 Found 147 hits with Last Name = 'esser' and Initial = 'r'
Found 147 hits with Last Name = 'esser' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Renin
(Homo sapiens (Human)) | BDBM50065428
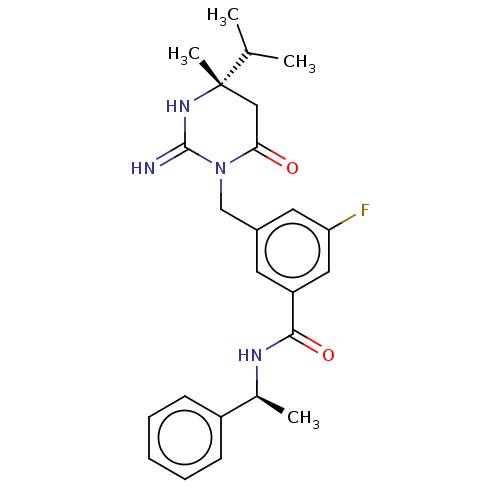
(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065395

(CHEMBL3401345)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)N2CC(CC2=O)c2cccc(Cl)c2)C(=N)N1 |r| Show InChI InChI=1S/C25H29ClN4O2/c1-16(2)25(3)13-23(32)30(24(27)28-25)14-17-6-4-9-21(10-17)29-15-19(12-22(29)31)18-7-5-8-20(26)11-18/h4-11,16,19H,12-15H2,1-3H3,(H2,27,28)/t19?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065426
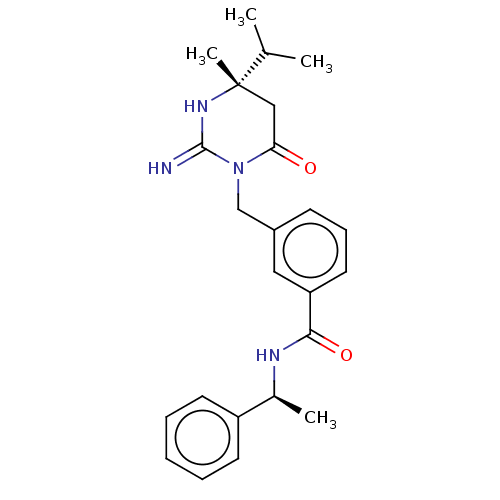
(CHEMBL3401348)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H30N4O2/c1-16(2)24(4)14-21(29)28(23(25)27-24)15-18-9-8-12-20(13-18)22(30)26-17(3)19-10-6-5-7-11-19/h5-13,16-17H,14-15H2,1-4H3,(H2,25,27)(H,26,30)/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065424
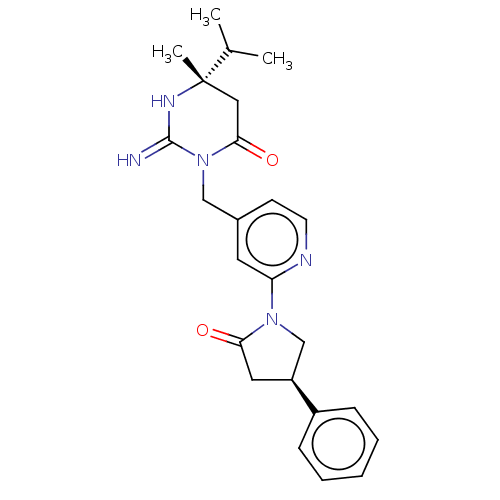
(CHEMBL3401346)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2ccnc(c2)N2C[C@H](CC2=O)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29N5O2/c1-16(2)24(3)13-22(31)29(23(25)27-24)14-17-9-10-26-20(11-17)28-15-19(12-21(28)30)18-7-5-4-6-8-18/h4-11,16,19H,12-15H2,1-3H3,(H2,25,27)/t19-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065425
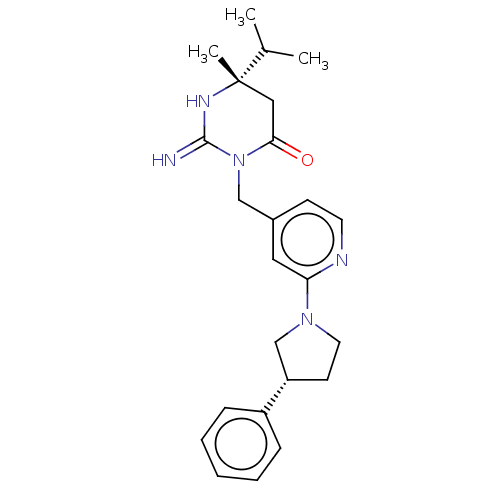
(CHEMBL3401347)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2ccnc(c2)N2CC[C@@H](C2)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H31N5O/c1-17(2)24(3)14-22(30)29(23(25)27-24)15-18-9-11-26-21(13-18)28-12-10-20(16-28)19-7-5-4-6-8-19/h4-9,11,13,17,20H,10,12,14-16H2,1-3H3,(H2,25,27)/t20-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065393

