2-benzothiazo...)Show InChI InChI=1S/C15H12FNOS/c1-9-3-4-10(2)13(7-9)17-15(18)12-6-5-11(16)8-14(12)19-17/h3-8H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD)
| Assay Description
Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... |
bioRxiv 2020: (2020)
Article DOI: 10.1101/2020.12.16.422677
BindingDB Entry DOI: 10.7270/Q26M39VR |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50050426

(6-(Naphthalen-1-ylaminomethyl)-pteridine-2,4-diami...)Show InChI InChI=1S/C17H15N7/c18-15-14-16(24-17(19)23-15)21-9-11(22-14)8-20-13-7-3-5-10-4-1-2-6-12(10)13/h1-7,9,20H,8H2,(H4,18,19,21,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600700

(CHEMBL5188304)Show SMILES NC(=O)C1CCN(CC1)C(=O)Cc1ccc(cc1)N(CCCO)Cc1cnc2nc(N)nc(N)c2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600692

(CHEMBL5197107)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N1CCCCC1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600689
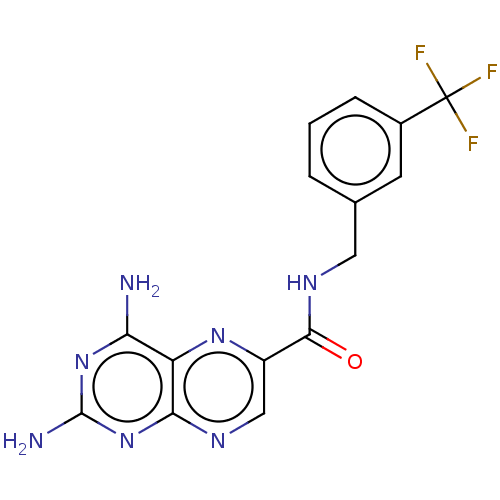
(CHEMBL5175397)Show SMILES Nc1nc(N)c2nc(cnc2n1)C(=O)NCc1cccc(c1)C(F)(F)F | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600688
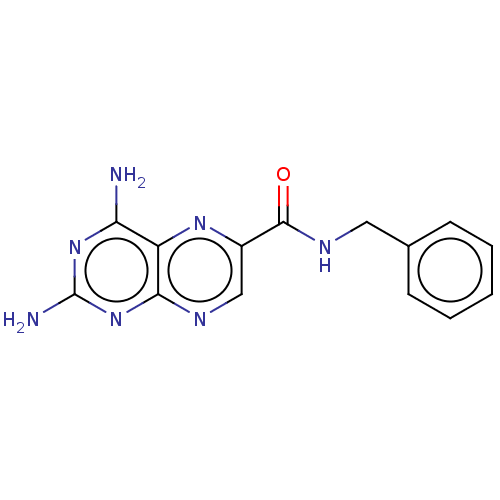
(CHEMBL5198855) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50050426

(6-(Naphthalen-1-ylaminomethyl)-pteridine-2,4-diami...)Show InChI InChI=1S/C17H15N7/c18-15-14-16(24-17(19)23-15)21-9-11(22-14)8-20-13-7-3-5-10-4-1-2-6-12(10)13/h1-7,9,20H,8H2,(H4,18,19,21,23,24) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50600683

(CHEMBL5184936)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(Cc1ccccc1)Cc1cnc2nc(N)nc(N)c2n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM46060

(2-(2,5-dimethylphenyl)-6-fluoranyl-1,2-benzothiazo...)Show InChI InChI=1S/C15H12FNOS/c1-9-3-4-10(2)13(7-9)17-15(18)12-6-5-11(16)8-14(12)19-17/h3-8H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD)
| Assay Description
Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... |
bioRxiv 2020: (2020)
Article DOI: 10.1101/2020.12.16.422677
BindingDB Entry DOI: 10.7270/Q26M39VR |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD)
| Assay Description
Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... |
bioRxiv 2020: (2020)
Article DOI: 10.1101/2020.12.16.422677
BindingDB Entry DOI: 10.7270/Q26M39VR |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50600679

(CHEMBL426 | Methotrexate | TCMDC-123832 | TCMDC-12...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600685

(CHEMBL5171609)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(CCCO)Cc1cnc2nc(N)nc(N)c2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50600679

(CHEMBL426 | Methotrexate | TCMDC-123832 | TCMDC-12...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM34233

(2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...)Show InChI InChI=1S/C13H9NOSe/c15-13-11-8-4-5-9-12(11)16-14(13)10-6-2-1-3-7-10/h1-9H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD)
| Assay Description
Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... |
bioRxiv 2020: (2020)
Article DOI: 10.1101/2020.12.16.422677
BindingDB Entry DOI: 10.7270/Q26M39VR |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600691
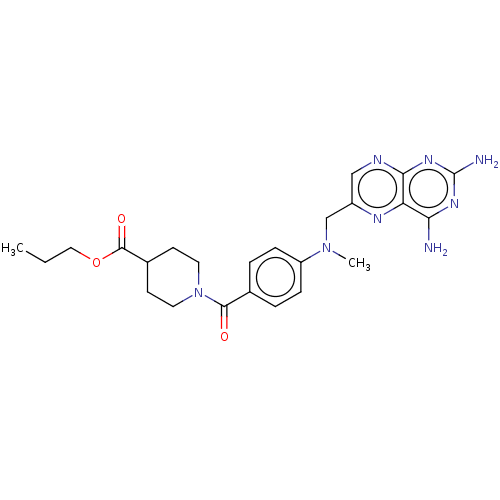
(CHEMBL5205559)Show SMILES CCCOC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(C)Cc1cnc2nc(N)nc(N)c2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50600694

(CHEMBL5204239)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NCCc3ccccc3)cnc2n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50600693

(CHEMBL5175012)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)c3ccccc3)cnc2n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM442806
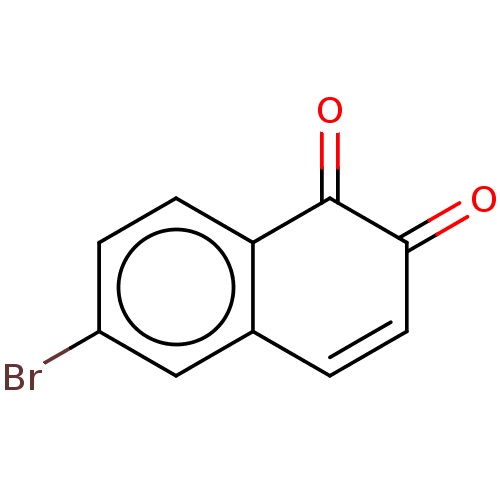
(Bonaphthone)Show InChI InChI=1S/C10H5BrO2/c11-7-2-3-8-6(5-7)1-4-9(12)10(8)13/h1-5H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD)
| Assay Description
Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... |
bioRxiv 2020: (2020)
Article DOI: 10.1101/2020.12.16.422677
BindingDB Entry DOI: 10.7270/Q26M39VR |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600682

(CHEMBL5193564)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(CC#C)Cc1cnc2nc(N)nc(N)c2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600690

(CHEMBL5206994)Show SMILES CCOC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(C)Cc1cnc2nc(N)nc(N)c2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600679

(CHEMBL426 | Methotrexate | TCMDC-123832 | TCMDC-12...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data