 Found 74 hits with Last Name = 'emery' and Initial = 'mg'
Found 74 hits with Last Name = 'emery' and Initial = 'mg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50382388

(CHEMBL2023973)Show SMILES CC(=C)[C@]1(C)SC(N[C@H]2C[C@@H]3CC[C@H]2C3)=NC1=O |r,c:16| Show InChI InChI=1S/C14H20N2OS/c1-8(2)14(3)12(17)16-13(18-14)15-11-7-9-4-5-10(11)6-9/h9-11H,1,4-7H2,2-3H3,(H,15,16,17)/t9-,10+,11+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone as substrate by scintillation proximity assay |
ACS Med Chem Lett 2: 824-827 (2011)
Article DOI: 10.1021/ml2001467
BindingDB Entry DOI: 10.7270/Q22N539Z |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50319660
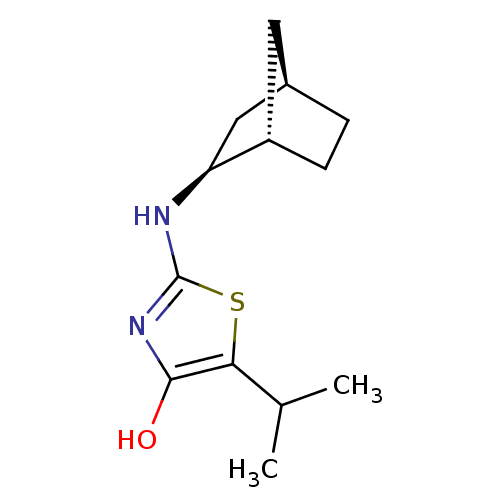
((S)-2-((1S,2S,4R)-Bicyclo[2.2.1]heptan-2-ylamino)-...)Show SMILES CC(C)c1sc(N[C@H]2C[C@@H]3CC[C@H]2C3)nc1O |r| Show InChI InChI=1S/C13H20N2OS/c1-7(2)11-12(16)15-13(17-11)14-10-6-8-3-4-9(10)5-8/h7-10,16H,3-6H2,1-2H3,(H,14,15)/t8-,9+,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay |
J Med Chem 53: 4481-7 (2010)
Article DOI: 10.1021/jm100242d
BindingDB Entry DOI: 10.7270/Q2WW7HVQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50319665

((S)-2-((1S,2S,4R)-Bicyclo[2.2.1]heptan-2-ylamino)-...)Show SMILES CC(C)[C@]1(C)SC(N[C@H]2C[C@@H]3CC[C@H]2C3)=NC1=O |r,c:16| Show InChI InChI=1S/C14H22N2OS/c1-8(2)14(3)12(17)16-13(18-14)15-11-7-9-4-5-10(11)6-9/h8-11H,4-7H2,1-3H3,(H,15,16,17)/t9-,10+,11+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay |
J Med Chem 53: 4481-7 (2010)
Article DOI: 10.1021/jm100242d
BindingDB Entry DOI: 10.7270/Q2WW7HVQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50319665

((S)-2-((1S,2S,4R)-Bicyclo[2.2.1]heptan-2-ylamino)-...)Show SMILES CC(C)[C@]1(C)SC(N[C@H]2C[C@@H]3CC[C@H]2C3)=NC1=O |r,c:16| Show InChI InChI=1S/C14H22N2OS/c1-8(2)14(3)12(17)16-13(18-14)15-11-7-9-4-5-10(11)6-9/h8-11H,4-7H2,1-3H3,(H,15,16,17)/t9-,10+,11+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone as substrate by scintillation proximity assay |
ACS Med Chem Lett 2: 824-827 (2011)
Article DOI: 10.1021/ml2001467
BindingDB Entry DOI: 10.7270/Q22N539Z |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50382382
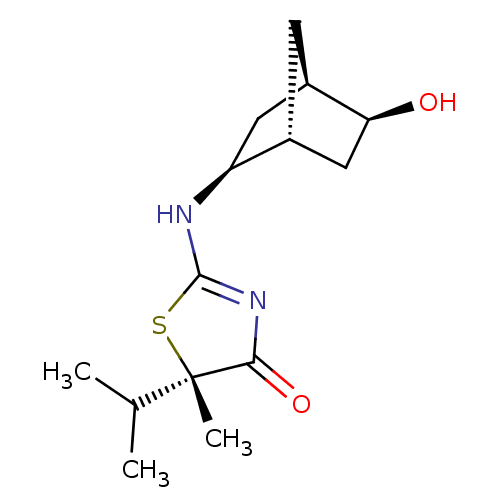
(CHEMBL2023966)Show SMILES CC(C)[C@]1(C)SC(N[C@H]2C[C@H]3C[C@@H]2C[C@@H]3O)=NC1=O |r,c:17| Show InChI InChI=1S/C14H22N2O2S/c1-7(2)14(3)12(18)16-13(19-14)15-10-5-9-4-8(10)6-11(9)17/h7-11,17H,4-6H2,1-3H3,(H,15,16,18)/t8-,9-,10+,11+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone as substrate by scintillation proximity assay |
ACS Med Chem Lett 2: 824-827 (2011)
Article DOI: 10.1021/ml2001467
BindingDB Entry DOI: 10.7270/Q22N539Z |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50319664

