 Found 109 hits with Last Name = 'evans' and Initial = 'dg'
Found 109 hits with Last Name = 'evans' and Initial = 'dg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311424
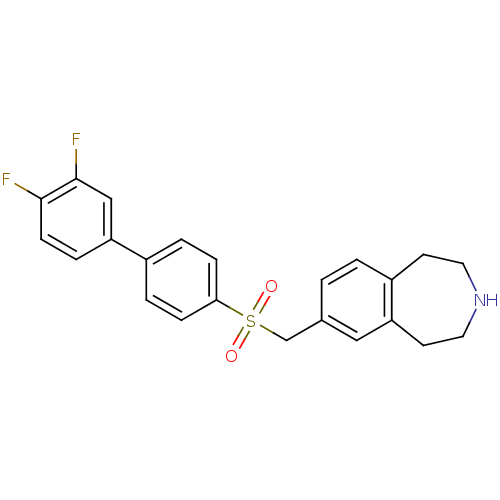
(7-((3',4'-difluorobiphenyl-4-ylsulfonyl)methyl)-2,...)Show SMILES Fc1ccc(cc1F)-c1ccc(cc1)S(=O)(=O)Cc1ccc2CCNCCc2c1 Show InChI InChI=1S/C23H21F2NO2S/c24-22-8-5-19(14-23(22)25)17-3-6-21(7-4-17)29(27,28)15-16-1-2-18-9-11-26-12-10-20(18)13-16/h1-8,13-14,26H,9-12,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311425
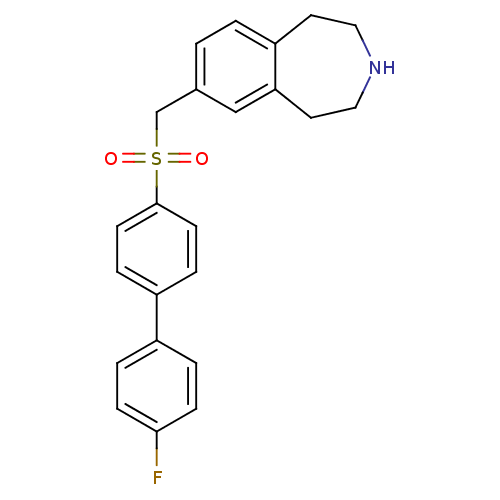
(7-((4'-fluorobiphenyl-4-ylsulfonyl)methyl)-2,3,4,5...)Show SMILES Fc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)Cc1ccc2CCNCCc2c1 Show InChI InChI=1S/C23H22FNO2S/c24-22-7-3-18(4-8-22)19-5-9-23(10-6-19)28(26,27)16-17-1-2-20-11-13-25-14-12-21(20)15-17/h1-10,15,25H,11-14,16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50311413
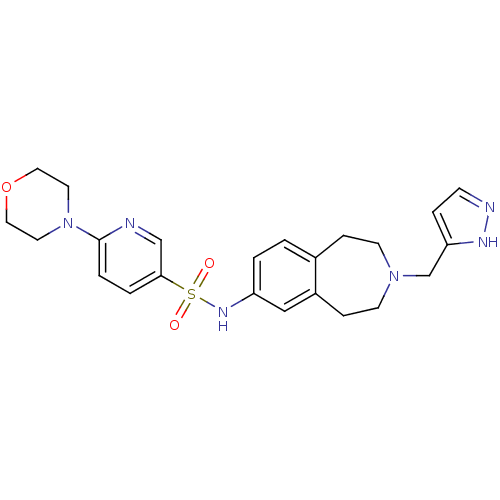
(CHEMBL1078595 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C23H28N6O3S/c30-33(31,22-3-4-23(24-16-22)29-11-13-32-14-12-29)27-20-2-1-18-6-9-28(10-7-19(18)15-20)17-21-5-8-25-26-21/h1-5,8,15-16,27H,6-7,9-14,17H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311414
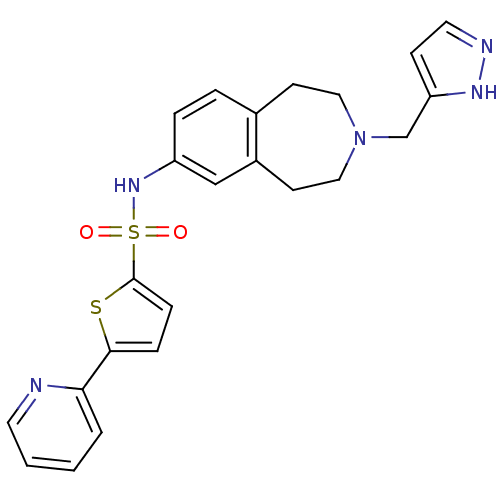
(CHEMBL1078492 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(s1)-c1ccccn1 Show InChI InChI=1S/C23H23N5O2S2/c29-32(30,23-7-6-22(31-23)21-3-1-2-11-24-21)27-19-5-4-17-9-13-28(14-10-18(17)15-19)16-20-8-12-25-26-20/h1-8,11-12,15,27H,9-10,13-14,16H2,(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311419

