 Found 334 hits with Last Name = 'feng' and Initial = 'q'
Found 334 hits with Last Name = 'feng' and Initial = 'q' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569138
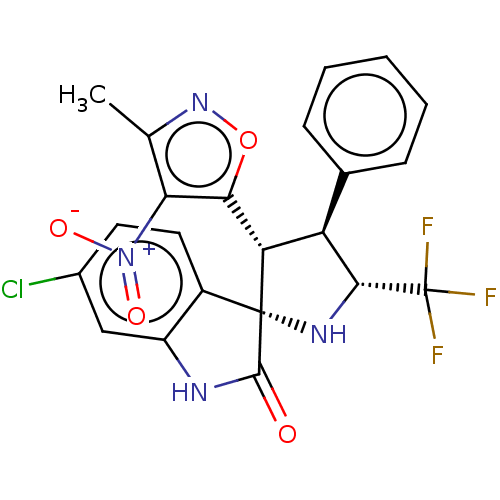
(CHEMBL4871070)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3cc(Cl)ccc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569140

(CHEMBL4863792)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3cc(Br)ccc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM31197
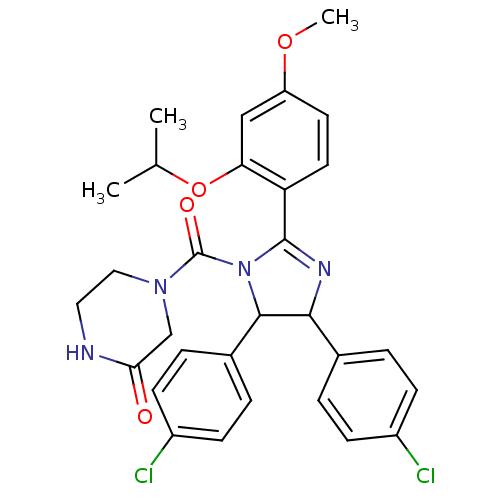
(CHEMBL211045 | Nutlin-3 | med.21724, Compound 186)Show SMILES COc1ccc(C2=NC(C(N2C(=O)N2CCNC(=O)C2)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(OC(C)C)c1 |t:6| Show InChI InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534504
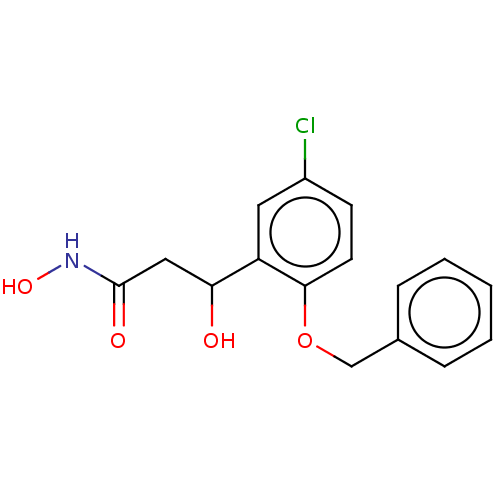
(CHEMBL4590676)Show InChI InChI=1S/C16H16ClNO4/c17-12-6-7-15(22-10-11-4-2-1-3-5-11)13(8-12)14(19)9-16(20)18-21/h1-8,14,19,21H,9-10H2,(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori urease using urea as substrate assessed as inhibition constant for enzyme-urea-inhibitor complex by non-linear fitt... |
Bioorg Med Chem 24: 4519-4527 (2016)
Article DOI: 10.1016/j.bmc.2016.07.052
BindingDB Entry DOI: 10.7270/Q24F1V76 |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534504

(CHEMBL4590676)Show InChI InChI=1S/C16H16ClNO4/c17-12-6-7-15(22-10-11-4-2-1-3-5-11)13(8-12)14(19)9-16(20)18-21/h1-8,14,19,21H,9-10H2,(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 404 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori urease using urea as substrate assessed as inhibition constant for enzyme-inhibitor complex by non-linear fitting a... |
Bioorg Med Chem 24: 4519-4527 (2016)
Article DOI: 10.1016/j.bmc.2016.07.052
BindingDB Entry DOI: 10.7270/Q24F1V76 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569136