((R,S)-2-((+/-)-exo-Bicyclo[2.2.1]heptan-2-ylamino)...)Show SMILES CC(C)C1(C)SC(N[C@H]2C[C@@H]3CC[C@H]2C3)=NC1=O |r,c:16| Show InChI InChI=1S/C14H22N2OS/c1-8(2)14(3)12(17)16-13(18-14)15-11-7-9-4-5-10(11)6-9/h8-11H,4-7H2,1-3H3,(H,15,16,17)/t9-,10+,11+,14?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay |
J Med Chem 53: 4481-7 (2010)
Article DOI: 10.1021/jm100242d
BindingDB Entry DOI: 10.7270/Q2WW7HVQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50382387
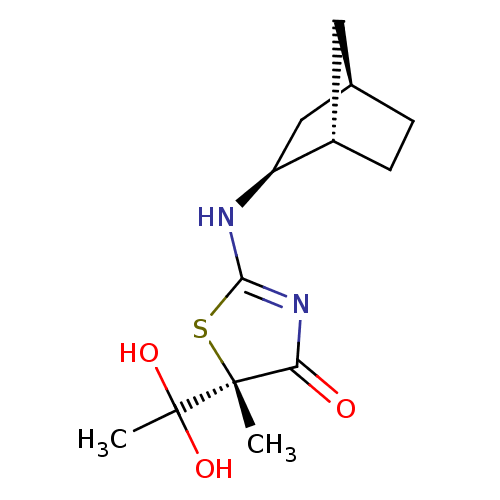
(CHEMBL2023972)Show SMILES CC(O)(O)[C@]1(C)SC(N[C@H]2C[C@@H]3CC[C@H]2C3)=NC1=O |r,c:17| Show InChI InChI=1S/C13H20N2O3S/c1-12(13(2,17)18)10(16)15-11(19-12)14-9-6-7-3-4-8(9)5-7/h7-9,17-18H,3-6H2,1-2H3,(H,14,15,16)/t7-,8+,9+,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone as substrate by scintillation proximity assay |
ACS Med Chem Lett 2: 824-827 (2011)
Article DOI: 10.1021/ml2001467
BindingDB Entry DOI: 10.7270/Q22N539Z |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50382383
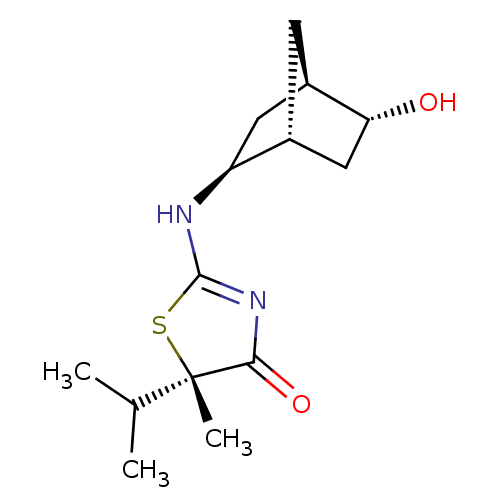
(CHEMBL2023967)Show SMILES CC(C)[C@]1(C)SC(N[C@H]2C[C@H]3C[C@@H]2C[C@H]3O)=NC1=O |r,c:17| Show InChI InChI=1S/C14H22N2O2S/c1-7(2)14(3)12(18)16-13(19-14)15-10-5-9-4-8(10)6-11(9)17/h7-11,17H,4-6H2,1-3H3,(H,15,16,18)/t8-,9-,10+,11-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone as substrate by scintillation proximity assay |
ACS Med Chem Lett 2: 824-827 (2011)
Article DOI: 10.1021/ml2001467
BindingDB Entry DOI: 10.7270/Q22N539Z |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50382389
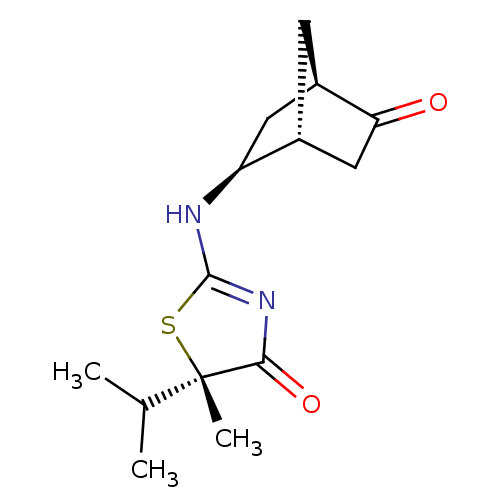
(CHEMBL2023968)Show SMILES CC(C)[C@]1(C)SC(N[C@H]2C[C@H]3C[C@@H]2CC3=O)=NC1=O |r,c:17| Show InChI InChI=1S/C14H20N2O2S/c1-7(2)14(3)12(18)16-13(19-14)15-10-5-9-4-8(10)6-11(9)17/h7-10H,4-6H2,1-3H3,(H,15,16,18)/t8-,9-,10+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone as substrate by scintillation proximity assay |
ACS Med Chem Lett 2: 824-827 (2011)
Article DOI: 10.1021/ml2001467
BindingDB Entry DOI: 10.7270/Q22N539Z |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50382386
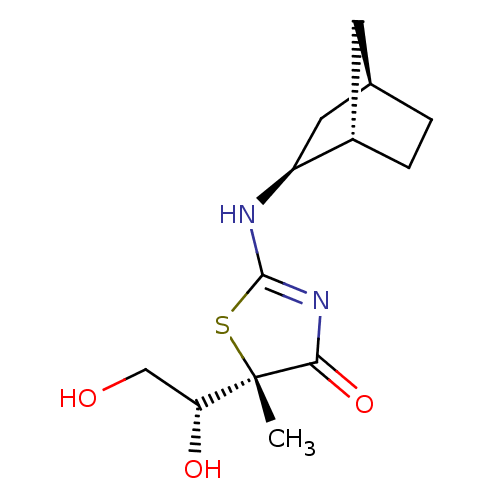
(CHEMBL2023971)Show SMILES C[C@]1(SC(N[C@H]2C[C@@H]3CC[C@H]2C3)=NC1=O)[C@H](O)CO |r,c:13| Show InChI InChI=1S/C13H20N2O3S/c1-13(10(17)6-16)11(18)15-12(19-13)14-9-5-7-2-3-8(9)4-7/h7-10,16-17H,2-6H2,1H3,(H,14,15,18)/t7-,8+,9+,10-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone as substrate by scintillation proximity assay |
ACS Med Chem Lett 2: 824-827 (2011)
Article DOI: 10.1021/ml2001467
BindingDB Entry DOI: 10.7270/Q22N539Z |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50319666