(CHEMBL1078464 | N-(4-fluorophenyl)-5-((2,3,4,5-tet...)Show SMILES Fc1ccc(Nc2ccc(cn2)S(=O)(=O)Cc2ccc3CCNCCc3c2)cc1 Show InChI InChI=1S/C22H22FN3O2S/c23-19-3-5-20(6-4-19)26-22-8-7-21(14-25-22)29(27,28)15-16-1-2-17-9-11-24-12-10-18(17)13-16/h1-8,13-14,24H,9-12,15H2,(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50311414

(CHEMBL1078492 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(s1)-c1ccccn1 Show InChI InChI=1S/C23H23N5O2S2/c29-32(30,23-7-6-22(31-23)21-3-1-2-11-24-21)27-19-5-4-17-9-13-28(14-10-18(17)15-19)16-20-8-12-25-26-20/h1-8,11-12,15,27H,9-10,13-14,16H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311415
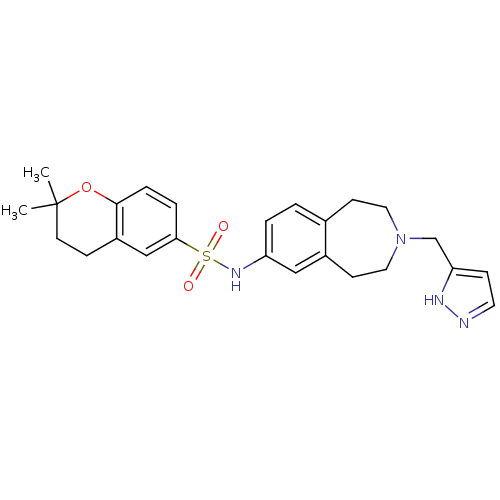
(CHEMBL1078490 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CC1(C)CCc2cc(ccc2O1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C25H30N4O3S/c1-25(2)11-7-20-16-23(5-6-24(20)32-25)33(30,31)28-21-4-3-18-9-13-29(14-10-19(18)15-21)17-22-8-12-26-27-22/h3-6,8,12,15-16,28H,7,9-11,13-14,17H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50311412
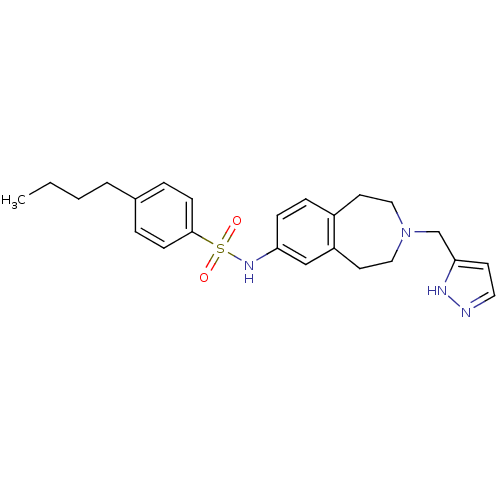
(CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CCCCc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C24H30N4O2S/c1-2-3-4-19-5-9-24(10-6-19)31(29,30)27-22-8-7-20-12-15-28(16-13-21(20)17-22)18-23-11-14-25-26-23/h5-11,14,17,27H,2-4,12-13,15-16,18H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311423
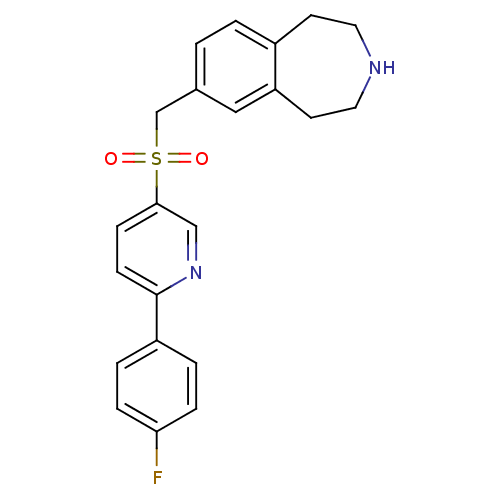
(7-((6-(4-fluorophenyl)pyridin-3-ylsulfonyl)methyl)...)Show SMILES Fc1ccc(cc1)-c1ccc(cn1)S(=O)(=O)Cc1ccc2CCNCCc2c1 Show InChI InChI=1S/C22H21FN2O2S/c23-20-5-3-18(4-6-20)22-8-7-21(14-25-22)28(26,27)15-16-1-2-17-9-11-24-12-10-19(17)13-16/h1-8,13-14,24H,9-12,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50311412

(CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CCCCc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C24H30N4O2S/c1-2-3-4-19-5-9-24(10-6-19)31(29,30)27-22-8-7-20-12-15-28(16-13-21(20)17-22)18-23-11-14-25-26-23/h5-11,14,17,27H,2-4,12-13,15-16,18H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 using diethoxyfluorescein as substrate |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311420
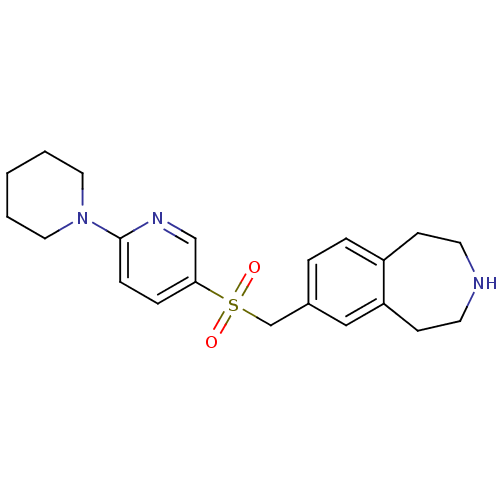
(7-((6-(piperidin-1-yl)pyridin-3-ylsulfonyl)methyl)...)Show SMILES O=S(=O)(Cc1ccc2CCNCCc2c1)c1ccc(nc1)N1CCCCC1 Show InChI InChI=1S/C21H27N3O2S/c25-27(26,16-17-4-5-18-8-10-22-11-9-19(18)14-17)20-6-7-21(23-15-20)24-12-2-1-3-13-24/h4-7,14-15,22H,1-3,8-13,16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311416
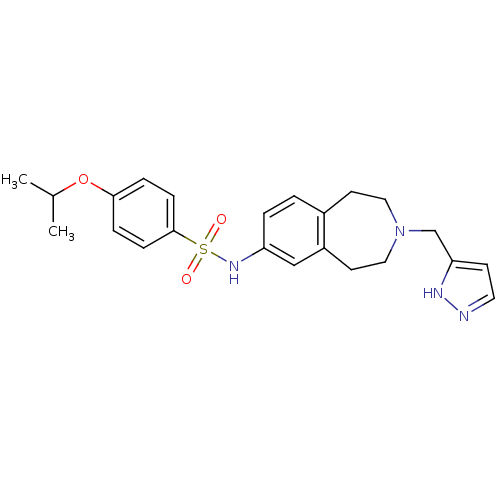
(CHEMBL1078208 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CC(C)Oc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C23H28N4O3S/c1-17(2)30-22-5-7-23(8-6-22)31(28,29)26-20-4-3-18-10-13-27(14-11-19(18)15-20)16-21-9-12-24-25-21/h3-9,12,15,17,26H,10-11,13-14,16H2,1-2H3,(H,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311421
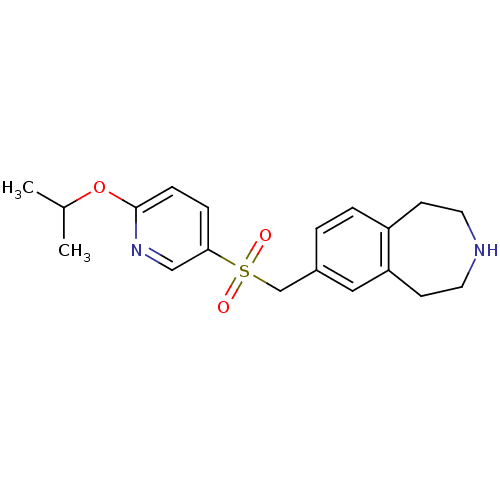
(7-((6-isopropoxypyridin-3-ylsulfonyl)methyl)-2,3,4...)Show InChI InChI=1S/C19H24N2O3S/c1-14(2)24-19-6-5-18(12-21-19)25(22,23)13-15-3-4-16-7-9-20-10-8-17(16)11-15/h3-6,11-12,14,20H,7-10,13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311422
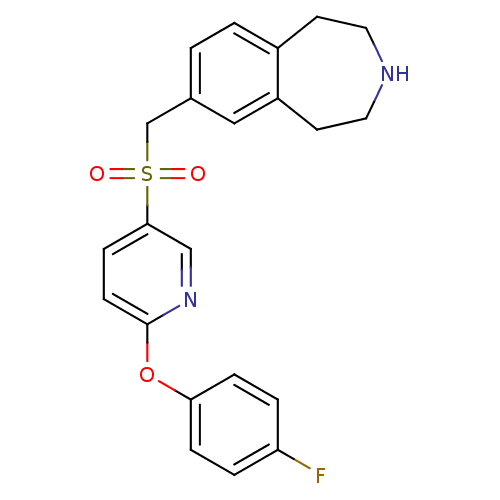
(7-((6-(4-fluorophenoxy)pyridin-3-ylsulfonyl)methyl...)Show SMILES Fc1ccc(Oc2ccc(cn2)S(=O)(=O)Cc2ccc3CCNCCc3c2)cc1 Show InChI InChI=1S/C22H21FN2O3S/c23-19-3-5-20(6-4-19)28-22-8-7-21(14-25-22)29(26,27)15-16-1-2-17-9-11-24-12-10-18(17)13-16/h1-8,13-14,24H,9-12,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50311412

(CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CCCCc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C24H30N4O2S/c1-2-3-4-19-5-9-24(10-6-19)31(29,30)27-22-8-7-20-12-15-28(16-13-21(20)17-22)18-23-11-14-25-26-23/h5-11,14,17,27H,2-4,12-13,15-16,18H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 using 7-{3-(4-phenylpiperazin-1-ylmethyl)benzyl}resorufin as substrate |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50311417
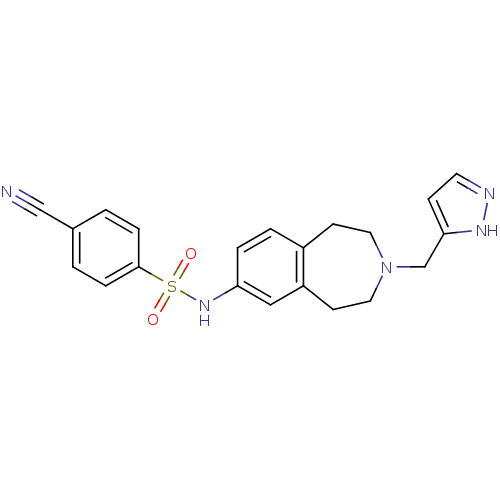
(CHEMBL1081860 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(cc1)C#N Show InChI InChI=1S/C21H21N5O2S/c22-14-16-1-5-21(6-2-16)29(27,28)25-19-4-3-17-8-11-26(12-9-18(17)13-19)15-20-7-10-23-24-20/h1-7,10,13,25H,8-9,11-12,15H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50311412

(CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CCCCc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C24H30N4O2S/c1-2-3-4-19-5-9-24(10-6-19)31(29,30)27-22-8-7-20-12-15-28(16-13-21(20)17-22)18-23-11-14-25-26-23/h5-11,14,17,27H,2-4,12-13,15-16,18H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311417

(CHEMBL1081860 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(cc1)C#N Show InChI InChI=1S/C21H21N5O2S/c22-14-16-1-5-21(6-2-16)29(27,28)25-19-4-3-17-8-11-26(12-9-18(17)13-19)15-20-7-10-23-24-20/h1-7,10,13,25H,8-9,11-12,15H2,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50311413

(CHEMBL1078595 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C23H28N6O3S/c30-33(31,22-3-4-23(24-16-22)29-11-13-32-14-12-29)27-20-2-1-18-6-9-28(10-7-19(18)15-20)17-21-5-8-25-26-21/h1-5,8,15-16,27H,6-7,9-14,17H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50311413

(CHEMBL1078595 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C23H28N6O3S/c30-33(31,22-3-4-23(24-16-22)29-11-13-32-14-12-29)27-20-2-1-18-6-9-28(10-7-19(18)15-20)17-21-5-8-25-26-21/h1-5,8,15-16,27H,6-7,9-14,17H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 using 7-{3-(4-phenylpiperazin-1-ylmethyl)benzyl}resorufin as substrate |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50311413

(CHEMBL1078595 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C23H28N6O3S/c30-33(31,22-3-4-23(24-16-22)29-11-13-32-14-12-29)27-20-2-1-18-6-9-28(10-7-19(18)15-20)17-21-5-8-25-26-21/h1-5,8,15-16,27H,6-7,9-14,17H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 using diethoxyfluorescein as substrate |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50311413

(CHEMBL1078595 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C23H28N6O3S/c30-33(31,22-3-4-23(24-16-22)29-11-13-32-14-12-29)27-20-2-1-18-6-9-28(10-7-19(18)15-20)17-21-5-8-25-26-21/h1-5,8,15-16,27H,6-7,9-14,17H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50311412

(CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CCCCc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C24H30N4O2S/c1-2-3-4-19-5-9-24(10-6-19)31(29,30)27-22-8-7-20-12-15-28(16-13-21(20)17-22)18-23-11-14-25-26-23/h5-11,14,17,27H,2-4,12-13,15-16,18H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50311413

(CHEMBL1078595 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C23H28N6O3S/c30-33(31,22-3-4-23(24-16-22)29-11-13-32-14-12-29)27-20-2-1-18-6-9-28(10-7-19(18)15-20)17-21-5-8-25-26-21/h1-5,8,15-16,27H,6-7,9-14,17H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311418

(CHEMBL1080047 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CC(C)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C17H24N4O2S/c1-13(2)24(22,23)20-16-4-3-14-6-9-21(10-7-15(14)11-16)12-17-5-8-18-19-17/h3-5,8,11,13,20H,6-7,9-10,12H2,1-2H3,(H,18,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50311426

(4-(5-((2,3,4,5-tetrahydro-1H-benzo[d]azepin-7-yl)m...)Show SMILES O=S(=O)(Cc1ccc2CCNCCc2c1)c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C20H25N3O3S/c24-27(25,15-16-1-2-17-5-7-21-8-6-18(17)13-16)19-3-4-20(22-14-19)23-9-11-26-12-10-23/h1-4,13-14,21H,5-12,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50311412

(CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CCCCc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C24H30N4O2S/c1-2-3-4-19-5-9-24(10-6-19)31(29,30)27-22-8-7-20-12-15-28(16-13-21(20)17-22)18-23-11-14-25-26-23/h5-11,14,17,27H,2-4,12-13,15-16,18H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50311419

(CHEMBL1078464 | N-(4-fluorophenyl)-5-((2,3,4,5-tet...)Show SMILES Fc1ccc(Nc2ccc(cn2)S(=O)(=O)Cc2ccc3CCNCCc3c2)cc1 Show InChI InChI=1S/C22H22FN3O2S/c23-19-3-5-20(6-4-19)26-22-8-7-21(14-25-22)29(27,28)15-16-1-2-17-9-11-24-12-10-18(17)13-16/h1-8,13-14,24H,9-12,15H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50311420

(7-((6-(piperidin-1-yl)pyridin-3-ylsulfonyl)methyl)...)Show SMILES O=S(=O)(Cc1ccc2CCNCCc2c1)c1ccc(nc1)N1CCCCC1 Show InChI InChI=1S/C21H27N3O2S/c25-27(26,16-17-4-5-18-8-10-22-11-9-19(18)14-17)20-6-7-21(23-15-20)24-12-2-1-3-13-24/h4-7,14-15,22H,1-3,8-13,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50415661
(CHEMBL1078462)Show InChI InChI=1S/C20H26N2O3S/c1-15(2)13-25-20-6-5-19(12-22-20)26(23,24)14-16-3-4-17-7-9-21-10-8-18(17)11-16/h3-6,11-12,15,21H,7-10,13-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50311421

(7-((6-isopropoxypyridin-3-ylsulfonyl)methyl)-2,3,4...)Show InChI InChI=1S/C19H24N2O3S/c1-14(2)24-19-6-5-18(12-21-19)25(22,23)13-15-3-4-16-7-9-20-10-8-17(16)11-15/h3-6,11-12,14,20H,7-10,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50311422

(7-((6-(4-fluorophenoxy)pyridin-3-ylsulfonyl)methyl...)Show SMILES Fc1ccc(Oc2ccc(cn2)S(=O)(=O)Cc2ccc3CCNCCc3c2)cc1 Show InChI InChI=1S/C22H21FN2O3S/c23-19-3-5-20(6-4-19)28-22-8-7-21(14-25-22)29(26,27)15-16-1-2-17-9-11-24-12-10-18(17)13-16/h1-8,13-14,24H,9-12,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50415662
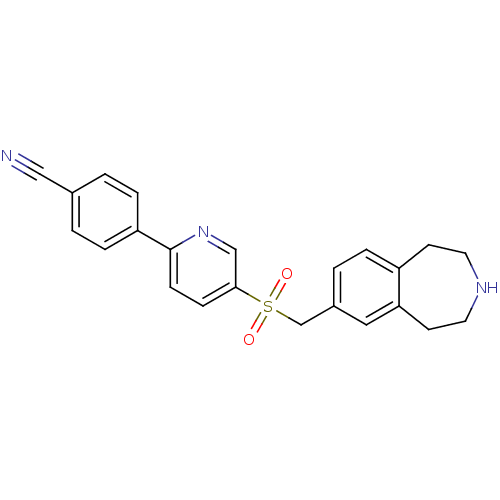
(CHEMBL1078269)Show SMILES O=S(=O)(Cc1ccc2CCNCCc2c1)c1ccc(nc1)-c1ccc(cc1)C#N Show InChI InChI=1S/C23H21N3O2S/c24-14-17-1-5-20(6-2-17)23-8-7-22(15-26-23)29(27,28)16-18-3-4-19-9-11-25-12-10-21(19)13-18/h1-8,13,15,25H,9-12,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50311423