(CHEMBL4874111)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccc(F)cc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569137

(CHEMBL4860936)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccc(Cl)cc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358256

(CHEMBL168946)Show SMILES COc1ccc(cc1)C(=O)Nc1ccccc1NC(=O)c1ccc(OC)cc1 Show InChI InChI=1S/C22H20N2O4/c1-27-17-11-7-15(8-12-17)21(25)23-19-5-3-4-6-20(19)24-22(26)16-9-13-18(28-2)14-10-16/h3-14H,1-2H3,(H,23,25)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114035
BindingDB Entry DOI: 10.7270/Q2Q52TMM |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569139
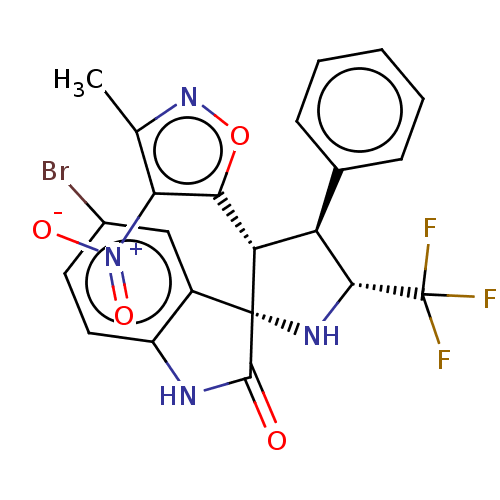
(CHEMBL4863780)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccc(Br)cc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569144

(CHEMBL4861981)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccc(F)cc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569143

(CHEMBL4876458)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2cccc(F)c2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569158

(CHEMBL4856044)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccco2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493364