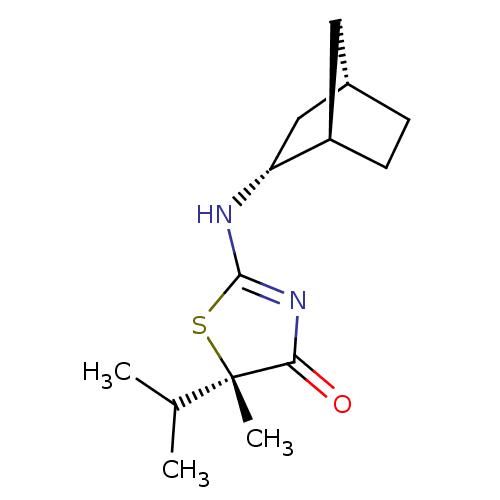
((S)-2-((1R,2R,4S)-Bicyclo[2.2.1]heptan-2-ylamino)-...)Show SMILES CC(C)[C@]1(C)SC(N[C@@H]2C[C@H]3CC[C@@H]2C3)=NC1=O |r,c:16| Show InChI InChI=1S/C14H22N2OS/c1-8(2)14(3)12(17)16-13(18-14)15-11-7-9-4-5-10(11)6-9/h8-11H,4-7H2,1-3H3,(H,15,16,17)/t9-,10+,11+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay |
J Med Chem 53: 4481-7 (2010)
Article DOI: 10.1021/jm100242d
BindingDB Entry DOI: 10.7270/Q2WW7HVQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50319667
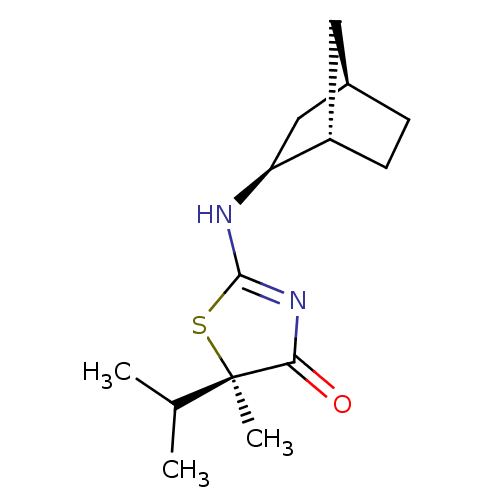
((R)-2-((1S,2S,4R)-Bicyclo[2.2.1]heptan-2-ylamino)-...)Show SMILES CC(C)[C@@]1(C)SC(N[C@H]2C[C@@H]3CC[C@H]2C3)=NC1=O |r,c:16| Show InChI InChI=1S/C14H22N2OS/c1-8(2)14(3)12(17)16-13(18-14)15-11-7-9-4-5-10(11)6-9/h8-11H,4-7H2,1-3H3,(H,15,16,17)/t9-,10+,11+,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay |
J Med Chem 53: 4481-7 (2010)
Article DOI: 10.1021/jm100242d
BindingDB Entry DOI: 10.7270/Q2WW7HVQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50382385
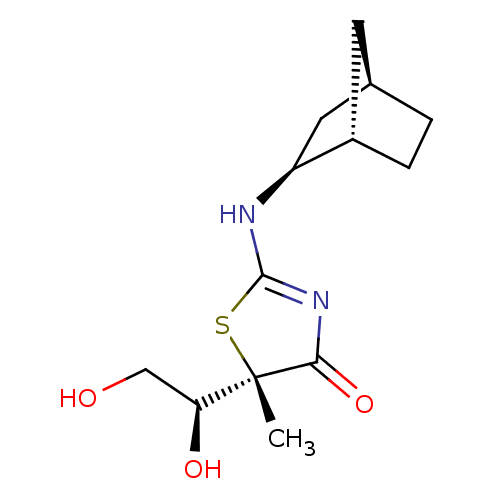
(CHEMBL2023970)Show SMILES C[C@]1(SC(N[C@H]2C[C@@H]3CC[C@H]2C3)=NC1=O)[C@@H](O)CO |r,c:13| Show InChI InChI=1S/C13H20N2O3S/c1-13(10(17)6-16)11(18)15-12(19-13)14-9-5-7-2-3-8(9)4-7/h7-10,16-17H,2-6H2,1H3,(H,14,15,18)/t7-,8+,9+,10+,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone as substrate by scintillation proximity assay |
ACS Med Chem Lett 2: 824-827 (2011)
Article DOI: 10.1021/ml2001467
BindingDB Entry DOI: 10.7270/Q22N539Z |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50319668
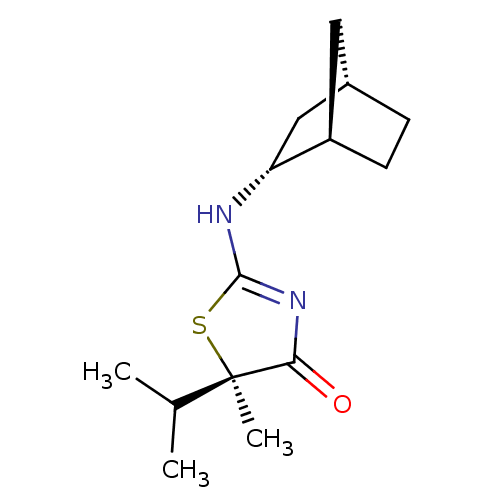
((R)-2-((1R,2R,4S)-Bicyclo[2.2.1]heptan-2-ylamino)-...)Show SMILES CC(C)[C@@]1(C)SC(N[C@@H]2C[C@H]3CC[C@@H]2C3)=NC1=O |r,c:16| Show InChI InChI=1S/C14H22N2OS/c1-8(2)14(3)12(17)16-13(18-14)15-11-7-9-4-5-10(11)6-9/h8-11H,4-7H2,1-3H3,(H,15,16,17)/t9-,10+,11+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 47.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay |
J Med Chem 53: 4481-7 (2010)
Article DOI: 10.1021/jm100242d
BindingDB Entry DOI: 10.7270/Q2WW7HVQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50319659
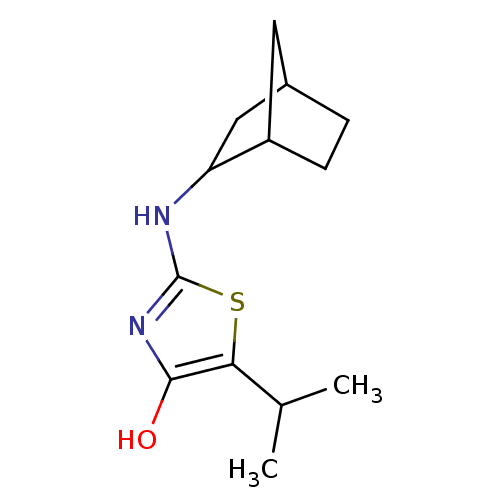
(2-(bicyclo[2.2.1]heptan-2-ylamino)-5-isopropylthia...)Show InChI InChI=1S/C13H20N2OS/c1-7(2)11-12(16)15-13(17-11)14-10-6-8-3-4-9(10)5-8/h7-10,16H,3-6H2,1-2H3,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay |
J Med Chem 53: 4481-7 (2010)
Article DOI: 10.1021/jm100242d
BindingDB Entry DOI: 10.7270/Q2WW7HVQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50319665

