

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031614 (CHEMBL3359758) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031612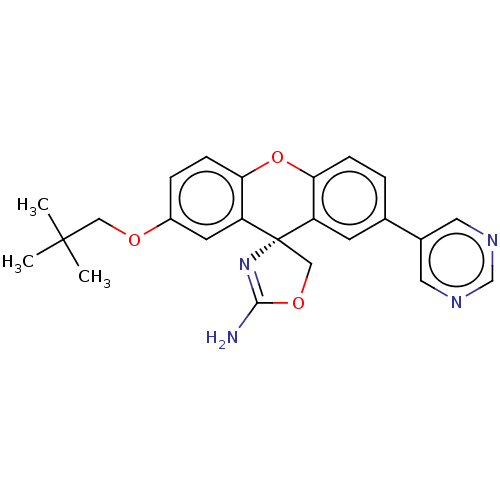 (CHEMBL3354688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031617 (CHEMBL3359760) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031619 (CHEMBL3359757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031627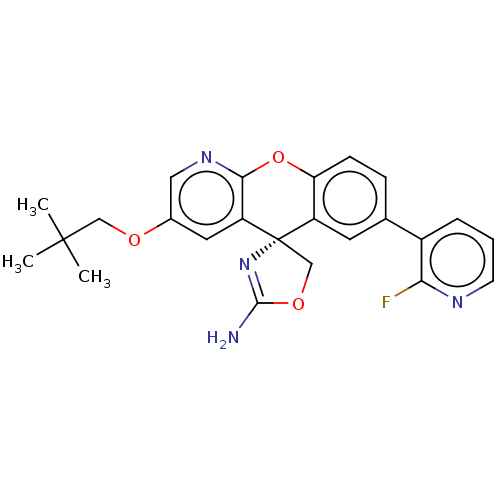 (CHEMBL3359749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031625 (CHEMBL3359751) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031626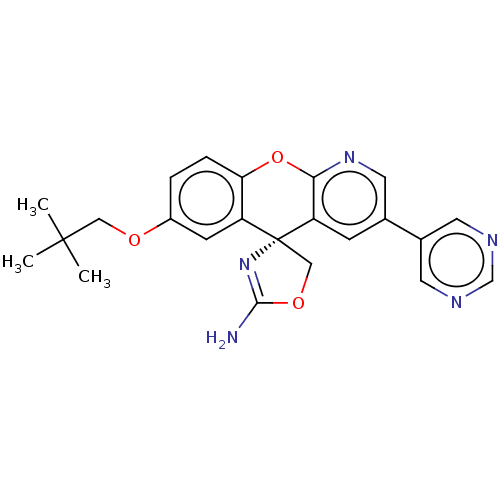 (CHEMBL3359750) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031624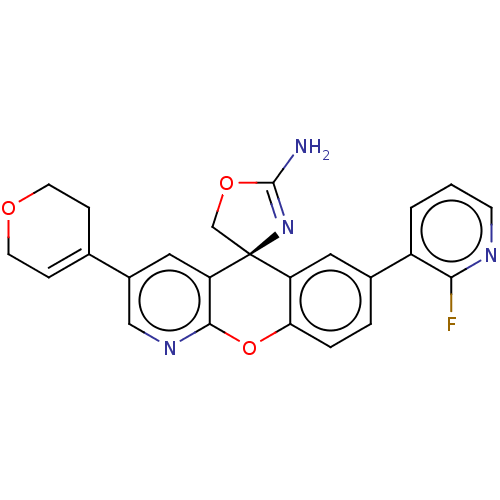 (CHEMBL3359752) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031628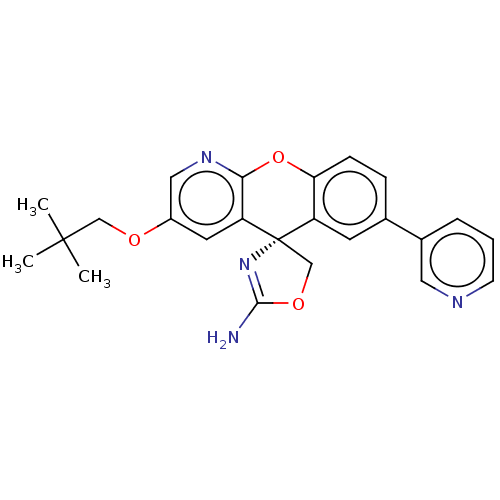 (CHEMBL3359748) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031629 (CHEMBL3359747) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031623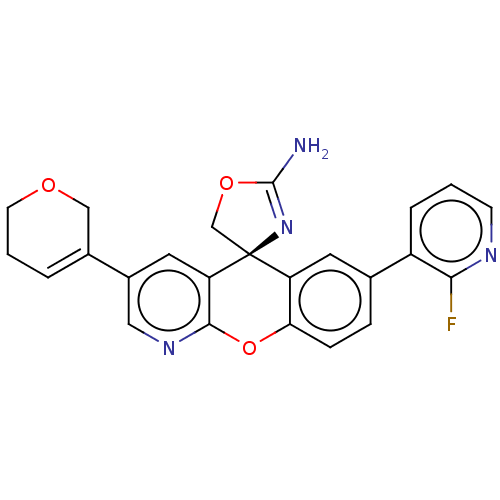 (CHEMBL3359753) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031622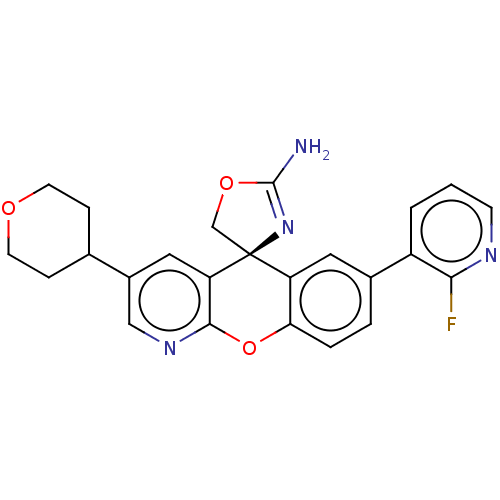 (CHEMBL3359754) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031621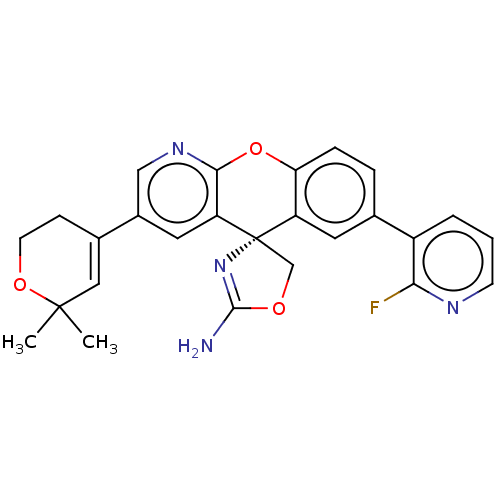 (CHEMBL3359755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031620 (CHEMBL3359756) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031618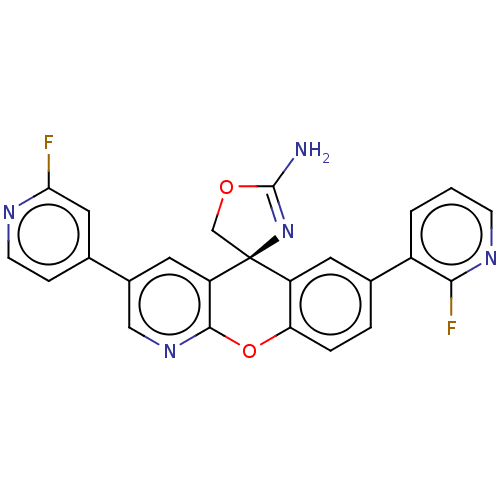 (CHEMBL3359759) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031616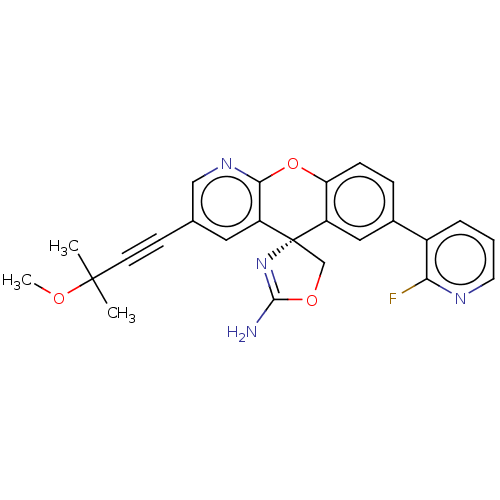 (CHEMBL3359761) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031615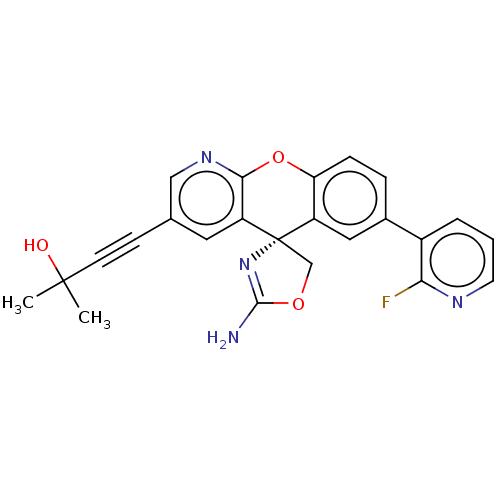 (CHEMBL3359762) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50031613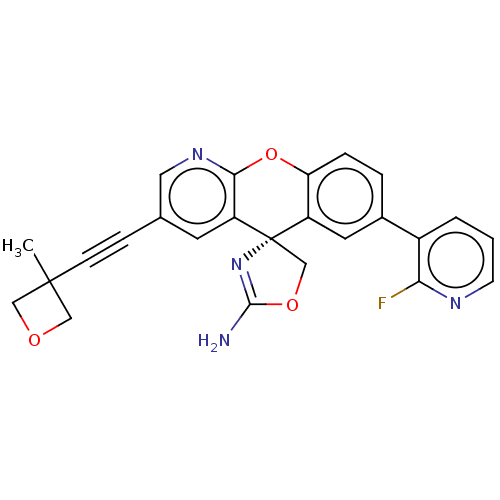 (CHEMBL3359763) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031627 (CHEMBL3359749) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) incubated for 60 mins prior to substrate