(CHEMBL3401344)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)N2C[C@H](CC2=O)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C25H29FN4O2/c1-16(2)25(3)13-23(32)30(24(27)28-25)14-17-9-20(26)12-21(10-17)29-15-19(11-22(29)31)18-7-5-4-6-8-18/h4-10,12,16,19H,11,13-15H2,1-3H3,(H2,27,28)/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065391
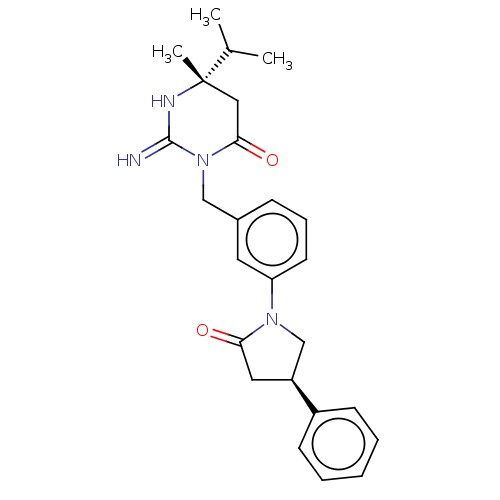
(CHEMBL3401342)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)N2C[C@H](CC2=O)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C25H30N4O2/c1-17(2)25(3)14-23(31)29(24(26)27-25)15-18-8-7-11-21(12-18)28-16-20(13-22(28)30)19-9-5-4-6-10-19/h4-12,17,20H,13-16H2,1-3H3,(H2,26,27)/t20-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50065390

(CHEMBL3401341)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C23H28N4O2/c1-16(2)23(3)13-20(28)27(22(24)26-23)15-18-10-7-11-19(12-18)21(29)25-14-17-8-5-4-6-9-17/h4-12,16H,13-15H2,1-3H3,(H2,24,26)(H,25,29)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50065389

(CHEMBL3401340)Show SMILES C[C@]1(CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1)c1ccc2sccc2c1 |r| Show InChI InChI=1S/C28H26N4O2S/c1-28(23-10-11-24-21(15-23)12-13-35-24)16-25(33)32(27(29)31-28)18-20-8-5-9-22(14-20)26(34)30-17-19-6-3-2-4-7-19/h2-15H,16-18H2,1H3,(H2,29,31)(H,30,34)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065427

(CHEMBL3401349)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)N[C@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H30N4O2/c1-16(2)24(4)14-21(29)28(23(25)27-24)15-18-9-8-12-20(13-18)22(30)26-17(3)19-10-6-5-7-11-19/h5-13,16-17H,14-15H2,1-4H3,(H2,25,27)(H,26,30)/t17-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50065428

(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CathD (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065392
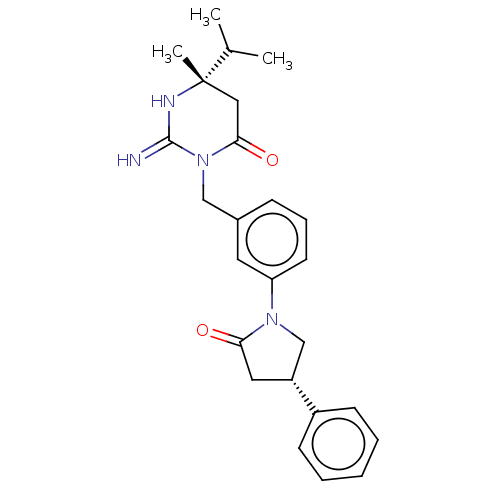
(CHEMBL3401343)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)N2C[C@@H](CC2=O)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C25H30N4O2/c1-17(2)25(3)14-23(31)29(24(26)27-25)15-18-8-7-11-21(12-18)28-16-20(13-22(28)30)19-9-5-4-6-10-19/h4-12,17,20H,13-16H2,1-3H3,(H2,26,27)/t20-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50065428

(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50065428

(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CathE (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50065390

(CHEMBL3401341)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C23H28N4O2/c1-16(2)23(3)13-20(28)27(22(24)26-23)15-18-10-7-11-19(12-18)21(29)25-14-17-8-5-4-6-9-17/h4-12,16H,13-15H2,1-3H3,(H2,24,26)(H,25,29)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CathD (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50065389

(CHEMBL3401340)Show SMILES C[C@]1(CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1)c1ccc2sccc2c1 |r| Show InChI InChI=1S/C28H26N4O2S/c1-28(23-10-11-24-21(15-23)12-13-35-24)16-25(33)32(27(29)31-28)18-20-8-5-9-22(14-20)26(34)30-17-19-6-3-2-4-7-19/h2-15H,16-18H2,1H3,(H2,29,31)(H,30,34)/t28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50065428