((S)-2-((1S,2S,4R)-Bicyclo[2.2.1]heptan-2-ylamino)-...)Show SMILES CC(C)[C@]1(C)SC(N[C@H]2C[C@@H]3CC[C@H]2C3)=NC1=O |r,c:16| Show InChI InChI=1S/C14H22N2OS/c1-8(2)14(3)12(17)16-13(18-14)15-11-7-9-4-5-10(11)6-9/h8-11H,4-7H2,1-3H3,(H,15,16,17)/t9-,10+,11+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 70.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone as substrate by scintillation proximity assay |
ACS Med Chem Lett 2: 824-827 (2011)
Article DOI: 10.1021/ml2001467
BindingDB Entry DOI: 10.7270/Q22N539Z |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50382382

(CHEMBL2023966)Show SMILES CC(C)[C@]1(C)SC(N[C@H]2C[C@H]3C[C@@H]2C[C@@H]3O)=NC1=O |r,c:17| Show InChI InChI=1S/C14H22N2O2S/c1-7(2)14(3)12(18)16-13(19-14)15-10-5-9-4-8(10)6-11(9)17/h7-11,17H,4-6H2,1-3H3,(H,15,16,18)/t8-,9-,10+,11+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone as substrate by scintillation proximity assay |
ACS Med Chem Lett 2: 824-827 (2011)
Article DOI: 10.1021/ml2001467
BindingDB Entry DOI: 10.7270/Q22N539Z |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50382383