(7-((6-(4-fluorophenyl)pyridin-3-ylsulfonyl)methyl)...)Show SMILES Fc1ccc(cc1)-c1ccc(cn1)S(=O)(=O)Cc1ccc2CCNCCc2c1 Show InChI InChI=1S/C22H21FN2O2S/c23-20-5-3-18(4-6-20)22-8-7-21(14-25-22)28(26,27)15-16-1-2-17-9-11-24-12-10-19(17)13-16/h1-8,13-14,24H,9-12,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50415663

(CHEMBL1078267)Show SMILES Clc1ccc(cc1)-c1ccc(cn1)S(=O)(=O)Cc1ccc2CCNCCc2c1 Show InChI InChI=1S/C22H21ClN2O2S/c23-20-5-3-18(4-6-20)22-8-7-21(14-25-22)28(26,27)15-16-1-2-17-9-11-24-12-10-19(17)13-16/h1-8,13-14,24H,9-12,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
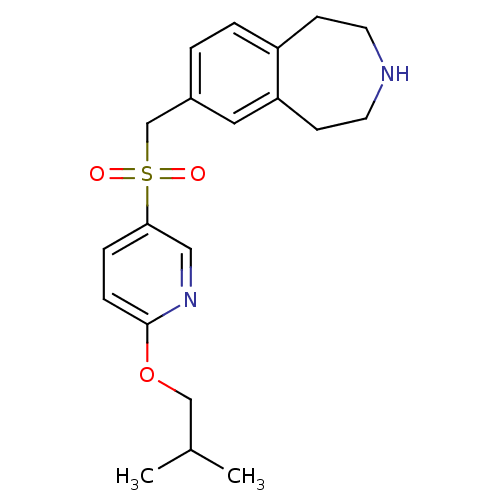
(Homo sapiens (Human)) | BDBM50311424

(7-((3',4'-difluorobiphenyl-4-ylsulfonyl)methyl)-2,...)Show SMILES Fc1ccc(cc1F)-c1ccc(cc1)S(=O)(=O)Cc1ccc2CCNCCc2c1 Show InChI InChI=1S/C23H21F2NO2S/c24-22-8-5-19(14-23(22)25)17-3-6-21(7-4-17)29(27,28)15-16-1-2-18-9-11-26-12-10-20(18)13-16/h1-8,13-14,26H,9-12,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50311425