(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50065390

(CHEMBL3401341)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C23H28N4O2/c1-16(2)23(3)13-20(28)27(22(24)26-23)15-18-10-7-11-19(12-18)21(29)25-14-17-8-5-4-6-9-17/h4-12,16H,13-15H2,1-3H3,(H2,24,26)(H,25,29)/t23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50065389

(CHEMBL3401340)Show SMILES C[C@]1(CC(=O)N(Cc2cccc(c2)C(=O)NCc2ccccc2)C(=N)N1)c1ccc2sccc2c1 |r| Show InChI InChI=1S/C28H26N4O2S/c1-28(23-10-11-24-21(15-23)12-13-35-24)16-25(33)32(27(29)31-28)18-20-8-5-9-22(14-20)26(34)30-17-19-6-3-2-4-7-19/h2-15H,16-18H2,1H3,(H2,29,31)(H,30,34)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CathD (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174708

(CHEMBL197375 | N-(2-(3-((2R,5S)-4-(4-fluorobenzyl)...)Show SMILES C[C@H]1CN([C@H](C)CN1Cc1ccc(F)cc1)C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C24H27ClFN3O2/c1-16-14-29(17(2)13-28(16)15-19-4-9-22(26)10-5-19)24(31)11-7-20-6-8-21(25)12-23(20)27-18(3)30/h4-12,16-17H,13-15H2,1-3H3,(H,27,30)/b11-7+/t16-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM98678
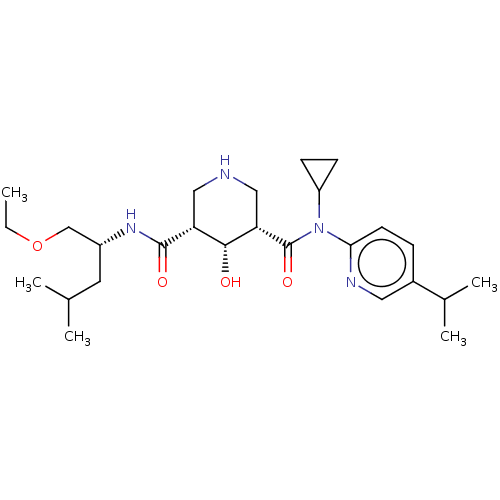
(US8497286, 154)Show SMILES CCOC[C@@H](CC(C)C)NC(=O)[C@@H]1CNC[C@@H]([C@@H]1O)C(=O)N(C1CC1)c1ccc(cn1)C(C)C |r| Show InChI InChI=1S/C26H42N4O4/c1-6-34-15-19(11-16(2)3)29-25(32)21-13-27-14-22(24(21)31)26(33)30(20-8-9-20)23-10-7-18(12-28-23)17(4)5/h7,10,12,16-17,19-22,24,27,31H,6,8-9,11,13-15H2,1-5H3,(H,29,32)/t19-,21-,22+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174703
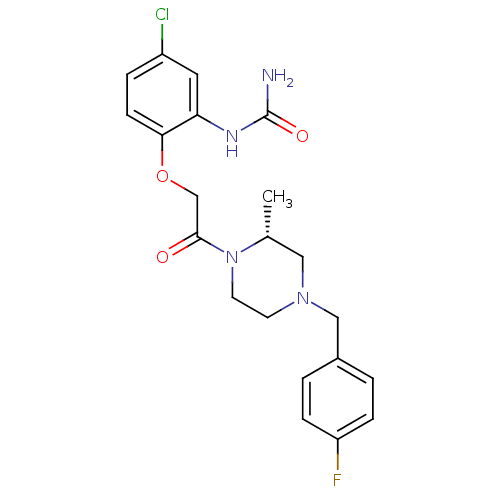
((R)-1-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)COc1ccc(Cl)cc1NC(N)=O Show InChI InChI=1S/C21H24ClFN4O3/c1-14-11-26(12-15-2-5-17(23)6-3-15)8-9-27(14)20(28)13-30-19-7-4-16(22)10-18(19)25-21(24)29/h2-7,10,14H,8-9,11-13H2,1H3,(H3,24,25,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50382334
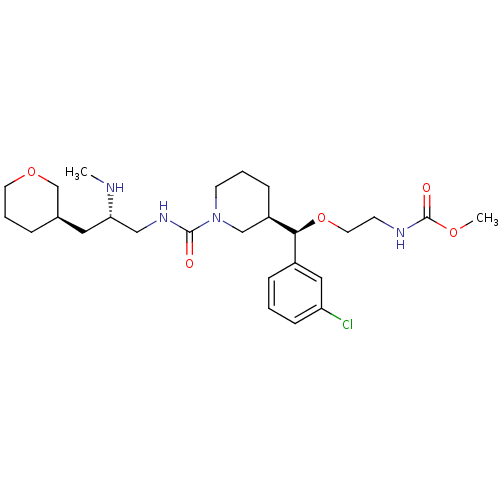
(CHEMBL1276678)Show SMILES CN[C@H](CNC(=O)N1CCC[C@H](C1)[C@@H](OCCNC(=O)OC)c1cccc(Cl)c1)C[C@H]1CCCOC1 |r| Show InChI InChI=1S/C26H41ClN4O5/c1-28-23(14-19-6-5-12-35-18-19)16-30-25(32)31-11-4-8-21(17-31)24(20-7-3-9-22(27)15-20)36-13-10-29-26(33)34-2/h3,7,9,15,19,21,23-24,28H,4-6,8,10-14,16-18H2,1-2H3,(H,29,33)(H,30,32)/t19-,21-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM17950