(CHEMBL2023967)Show SMILES CC(C)[C@]1(C)SC(N[C@H]2C[C@H]3C[C@@H]2C[C@H]3O)=NC1=O |r,c:17| Show InChI InChI=1S/C14H22N2O2S/c1-7(2)14(3)12(18)16-13(19-14)15-10-5-9-4-8(10)6-11(9)17/h7-11,17H,4-6H2,1-3H3,(H,15,16,18)/t8-,9-,10+,11-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone as substrate by scintillation proximity assay |
ACS Med Chem Lett 2: 824-827 (2011)
Article DOI: 10.1021/ml2001467
BindingDB Entry DOI: 10.7270/Q22N539Z |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50319661
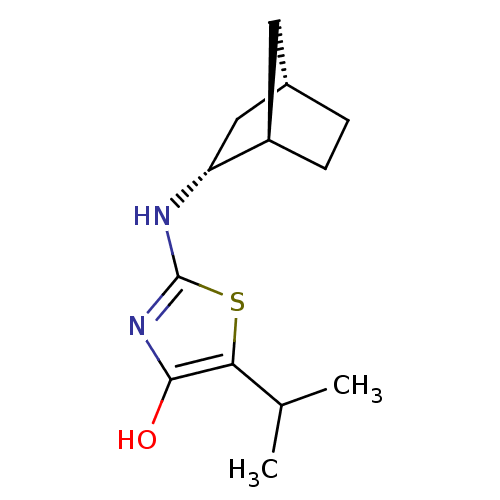
((R)-2-((1R,2R,4S)-Bicyclo[2.2.1]heptan-2-ylamino)-...)Show SMILES CC(C)c1sc(N[C@@H]2C[C@H]3CC[C@@H]2C3)nc1O |r| Show InChI InChI=1S/C13H20N2OS/c1-7(2)11-12(16)15-13(17-11)14-10-6-8-3-4-9(10)5-8/h7-10,16H,3-6H2,1-2H3,(H,14,15)/t8-,9+,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay |
J Med Chem 53: 4481-7 (2010)
Article DOI: 10.1021/jm100242d
BindingDB Entry DOI: 10.7270/Q2WW7HVQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50382389

(CHEMBL2023968)Show SMILES CC(C)[C@]1(C)SC(N[C@H]2C[C@H]3C[C@@H]2CC3=O)=NC1=O |r,c:17| Show InChI InChI=1S/C14H20N2O2S/c1-7(2)14(3)12(18)16-13(19-14)15-10-5-9-4-8(10)6-11(9)17/h7-10H,4-6H2,1-3H3,(H,15,16,18)/t8-,9-,10+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone as substrate by scintillation proximity assay |
ACS Med Chem Lett 2: 824-827 (2011)
Article DOI: 10.1021/ml2001467
BindingDB Entry DOI: 10.7270/Q22N539Z |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50319660

((S)-2-((1S,2S,4R)-Bicyclo[2.2.1]heptan-2-ylamino)-...)Show SMILES CC(C)c1sc(N[C@H]2C[C@@H]3CC[C@H]2C3)nc1O |r| Show InChI InChI=1S/C13H20N2OS/c1-7(2)11-12(16)15-13(17-11)14-10-6-8-3-4-9(10)5-8/h7-10,16H,3-6H2,1-2H3,(H,14,15)/t8-,9+,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 329 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay |
J Med Chem 53: 4481-7 (2010)
Article DOI: 10.1021/jm100242d
BindingDB Entry DOI: 10.7270/Q2WW7HVQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50382384
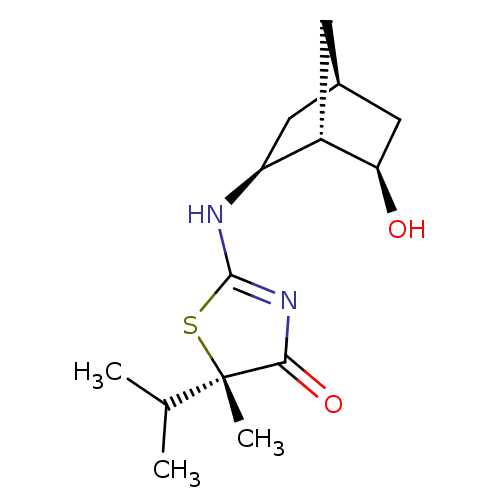
(CHEMBL2023969)Show SMILES CC(C)[C@]1(C)SC(N[C@H]2C[C@@H]3C[C@@H](O)[C@H]2C3)=NC1=O |r,c:17| Show InChI InChI=1S/C14H22N2O2S/c1-7(2)14(3)12(18)16-13(19-14)15-10-5-8-4-9(10)11(17)6-8/h7-11,17H,4-6H2,1-3H3,(H,15,16,18)/t8-,9+,10+,11-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 449 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone as substrate by scintillation proximity assay |
ACS Med Chem Lett 2: 824-827 (2011)
Article DOI: 10.1021/ml2001467
BindingDB Entry DOI: 10.7270/Q22N539Z |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50319661