(7-((4'-fluorobiphenyl-4-ylsulfonyl)methyl)-2,3,4,5...)Show SMILES Fc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)Cc1ccc2CCNCCc2c1 Show InChI InChI=1S/C23H22FNO2S/c24-22-7-3-18(4-8-22)19-5-9-23(10-6-19)28(26,27)16-17-1-2-20-11-13-25-14-12-21(20)15-17/h1-10,15,25H,11-14,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50415664
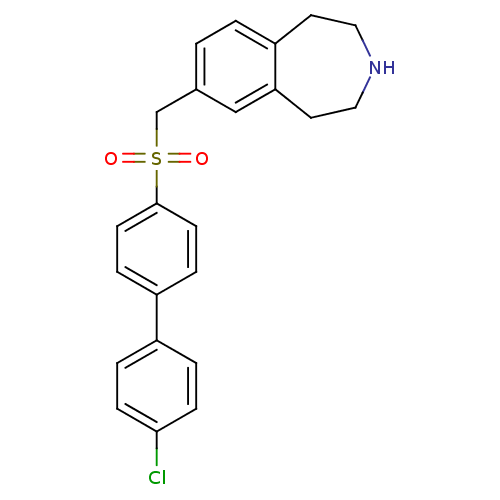
(CHEMBL1078344)Show SMILES Clc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)Cc1ccc2CCNCCc2c1 Show InChI InChI=1S/C23H22ClNO2S/c24-22-7-3-18(4-8-22)19-5-9-23(10-6-19)28(26,27)16-17-1-2-20-11-13-25-14-12-21(20)15-17/h1-10,15,25H,11-14,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50415665
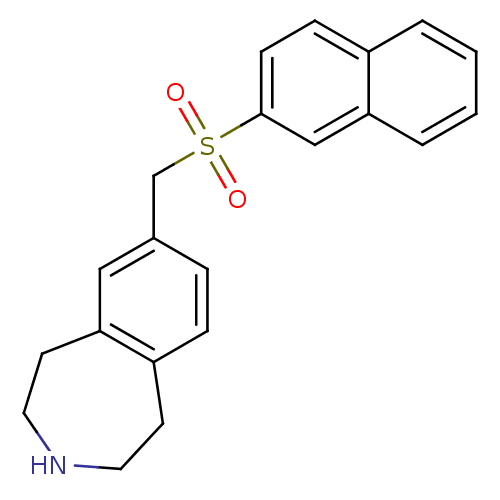
(CHEMBL1078343)Show InChI InChI=1S/C21H21NO2S/c23-25(24,21-8-7-17-3-1-2-4-19(17)14-21)15-16-5-6-18-9-11-22-12-10-20(18)13-16/h1-8,13-14,22H,9-12,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50415666
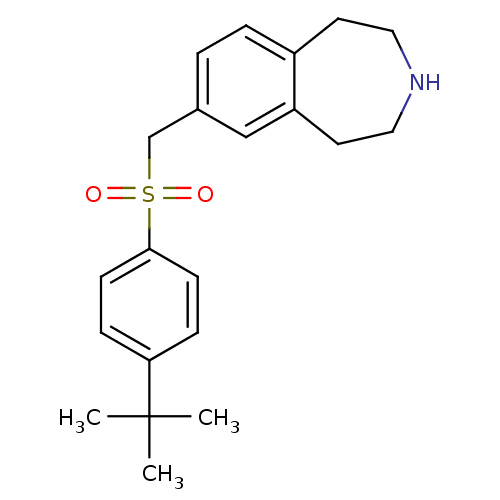
(CHEMBL1078342)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)Cc1ccc2CCNCCc2c1 Show InChI InChI=1S/C21H27NO2S/c1-21(2,3)19-6-8-20(9-7-19)25(23,24)15-16-4-5-17-10-12-22-13-11-18(17)14-16/h4-9,14,22H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50415667
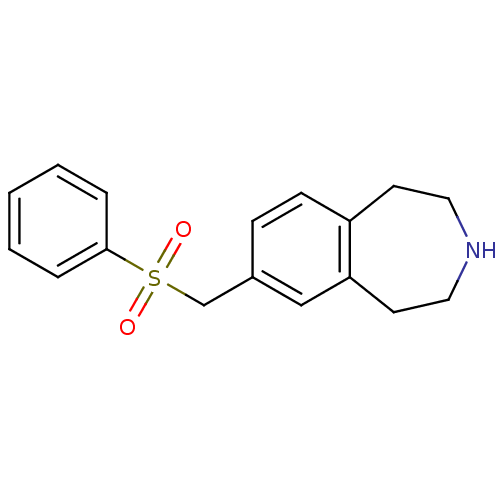
(CHEMBL1077578)Show InChI InChI=1S/C17H19NO2S/c19-21(20,17-4-2-1-3-5-17)13-14-6-7-15-8-10-18-11-9-16(15)12-14/h1-7,12,18H,8-11,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50311426

(4-(5-((2,3,4,5-tetrahydro-1H-benzo[d]azepin-7-yl)m...)Show SMILES O=S(=O)(Cc1ccc2CCNCCc2c1)c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C20H25N3O3S/c24-27(25,15-16-1-2-17-5-7-21-8-6-18(17)13-16)19-3-4-20(22-14-19)23-9-11-26-12-10-23/h1-4,13-14,21H,5-12,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50311413

(CHEMBL1078595 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(nc1)N1CCOCC1 Show InChI InChI=1S/C23H28N6O3S/c30-33(31,22-3-4-23(24-16-22)29-11-13-32-14-12-29)27-20-2-1-18-6-9-28(10-7-19(18)15-20)17-21-5-8-25-26-21/h1-5,8,15-16,27H,6-7,9-14,17H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50311414