((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174707

((R)-1-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(N)=O Show InChI InChI=1S/C22H24ClFN4O2/c1-15-13-27(14-16-2-7-19(24)8-3-16)10-11-28(15)21(29)9-5-17-4-6-18(23)12-20(17)26-22(25)30/h2-9,12,15H,10-11,13-14H2,1H3,(H3,25,26,30)/b9-5+/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50392953
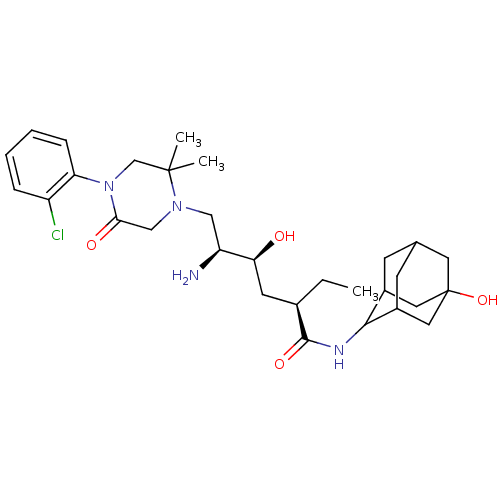
(CHEMBL2152353)Show SMILES CC[C@H](C[C@H](O)[C@@H](N)CN1CC(=O)N(CC1(C)C)c1ccccc1Cl)C(=O)NC1C2CC3CC1CC(O)(C3)C2 |r,wU:4.4,2.2,wD:6.6,TLB:38:35:28.29.30:32,36:35:28:30.31.32,27:28:34.38.35:30.31.32,27:28:32:34.35.37,THB:38:29:32:34.35.37,37:35:28:30.31.32,37:31:28:34.38.35,(13.51,-6.54,;12.17,-7.31,;12.17,-8.85,;10.84,-9.62,;9.51,-8.85,;9.51,-7.31,;8.17,-9.62,;6.84,-8.85,;8.17,-11.16,;6.84,-11.93,;5.5,-11.17,;4.16,-11.94,;2.83,-11.17,;4.18,-13.47,;5.51,-14.24,;6.84,-13.48,;7.61,-14.81,;8.38,-13.48,;2.85,-14.25,;1.51,-13.49,;.18,-14.26,;.18,-15.8,;1.52,-16.57,;2.85,-15.8,;4.19,-16.57,;13.51,-9.62,;13.51,-11.16,;14.84,-8.86,;16.17,-9.63,;17.67,-9.21,;17.67,-7.62,;18.71,-6.39,;17.36,-6.87,;17.37,-8.35,;18.7,-8.84,;20.09,-8.5,;21.42,-9.26,;20.1,-6.97,;19.08,-9.77,)| Show InChI InChI=1S/C30H45ClN4O4/c1-4-19(28(38)33-27-20-9-18-10-21(27)14-30(39,12-18)13-20)11-25(36)23(32)15-34-16-26(37)35(17-29(34,2)3)24-8-6-5-7-22(24)31/h5-8,18-21,23,25,27,36,39H,4,9-17,32H2,1-3H3,(H,33,38)/t18?,19-,20?,21?,23+,25+,27?,30?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174709
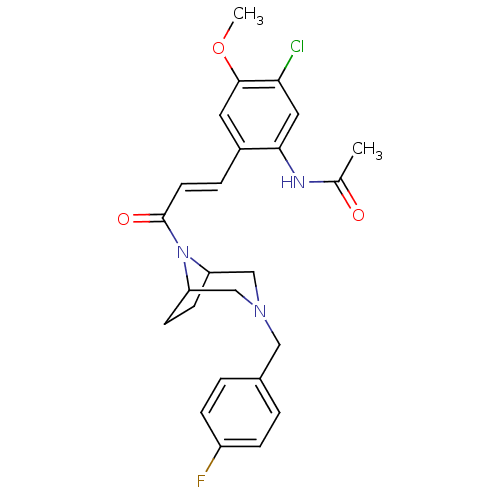
(CHEMBL372807 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES COc1cc(\C=C\C(=O)N2C3CCC2CN(Cc2ccc(F)cc2)C3)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C25H27ClFN3O3/c1-16(31)28-23-12-22(26)24(33-2)11-18(23)5-10-25(32)30-20-8-9-21(30)15-29(14-20)13-17-3-6-19(27)7-4-17/h3-7,10-12,20-21H,8-9,13-15H2,1-2H3,(H,28,31)/b10-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50347010
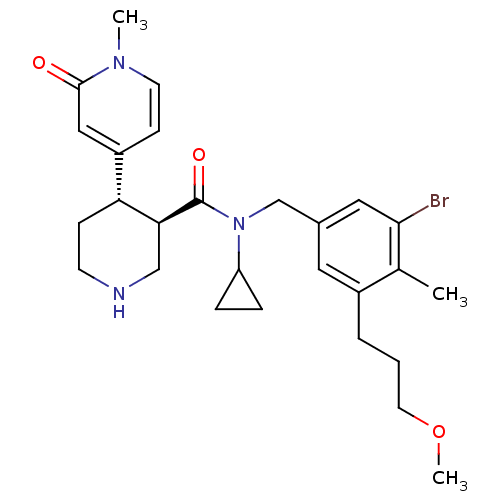
(CHEMBL1796063)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccn(C)c(=O)c2)cc(Br)c1C |r| Show InChI InChI=1S/C27H36BrN3O3/c1-18-20(5-4-12-34-3)13-19(14-25(18)28)17-31(22-6-7-22)27(33)24-16-29-10-8-23(24)21-9-11-30(2)26(32)15-21/h9,11,13-15,22-24,29H,4-8,10,12,16-17H2,1-3H3/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174711
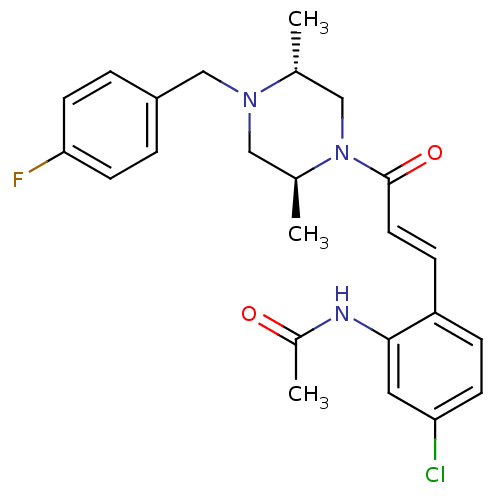
(CHEMBL197345 | N-(2-(3-((2S,5R)-4-(4-fluorobenzyl)...)Show SMILES C[C@@H]1CN([C@@H](C)CN1Cc1ccc(F)cc1)C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C24H27ClFN3O2/c1-16-14-29(17(2)13-28(16)15-19-4-9-22(26)10-5-19)24(31)11-7-20-6-8-21(25)12-23(20)27-18(3)30/h4-12,16-17H,13-15H2,1-3H3,(H,27,30)/b11-7+/t16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50505088