((R)-2-((1R,2R,4S)-Bicyclo[2.2.1]heptan-2-ylamino)-...)Show SMILES CC(C)c1sc(N[C@@H]2C[C@H]3CC[C@@H]2C3)nc1O |r| Show InChI InChI=1S/C13H20N2OS/c1-7(2)11-12(16)15-13(17-11)14-10-6-8-3-4-9(10)5-8/h7-10,16H,3-6H2,1-2H3,(H,14,15)/t8-,9+,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay |
J Med Chem 53: 4481-7 (2010)
Article DOI: 10.1021/jm100242d
BindingDB Entry DOI: 10.7270/Q2WW7HVQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50363928
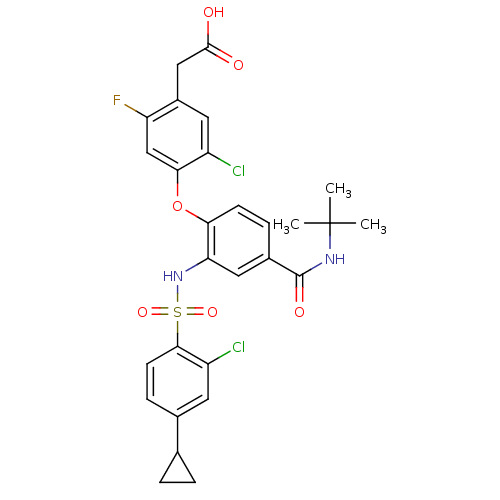
(CHEMBL1951575)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 Show InChI InChI=1S/C28H27Cl2FN2O6S/c1-28(2,3)32-27(36)17-6-8-23(39-24-14-21(31)18(11-19(24)29)13-26(34)35)22(12-17)33-40(37,38)25-9-7-16(10-20(25)30)15-4-5-15/h6-12,14-15,33H,4-5,13H2,1-3H3,(H,32,36)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Competitive inhibition of CYP2C8 in human liver microsomes assessed as paclitaxel 6-hydroxylation after 20 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50363928

(CHEMBL1951575)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 Show InChI InChI=1S/C28H27Cl2FN2O6S/c1-28(2,3)32-27(36)17-6-8-23(39-24-14-21(31)18(11-19(24)29)13-26(34)35)22(12-17)33-40(37,38)25-9-7-16(10-20(25)30)15-4-5-15/h6-12,14-15,33H,4-5,13H2,1-3H3,(H,32,36)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Biphasic inhibition of CYP2C8 in human liver microsomes assessed as montelukast 36-hydroxylation after 20 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50088490

(CHEMBL3526979)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(=O)O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 |r| Show InChI InChI=1S/C34H35Cl2FN2O12S/c1-34(2,3)38-31(44)17-6-8-23(22(12-17)39-52(47,48)25-9-7-16(10-20(25)36)15-4-5-15)49-24-14-21(37)18(11-19(24)35)13-26(40)50-33-29(43)27(41)28(42)30(51-33)32(45)46/h6-12,14-15,27-30,33,39,41-43H,4-5,13H2,1-3H3,(H,38,44)(H,45,46)/t27-,28-,29+,30-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Linear mixed inhibition of CYP2C8 in human liver microsomes assessed as paclitaxel 6-hydroxylation after 20 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50363928

(CHEMBL1951575)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 Show InChI InChI=1S/C28H27Cl2FN2O6S/c1-28(2,3)32-27(36)17-6-8-23(39-24-14-21(31)18(11-19(24)29)13-26(34)35)22(12-17)33-40(37,38)25-9-7-16(10-20(25)30)15-4-5-15/h6-12,14-15,33H,4-5,13H2,1-3H3,(H,32,36)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Competitive inhibition of CYP2C8 in human liver microsomes assessed as rosiglitazone demethylation after 20 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50088490

(CHEMBL3526979)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(=O)O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 |r| Show InChI InChI=1S/C34H35Cl2FN2O12S/c1-34(2,3)38-31(44)17-6-8-23(22(12-17)39-52(47,48)25-9-7-16(10-20(25)36)15-4-5-15)49-24-14-21(37)18(11-19(24)35)13-26(40)50-33-29(43)27(41)28(42)30(51-33)32(45)46/h6-12,14-15,27-30,33,39,41-43H,4-5,13H2,1-3H3,(H,38,44)(H,45,46)/t27-,28-,29+,30-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Linear mixed inhibition of CYP2C8 in human liver microsomes assessed as rosiglitazone demethylation after 20 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50088490

(CHEMBL3526979)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(=O)O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 |r| Show InChI InChI=1S/C34H35Cl2FN2O12S/c1-34(2,3)38-31(44)17-6-8-23(22(12-17)39-52(47,48)25-9-7-16(10-20(25)36)15-4-5-15)49-24-14-21(37)18(11-19(24)35)13-26(40)50-33-29(43)27(41)28(42)30(51-33)32(45)46/h6-12,14-15,27-30,33,39,41-43H,4-5,13H2,1-3H3,(H,38,44)(H,45,46)/t27-,28-,29+,30-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Biphasic inhibition of CYP2C8 in human liver microsomes assessed as montelukast 36-hydroxylation after 20 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50363928

(CHEMBL1951575)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 Show InChI InChI=1S/C28H27Cl2FN2O6S/c1-28(2,3)32-27(36)17-6-8-23(39-24-14-21(31)18(11-19(24)29)13-26(34)35)22(12-17)33-40(37,38)25-9-7-16(10-20(25)30)15-4-5-15/h6-12,14-15,33H,4-5,13H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2E1
(Homo sapiens (Human)) | BDBM50088490

(CHEMBL3526979)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(=O)O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 |r| Show InChI InChI=1S/C34H35Cl2FN2O12S/c1-34(2,3)38-31(44)17-6-8-23(22(12-17)39-52(47,48)25-9-7-16(10-20(25)36)15-4-5-15)49-24-14-21(37)18(11-19(24)35)13-26(40)50-33-29(43)27(41)28(42)30(51-33)32(45)46/h6-12,14-15,27-30,33,39,41-43H,4-5,13H2,1-3H3,(H,38,44)(H,45,46)/t27-,28-,29+,30-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2E1
(Homo sapiens (Human)) | BDBM50363928