addition measured after 60 mins by fluorescence assay | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031628 (CHEMBL3359748) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) incubated for 60 mins prior to substrate addition measured after 60 mins by fluorescence assay | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031613 (CHEMBL3359763) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) incubated for 60 mins prior to substrate addition measured after 60 mins by fluorescence assay | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031629 (CHEMBL3359747) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) incubated for 60 mins prior to substrate addition measured after 60 mins by fluorescence assay | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031614 (CHEMBL3359758) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) incubated for 60 mins prior to substrate addition measured after 60 mins by fluorescence assay | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031619 (CHEMBL3359757) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) incubated for 60 mins prior to substrate addition measured after 60 mins by fluorescence assay | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031621 (CHEMBL3359755) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) incubated for 60 mins prior to substrate addition measured after 60 mins by fluorescence assay | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031623 (CHEMBL3359753) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) incubated for 60 mins prior to substrate addition measured after 60 mins by fluorescence assay | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031615 (CHEMBL3359762) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) incubated for 60 mins prior to substrate addition measured after 60 mins by fluorescence assay | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031624 (CHEMBL3359752) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) incubated for 60 mins prior to substrate addition measured after 60 mins by fluorescence assay | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031625 (CHEMBL3359751) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) incubated for 60 mins prior to substrate addition measured after 60 mins by fluorescence assay | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50397712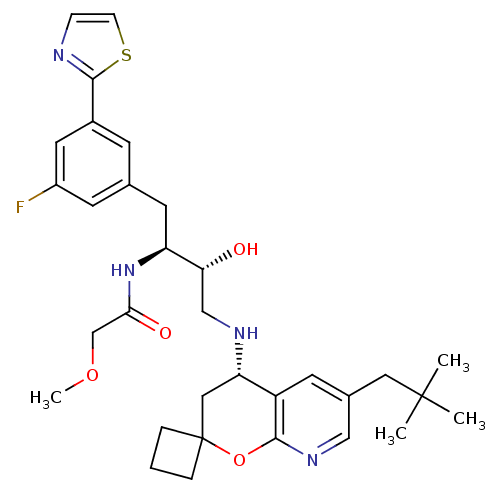 (CHEMBL2181902) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Glu-Ile-Asp-Leu-Met-Val-Leu-Asp as substrate incubated for 60 mins prior to substrate addition measured a... | J Med Chem 55: 9025-44 (2012) Article DOI: 10.1021/jm300118s BindingDB Entry DOI: 10.7270/Q298885R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50397708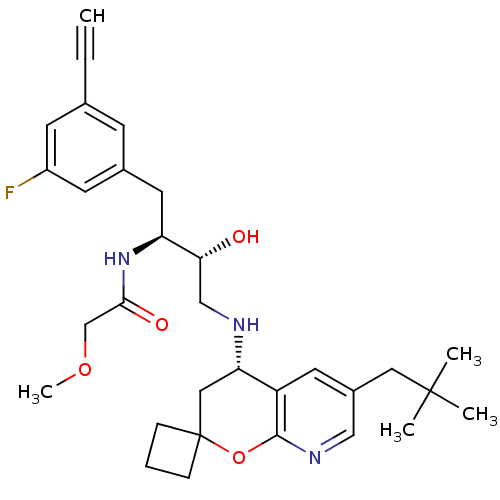 (CHEMBL2181906) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Glu-Ile-Asp-Leu-Met-Val-Leu-Asp as substrate incubated for 60 mins prior to substrate addition measured a... | J Med Chem 55: 9025-44 (2012) Article DOI: 10.1021/jm300118s BindingDB Entry DOI: 10.7270/Q298885R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031612 (CHEMBL3354688) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) incubated for 60 mins prior to substrate addition measured after 60 mins by fluorescence assay | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031618 (CHEMBL3359759) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) incubated for 60 mins prior to substrate addition measured after 60 mins by fluorescence assay | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031627 (CHEMBL3359749) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells assessed as Abeta40 level after overnight incubation by sandwich ELISA | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031616 (CHEMBL3359761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells assessed as Abeta40 level after overnight incubation by sandwich ELISA | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50397707 (CHEMBL2181907) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Glu-Ile-Asp-Leu-Met-Val-Leu-Asp as substrate incubated for 60 mins prior to substrate addition measured a... | J Med Chem 55: 9025-44 (2012) Article DOI: 10.1021/jm300118s BindingDB Entry DOI: 10.7270/Q298885R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50397697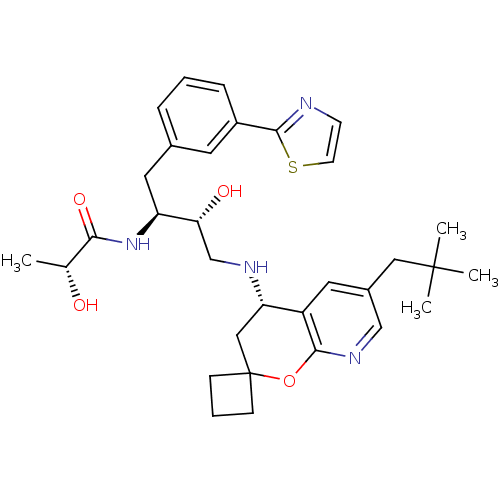 (CHEMBL2181917) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Glu-Ile-Asp-Leu-Met-Val-Leu-Asp as substrate incubated for 60 mins prior to substrate addition measured a... | J Med Chem 55: 9025-44 (2012) Article DOI: 10.1021/jm300118s BindingDB Entry DOI: 10.7270/Q298885R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031620 (CHEMBL3359756) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) incubated for 60 mins prior to substrate addition measured after 60 mins by fluorescence assay | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50397688 (CHEMBL2181896) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Glu-Ile-Asp-Leu-Met-Val-Leu-Asp as substrate incubated for 60 mins prior to substrate addition measured a... | J Med Chem 55: 9025-44 (2012) Article DOI: 10.1021/jm300118s BindingDB Entry DOI: 10.7270/Q298885R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50397709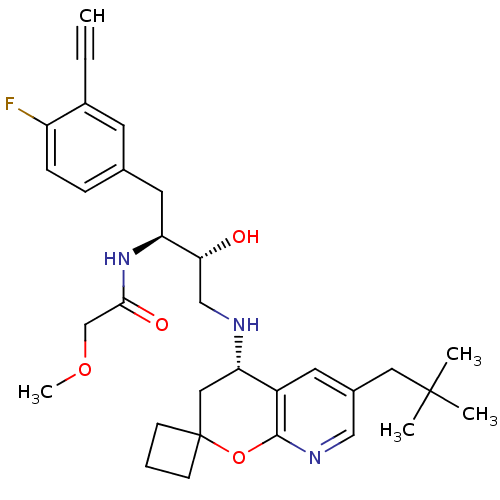 (CHEMBL2181905) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Glu-Ile-Asp-Leu-Met-Val-Leu-Asp as substrate incubated for 60 mins prior to substrate addition measured a... | J Med Chem 55: 9025-44 (2012) Article DOI: 10.1021/jm300118s BindingDB Entry DOI: 10.7270/Q298885R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50397706 (CHEMBL2181908) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Glu-Ile-Asp-Leu-Met-Val-Leu-Asp as substrate incubated for 60 mins prior to substrate addition measured a... | J Med Chem 55: 9025-44 (2012) Article DOI: 10.1021/jm300118s BindingDB Entry DOI: 10.7270/Q298885R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031628 (CHEMBL3359748) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells assessed as Abeta40 level after overnight incubation by sandwich ELISA | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50397692 (CHEMBL2181892) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 using Glu-Ile-Asp-Leu-Met-Val-Leu-Asp as substrate incubated for 60 mins prior to substrate addition measured a... | J Med Chem 55: 9025-44 (2012) Article DOI: 10.1021/jm300118s BindingDB Entry DOI: 10.7270/Q298885R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031613 (CHEMBL3359763) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells assessed as Abeta40 level after overnight incubation by sandwich ELISA | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031629 (CHEMBL3359747) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells assessed as Abeta40 level after overnight incubation by sandwich ELISA | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031617 (CHEMBL3359760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells assessed as Abeta40 level after overnight incubation by sandwich ELISA | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031616 (CHEMBL3359761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) incubated for 60 mins prior to substrate addition measured after 60 mins by fluorescence assay | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031617 (CHEMBL3359760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) incubated for 60 mins prior to substrate addition measured after 60 mins by fluorescence assay | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50031613 (CHEMBL3359763) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50031624 (CHEMBL3359752) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells assessed as Abeta40 level after overnight incubation by sandwich ELISA | J Med Chem 57: 9811-31 (2014) Article DOI: 10.1021/jm5012676 BindingDB Entry DOI: 10.7270/Q20G3MRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 164 total ) | Next | Last >> |