(CHEMBL4466914)Show SMILES [Br-].[Br-].C[N+](C)(CC#CCOC1=NOCC1)Cc1cc(F)c(\N=N\c2c(F)cc(C[N+](C)(C)CC#CCOC3=NOCC3)cc2F)c(F)c1 |t:8,36| Show InChI InChI=1S/C32H36F4N6O4/c1-41(2,11-5-7-13-43-29-9-15-45-39-29)21-23-17-25(33)31(26(34)18-23)37-38-32-27(35)19-24(20-28(32)36)22-42(3,4)12-6-8-14-44-30-10-16-46-40-30/h17-20H,9-16,21-22H2,1-4H3/q+2/b38-37+ | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in HEK293T cells co-expressing Galpha subunit and PLC-beta3 by split luciferase complement... |
J Med Chem 62: 3009-3020 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01822
BindingDB Entry DOI: 10.7270/Q2668HG2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50448377

(CHEMBL3121473)Show InChI InChI=1S/C10H17N2O2/c1-12(2,3)7-4-5-8-13-10-6-9-14-11-10/h6-9H2,1-3H3/q+1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in HEK293T cells co-expressing Galpha subunit and PLC-beta3 by split luciferase complement... |
J Med Chem 62: 3009-3020 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01822
BindingDB Entry DOI: 10.7270/Q2668HG2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174714
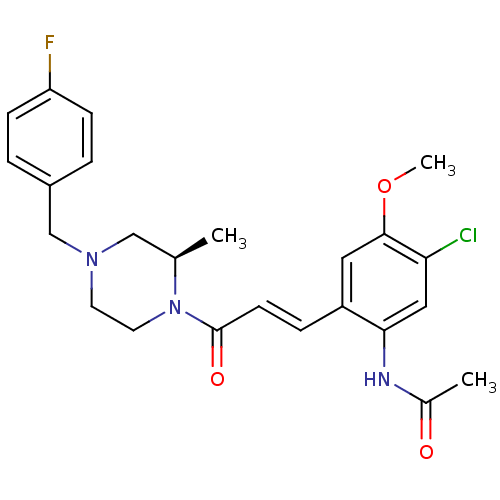
((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES COc1cc(\C=C\C(=O)N2CCN(Cc3ccc(F)cc3)C[C@H]2C)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C24H27ClFN3O3/c1-16-14-28(15-18-4-7-20(26)8-5-18)10-11-29(16)24(31)9-6-19-12-23(32-3)21(25)13-22(19)27-17(2)30/h4-9,12-13,16H,10-11,14-15H2,1-3H3,(H,27,30)/b9-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174703