(CHEMBL1951575)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 Show InChI InChI=1S/C28H27Cl2FN2O6S/c1-28(2,3)32-27(36)17-6-8-23(39-24-14-21(31)18(11-19(24)29)13-26(34)35)22(12-17)33-40(37,38)25-9-7-16(10-20(25)30)15-4-5-15/h6-12,14-15,33H,4-5,13H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50088490

(CHEMBL3526979)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(=O)O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 |r| Show InChI InChI=1S/C34H35Cl2FN2O12S/c1-34(2,3)38-31(44)17-6-8-23(22(12-17)39-52(47,48)25-9-7-16(10-20(25)36)15-4-5-15)49-24-14-21(37)18(11-19(24)35)13-26(40)50-33-29(43)27(41)28(42)30(51-33)32(45)46/h6-12,14-15,27-30,33,39,41-43H,4-5,13H2,1-3H3,(H,38,44)(H,45,46)/t27-,28-,29+,30-,33+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50363928

(CHEMBL1951575)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 Show InChI InChI=1S/C28H27Cl2FN2O6S/c1-28(2,3)32-27(36)17-6-8-23(39-24-14-21(31)18(11-19(24)29)13-26(34)35)22(12-17)33-40(37,38)25-9-7-16(10-20(25)30)15-4-5-15/h6-12,14-15,33H,4-5,13H2,1-3H3,(H,32,36)(H,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50088490

(CHEMBL3526979)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(=O)O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 |r| Show InChI InChI=1S/C34H35Cl2FN2O12S/c1-34(2,3)38-31(44)17-6-8-23(22(12-17)39-52(47,48)25-9-7-16(10-20(25)36)15-4-5-15)49-24-14-21(37)18(11-19(24)35)13-26(40)50-33-29(43)27(41)28(42)30(51-33)32(45)46/h6-12,14-15,27-30,33,39,41-43H,4-5,13H2,1-3H3,(H,38,44)(H,45,46)/t27-,28-,29+,30-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using (S)-Mephenytoin as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088490

(CHEMBL3526979)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(=O)O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 |r| Show InChI InChI=1S/C34H35Cl2FN2O12S/c1-34(2,3)38-31(44)17-6-8-23(22(12-17)39-52(47,48)25-9-7-16(10-20(25)36)15-4-5-15)49-24-14-21(37)18(11-19(24)35)13-26(40)50-33-29(43)27(41)28(42)30(51-33)32(45)46/h6-12,14-15,27-30,33,39,41-43H,4-5,13H2,1-3H3,(H,38,44)(H,45,46)/t27-,28-,29+,30-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50363928

(CHEMBL1951575)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 Show InChI InChI=1S/C28H27Cl2FN2O6S/c1-28(2,3)32-27(36)17-6-8-23(39-24-14-21(31)18(11-19(24)29)13-26(34)35)22(12-17)33-40(37,38)25-9-7-16(10-20(25)30)15-4-5-15/h6-12,14-15,33H,4-5,13H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using (S)-Mephenytoin as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50088490

(CHEMBL3526979)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(=O)O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 |r| Show InChI InChI=1S/C34H35Cl2FN2O12S/c1-34(2,3)38-31(44)17-6-8-23(22(12-17)39-52(47,48)25-9-7-16(10-20(25)36)15-4-5-15)49-24-14-21(37)18(11-19(24)35)13-26(40)50-33-29(43)27(41)28(42)30(51-33)32(45)46/h6-12,14-15,27-30,33,39,41-43H,4-5,13H2,1-3H3,(H,38,44)(H,45,46)/t27-,28-,29+,30-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50363928

(CHEMBL1951575)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 Show InChI InChI=1S/C28H27Cl2FN2O6S/c1-28(2,3)32-27(36)17-6-8-23(39-24-14-21(31)18(11-19(24)29)13-26(34)35)22(12-17)33-40(37,38)25-9-7-16(10-20(25)30)15-4-5-15/h6-12,14-15,33H,4-5,13H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50088490

(CHEMBL3526979)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(=O)O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 |r| Show InChI InChI=1S/C34H35Cl2FN2O12S/c1-34(2,3)38-31(44)17-6-8-23(22(12-17)39-52(47,48)25-9-7-16(10-20(25)36)15-4-5-15)49-24-14-21(37)18(11-19(24)35)13-26(40)50-33-29(43)27(41)28(42)30(51-33)32(45)46/h6-12,14-15,27-30,33,39,41-43H,4-5,13H2,1-3H3,(H,38,44)(H,45,46)/t27-,28-,29+,30-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 in human liver microsomes using bupropion as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50363928

(CHEMBL1951575)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 Show InChI InChI=1S/C28H27Cl2FN2O6S/c1-28(2,3)32-27(36)17-6-8-23(39-24-14-21(31)18(11-19(24)29)13-26(34)35)22(12-17)33-40(37,38)25-9-7-16(10-20(25)30)15-4-5-15/h6-12,14-15,33H,4-5,13H2,1-3H3,(H,32,36)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 in human liver microsomes using bupropion as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50088490

