

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-galactosidase (Escherichia coli (Enterobacteria)) | BDBM50182795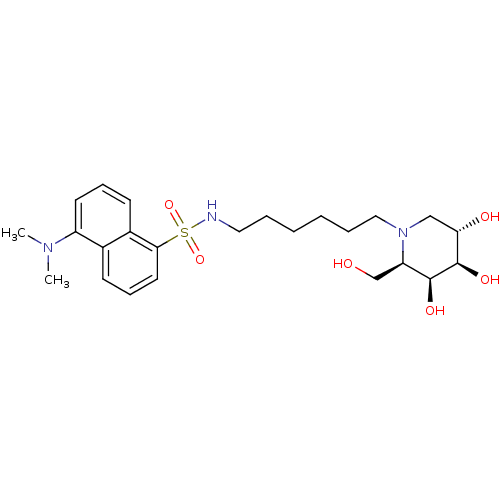 (5-(dimethylamino)-N-(6-((2R,3S,4R,5S)-3,4,5-trihyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-galactosidase assessed as inhibition of nitrophenolate release by spectrophotometry | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli (Enterobacteria)) | BDBM50356096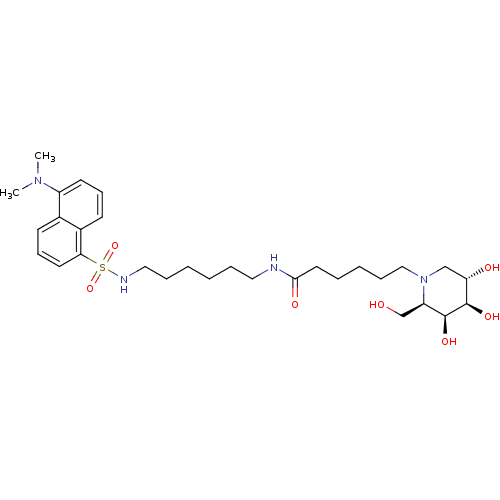 (CHEMBL1911831) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-galactosidase assessed as inhibition of nitrophenolate release by spectrophotometry | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50182795 (5-(dimethylamino)-N-(6-((2R,3S,4R,5S)-3,4,5-trihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-galactosidase assessed as inhibition of hydrolyzed 4-methylumbelliferone production after 30 mins by luminescence ... | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50246569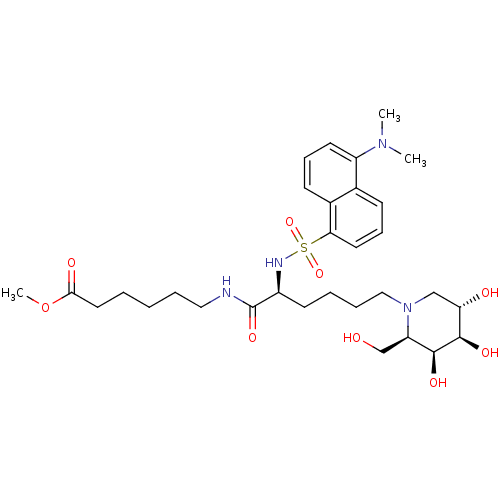 (CHEMBL505422 | Methyl 6-[N2-dansyl-N6-(1,5-dideoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-galactosidase assessed as inhibition of hydrolyzed 4-methylumbelliferone production after 30 mins by luminescence ... | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50356096 (CHEMBL1911831) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-galactosidase assessed as inhibition of hydrolyzed 4-methylumbelliferone production after 30 mins by luminescence ... | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50356097 (CHEMBL461161) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-galactosidase assessed as inhibition of hydrolyzed 4-methylumbelliferone production after 30 mins by luminescence ... | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50182795 (5-(dimethylamino)-N-(6-((2R,3S,4R,5S)-3,4,5-trihyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of green coffee bean alpha-galactosidase assessed as inhibition of nitrophenolate release by spectrophotometry | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50356096 (CHEMBL1911831) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of green coffee bean alpha-galactosidase assessed as inhibition of nitrophenolate release by spectrophotometry | Bioorg Med Chem Lett 21: 6872-5 (2011) Article DOI: 10.1016/j.bmcl.2011.09.012 BindingDB Entry DOI: 10.7270/Q24B31RQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50246569 (CHEMBL505422 | Methyl 6-[N2-dansyl-N6-(1,5-dideoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta galactosidase | Bioorg Med Chem 16: 10216-20 (2008) Article DOI: 10.1016/j.bmc.2008.10.054 BindingDB Entry DOI: 10.7270/Q2DZ085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||