((R)-1-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)COc1ccc(Cl)cc1NC(N)=O Show InChI InChI=1S/C21H24ClFN4O3/c1-14-11-26(12-15-2-5-17(23)6-3-15)8-9-27(14)20(28)13-30-19-7-4-16(22)10-18(19)25-21(24)29/h2-7,10,14H,8-9,11-13H2,1H3,(H3,24,25,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory effect on transwell chemotaxis induced by 1 nM MIP-1alpha in mouse pre-B cells transfected with human CCR1 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174720
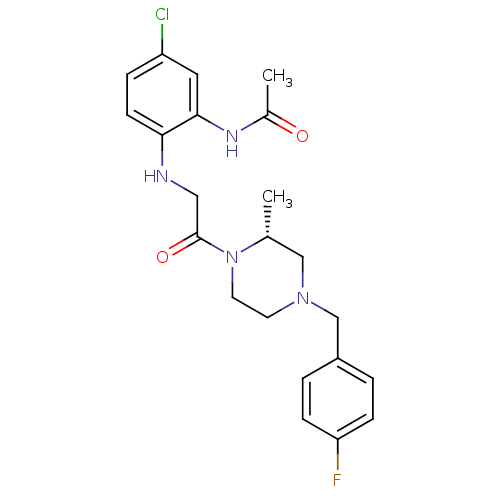
((R)-N-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)CNc1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C22H26ClFN4O2/c1-15-13-27(14-17-3-6-19(24)7-4-17)9-10-28(15)22(30)12-25-20-8-5-18(23)11-21(20)26-16(2)29/h3-8,11,15,25H,9-10,12-14H2,1-2H3,(H,26,29)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Mus musculus) | BDBM50174709

(CHEMBL372807 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES COc1cc(\C=C\C(=O)N2C3CCC2CN(Cc2ccc(F)cc2)C3)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C25H27ClFN3O3/c1-16(31)28-23-12-22(26)24(33-2)11-18(23)5-10-25(32)30-20-8-9-21(30)15-29(14-20)13-17-3-6-19(27)7-4-17/h3-7,10-12,20-21H,8-9,13-15H2,1-2H3,(H,28,31)/b10-5+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at mouse CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174714

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES COc1cc(\C=C\C(=O)N2CCN(Cc3ccc(F)cc3)C[C@H]2C)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C24H27ClFN3O3/c1-16-14-28(15-18-4-7-20(26)8-5-18)10-11-29(16)24(31)9-6-19-12-23(32-3)21(25)13-22(19)27-17(2)30/h4-9,12-13,16H,10-11,14-15H2,1-3H3,(H,27,30)/b9-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50505087
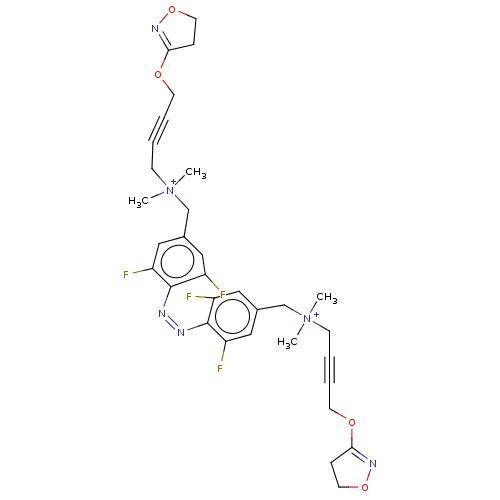
(CHEMBL4514215)Show SMILES [Br-].[Br-].C[N+](C)(CC#CCOC1=NOCC1)Cc1cc(F)c(\N=N/c2c(F)cc(C[N+](C)(C)CC#CCOC3=NOCC3)cc2F)c(F)c1 |t:8,36| Show InChI InChI=1S/C32H36F4N6O4/c1-41(2,11-5-7-13-43-29-9-15-45-39-29)21-23-17-25(33)31(26(34)18-23)37-38-32-27(35)19-24(20-28(32)36)22-42(3,4)12-6-8-14-44-30-10-16-46-40-30/h17-20H,9-16,21-22H2,1-4H3/q+2/b38-37- | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in HEK293T cells co-expressing Galpha subunit and PLC-beta3 by split luciferase complement... |
J Med Chem 62: 3009-3020 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01822
BindingDB Entry DOI: 10.7270/Q2668HG2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174718