(CHEMBL3526979)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(=O)O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 |r| Show InChI InChI=1S/C34H35Cl2FN2O12S/c1-34(2,3)38-31(44)17-6-8-23(22(12-17)39-52(47,48)25-9-7-16(10-20(25)36)15-4-5-15)49-24-14-21(37)18(11-19(24)35)13-26(40)50-33-29(43)27(41)28(42)30(51-33)32(45)46/h6-12,14-15,27-30,33,39,41-43H,4-5,13H2,1-3H3,(H,38,44)(H,45,46)/t27-,28-,29+,30-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50363928

(CHEMBL1951575)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 Show InChI InChI=1S/C28H27Cl2FN2O6S/c1-28(2,3)32-27(36)17-6-8-23(39-24-14-21(31)18(11-19(24)29)13-26(34)35)22(12-17)33-40(37,38)25-9-7-16(10-20(25)30)15-4-5-15/h6-12,14-15,33H,4-5,13H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088490

(CHEMBL3526979)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(=O)O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 |r| Show InChI InChI=1S/C34H35Cl2FN2O12S/c1-34(2,3)38-31(44)17-6-8-23(22(12-17)39-52(47,48)25-9-7-16(10-20(25)36)15-4-5-15)49-24-14-21(37)18(11-19(24)35)13-26(40)50-33-29(43)27(41)28(42)30(51-33)32(45)46/h6-12,14-15,27-30,33,39,41-43H,4-5,13H2,1-3H3,(H,38,44)(H,45,46)/t27-,28-,29+,30-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50363928

(CHEMBL1951575)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 Show InChI InChI=1S/C28H27Cl2FN2O6S/c1-28(2,3)32-27(36)17-6-8-23(39-24-14-21(31)18(11-19(24)29)13-26(34)35)22(12-17)33-40(37,38)25-9-7-16(10-20(25)30)15-4-5-15/h6-12,14-15,33H,4-5,13H2,1-3H3,(H,32,36)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50088489

(CHEMBL3527324)Show SMILES CC(C)(CO)NC(=O)c1ccc(Oc2cc(F)c(CC(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 Show InChI InChI=1S/C28H27Cl2FN2O7S/c1-28(2,14-34)32-27(37)17-5-7-23(40-24-13-21(31)18(10-19(24)29)12-26(35)36)22(11-17)33-41(38,39)25-8-6-16(9-20(25)30)15-3-4-15/h5-11,13,15,33-34H,3-4,12,14H2,1-2H3,(H,32,37)(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2C8 (unknown origin) treated with AMG 853 |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2J2
(Homo sapiens (Human)) | BDBM50088489

(CHEMBL3527324)Show SMILES CC(C)(CO)NC(=O)c1ccc(Oc2cc(F)c(CC(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 Show InChI InChI=1S/C28H27Cl2FN2O7S/c1-28(2,14-34)32-27(37)17-5-7-23(40-24-13-21(31)18(10-19(24)29)12-26(35)36)22(11-17)33-41(38,39)25-8-6-16(9-20(25)30)15-3-4-15/h5-11,13,15,33-34H,3-4,12,14H2,1-2H3,(H,32,37)(H,35,36) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CYP2J2 (unknown origin) treated with AMG 853 |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50319665

((S)-2-((1S,2S,4R)-Bicyclo[2.2.1]heptan-2-ylamino)-...)Show SMILES CC(C)[C@]1(C)SC(N[C@H]2C[C@@H]3CC[C@H]2C3)=NC1=O |r,c:16| Show InChI InChI=1S/C14H22N2OS/c1-8(2)14(3)12(17)16-13(18-14)15-11-7-9-4-5-10(11)6-9/h8-11H,4-7H2,1-3H3,(H,15,16,17)/t9-,10+,11+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10.1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells |
ACS Med Chem Lett 2: 824-827 (2011)
Article DOI: 10.1021/ml2001467
BindingDB Entry DOI: 10.7270/Q22N539Z |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50319665

((S)-2-((1S,2S,4R)-Bicyclo[2.2.1]heptan-2-ylamino)-...)Show SMILES CC(C)[C@]1(C)SC(N[C@H]2C[C@@H]3CC[C@H]2C3)=NC1=O |r,c:16| Show InChI InChI=1S/C14H22N2OS/c1-8(2)14(3)12(17)16-13(18-14)15-11-7-9-4-5-10(11)6-9/h8-11H,4-7H2,1-3H3,(H,15,16,17)/t9-,10+,11+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cortisone human 17beta-HSD1 expressed in HEK293 cells after 30 mins by scintillation proximity assay |
J Med Chem 53: 4481-7 (2010)
Article DOI: 10.1021/jm100242d
BindingDB Entry DOI: 10.7270/Q2WW7HVQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50382389

(CHEMBL2023968)Show SMILES CC(C)[C@]1(C)SC(N[C@H]2C[C@H]3C[C@@H]2CC3=O)=NC1=O |r,c:17| Show InChI InChI=1S/C14H20N2O2S/c1-7(2)14(3)12(18)16-13(19-14)15-10-5-9-4-8(10)6-11(9)17/h7-10H,4-6H2,1-3H3,(H,15,16,18)/t8-,9-,10+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells |
ACS Med Chem Lett 2: 824-827 (2011)
Article DOI: 10.1021/ml2001467
BindingDB Entry DOI: 10.7270/Q22N539Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data