(CHEMBL1078492 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(s1)-c1ccccn1 Show InChI InChI=1S/C23H23N5O2S2/c29-32(30,23-7-6-22(31-23)21-3-1-2-11-24-21)27-19-5-4-17-9-13-28(14-10-18(17)15-19)16-20-8-12-25-26-20/h1-8,11-12,15,27H,9-10,13-14,16H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50415668
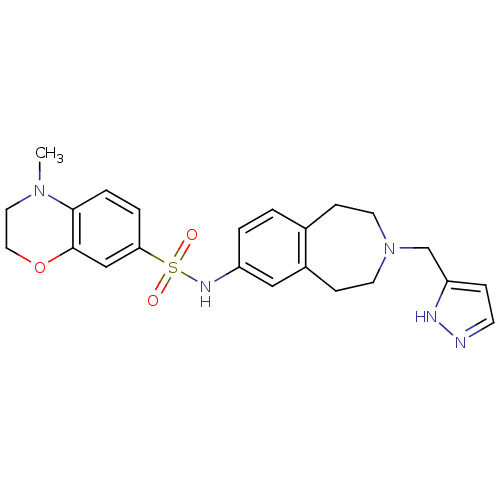
(CHEMBL1078491)Show SMILES CN1CCOc2cc(ccc12)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C23H27N5O3S/c1-27-12-13-31-23-15-21(4-5-22(23)27)32(29,30)26-19-3-2-17-7-10-28(11-8-18(17)14-19)16-20-6-9-24-25-20/h2-6,9,14-15,26H,7-8,10-13,16H2,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50311415

(CHEMBL1078490 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CC1(C)CCc2cc(ccc2O1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C25H30N4O3S/c1-25(2)11-7-20-16-23(5-6-24(20)32-25)33(30,31)28-21-4-3-18-9-13-29(14-10-19(18)15-21)17-22-8-12-26-27-22/h3-6,8,12,15-16,28H,7,9-11,13-14,17H2,1-2H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 631 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50311416

(CHEMBL1078208 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...)Show SMILES CC(C)Oc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C23H28N4O3S/c1-17(2)30-22-5-7-23(8-6-22)31(28,29)26-20-4-3-18-10-13-27(14-11-19(18)15-20)16-21-9-12-24-25-21/h3-9,12,15,17,26H,10-11,13-14,16H2,1-2H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50415669

(CHEMBL1078207)Show SMILES O=S(=O)(Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C26H26N4O2S/c31-33(32,26-10-7-21(8-11-26)20-4-2-1-3-5-20)29-24-9-6-22-13-16-30(17-14-23(22)18-24)19-25-12-15-27-28-25/h1-12,15,18,29H,13-14,16-17,19H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50415670
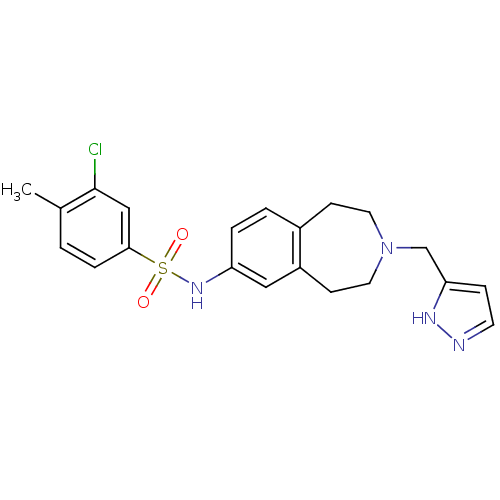
(CHEMBL1079311)Show SMILES Cc1ccc(cc1Cl)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C21H23ClN4O2S/c1-15-2-5-20(13-21(15)22)29(27,28)25-18-4-3-16-7-10-26(11-8-17(16)12-18)14-19-6-9-23-24-19/h2-6,9,12-13,25H,7-8,10-11,14H2,1H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50415671
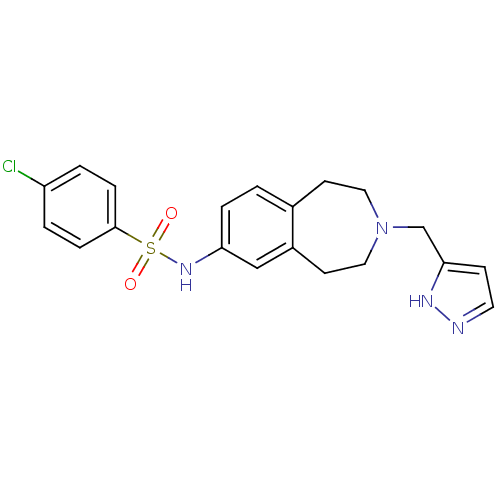
(CHEMBL1078104)Show SMILES Clc1ccc(cc1)S(=O)(=O)Nc1ccc2CCN(Cc3ccn[nH]3)CCc2c1 Show InChI InChI=1S/C20H21ClN4O2S/c21-17-2-5-20(6-3-17)28(26,27)24-18-4-1-15-8-11-25(12-9-16(15)13-18)14-19-7-10-22-23-19/h1-7,10,13,24H,8-9,11-12,14H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human gherlin receptor |
Bioorg Med Chem Lett 19: 6452-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.027
BindingDB Entry DOI: 10.7270/Q2DV1K0C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data