(CHEMBL200680 | N-(6-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES CC(=O)Nc1cc2ncccc2cc1\C=C\C(=O)N1C2CCC1CN(Cc1ccc(F)cc1)C2 Show InChI InChI=1S/C27H27FN4O2/c1-18(33)30-26-14-25-20(3-2-12-29-25)13-21(26)6-11-27(34)32-23-9-10-24(32)17-31(16-23)15-19-4-7-22(28)8-5-19/h2-8,11-14,23-24H,9-10,15-17H2,1H3,(H,30,33)/b11-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174709

(CHEMBL372807 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES COc1cc(\C=C\C(=O)N2C3CCC2CN(Cc2ccc(F)cc2)C3)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C25H27ClFN3O3/c1-16(31)28-23-12-22(26)24(33-2)11-18(23)5-10-25(32)30-20-8-9-21(30)15-29(14-20)13-17-3-6-19(27)7-4-17/h3-7,10-12,20-21H,8-9,13-15H2,1-2H3,(H,28,31)/b10-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174709

(CHEMBL372807 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES COc1cc(\C=C\C(=O)N2C3CCC2CN(Cc2ccc(F)cc2)C3)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C25H27ClFN3O3/c1-16(31)28-23-12-22(26)24(33-2)11-18(23)5-10-25(32)30-20-8-9-21(30)15-29(14-20)13-17-3-6-19(27)7-4-17/h3-7,10-12,20-21H,8-9,13-15H2,1-2H3,(H,28,31)/b10-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory effect on transwell chemotaxis induced by 1 nM MIP-1alpha in mouse pre-B cells transfected with human CCR1 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174710
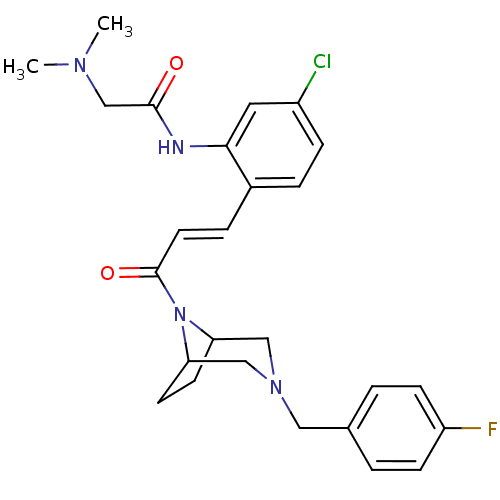
(CHEMBL200242 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES CN(C)CC(=O)Nc1cc(Cl)ccc1\C=C\C(=O)N1C2CCC1CN(Cc1ccc(F)cc1)C2 Show InChI InChI=1S/C26H30ClFN4O2/c1-30(2)17-25(33)29-24-13-20(27)7-5-19(24)6-12-26(34)32-22-10-11-23(32)16-31(15-22)14-18-3-8-21(28)9-4-18/h3-9,12-13,22-23H,10-11,14-17H2,1-2H3,(H,29,33)/b12-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174708

(CHEMBL197375 | N-(2-(3-((2R,5S)-4-(4-fluorobenzyl)...)Show SMILES C[C@H]1CN([C@H](C)CN1Cc1ccc(F)cc1)C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C24H27ClFN3O2/c1-16-14-29(17(2)13-28(16)15-19-4-9-22(26)10-5-19)24(31)11-7-20-6-8-21(25)12-23(20)27-18(3)30/h4-12,16-17H,13-15H2,1-3H3,(H,27,30)/b11-7+/t16-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174704

((R)-N-(2-(1-(4-fluorobenzyl)-3-methylpiperazine-4-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)c1cc2cc(Cl)cc(NC(C)=O)c2[nH]1 Show InChI InChI=1S/C23H24ClFN4O2/c1-14-12-28(13-16-3-5-19(25)6-4-16)7-8-29(14)23(31)21-10-17-9-18(24)11-20(22(17)27-21)26-15(2)30/h3-6,9-11,14,27H,7-8,12-13H2,1-2H3,(H,26,30)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory effect on transwell chemotaxis induced by 1 nM MIP-1alpha in mouse pre-B cells transfected with human CCR1 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174710

(CHEMBL200242 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES CN(C)CC(=O)Nc1cc(Cl)ccc1\C=C\C(=O)N1C2CCC1CN(Cc1ccc(F)cc1)C2 Show InChI InChI=1S/C26H30ClFN4O2/c1-30(2)17-25(33)29-24-13-20(27)7-5-19(24)6-12-26(34)32-22-10-11-23(32)16-31(15-22)14-18-3-8-21(28)9-4-18/h3-9,12-13,22-23H,10-11,14-17H2,1-2H3,(H,29,33)/b12-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174706
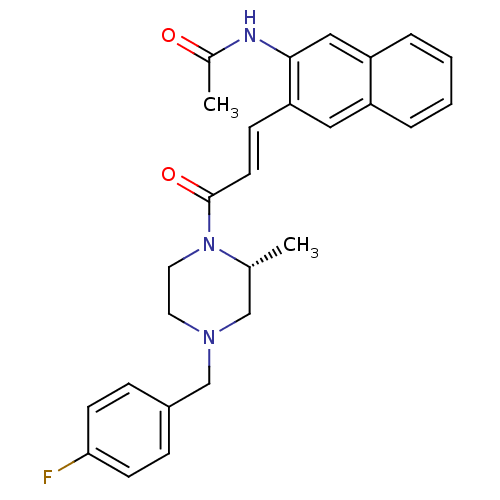
((R)-N-(3-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1cc2ccccc2cc1NC(C)=O Show InChI InChI=1S/C27H28FN3O2/c1-19-17-30(18-21-7-10-25(28)11-8-21)13-14-31(19)27(33)12-9-24-15-22-5-3-4-6-23(22)16-26(24)29-20(2)32/h3-12,15-16,19H,13-14,17-18H2,1-2H3,(H,29,32)/b12-9+/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50505086
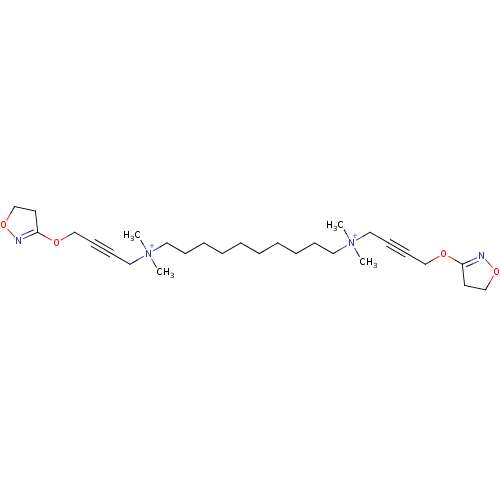
(CHEMBL4571387)Show SMILES [Br-].[Br-].C[N+](C)(CCCCCCCCCC[N+](C)(C)CC#CCOC1=NOCC1)CC#CCOC1=NOCC1 |t:21,32| Show InChI InChI=1S/C28H48N4O4/c1-31(2,21-13-15-23-33-27-17-25-35-29-27)19-11-9-7-5-6-8-10-12-20-32(3,4)22-14-16-24-34-28-18-26-36-30-28/h5-12,17-26H2,1-4H3/q+2 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in HEK293T cells co-expressing Galpha subunit and PLC-beta3 by split luciferase complement... |
J Med Chem 62: 3009-3020 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01822
BindingDB Entry DOI: 10.7270/Q2668HG2 |
More data for this
Ligand-Target Pair | |
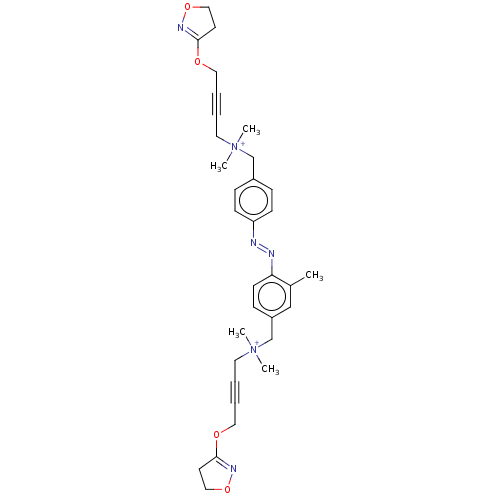
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50505085
(CHEMBL4470357)Show SMILES [Br-].[Br-].Cc1cc(C[N+](C)(C)CC#CCOC2=NOCC2)ccc1\N=N\c1ccc(C[N+](C)(C)CC#CCOC2=NOCC2)cc1 |t:13,38| Show InChI InChI=1S/C33H42N6O4/c1-27-24-29(26-39(4,5)19-7-9-21-41-33-17-23-43-37-33)12-15-31(27)35-34-30-13-10-28(11-14-30)25-38(2,3)18-6-8-20-40-32-16-22-42-36-32/h10-15,24H,16-23,25-26H2,1-5H3/q+2/b35-34+ | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M1 receptor expressed in HEK293T cells co-expressing Galpha subunit and PLC-beta3 by split luciferase complement... |
J Med Chem 62: 3009-3020 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01822
BindingDB Entry DOI: 10.7270/Q2668HG2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174707

((R)-1-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(N)=O Show InChI InChI=1S/C22H24ClFN4O2/c1-15-13-27(14-16-2-7-19(24)8-3-16)10-11-28(15)21(29)9-5-17-4-6-18(23)12-20(17)26-22(25)30/h2-9,12,15H,10-11,13-14H2,1H3,(H3,25,26,30)/b9-5+/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data