(CHEMBL2425480)Show InChI InChI=1S/C9H10ClNO4/c10-5-1-2-7(12)6(3-5)8(13)4-9(14)11-15/h1-3,8,12-13,15H,4H2,(H,11,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori urease using urea as substrate assessed as inhibition constant for enzyme-urea-inhibitor complex by non-linear fitt... |
Bioorg Med Chem 24: 4519-4527 (2016)
Article DOI: 10.1016/j.bmc.2016.07.052
BindingDB Entry DOI: 10.7270/Q24F1V76 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569135
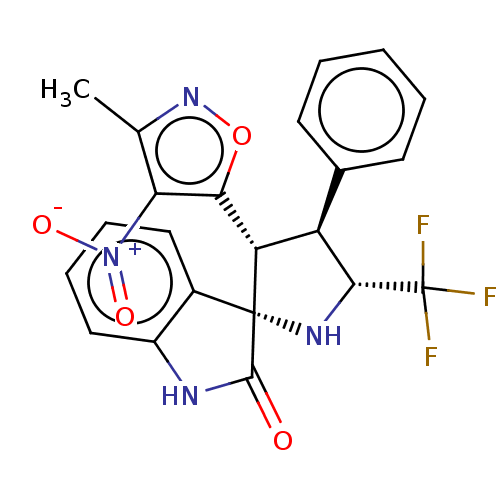
(CHEMBL4874952)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569159
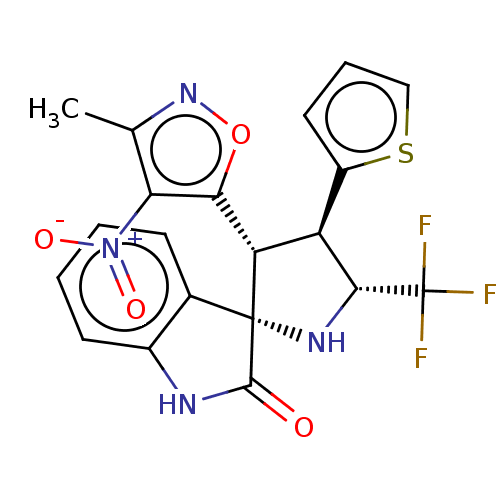
(CHEMBL4870214)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2cccs2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569142
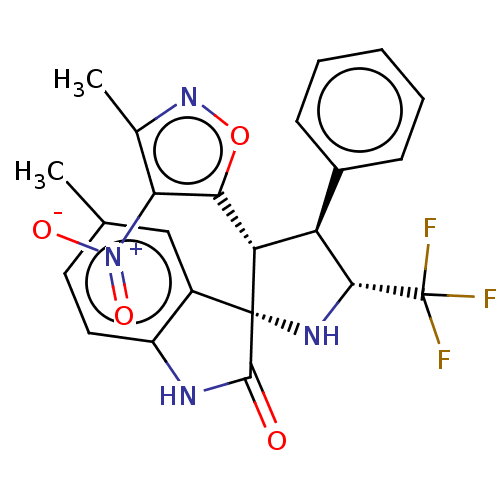
(CHEMBL4877534)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccc(C)cc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569152
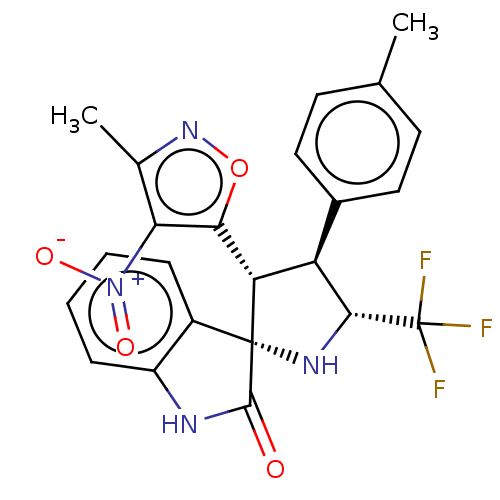
(CHEMBL4859985)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccc(C)cc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569149
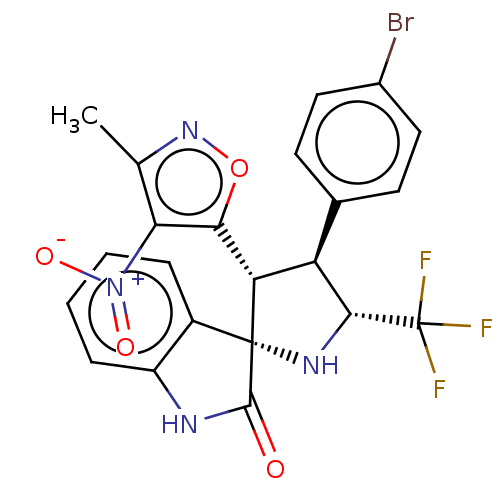
(CHEMBL4877092)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccc(Br)cc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569154
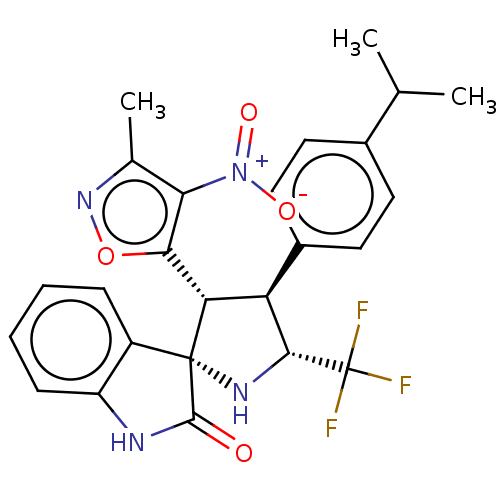
(CHEMBL4860559)Show SMILES CC(C)c1ccc(cc1)[C@H]1[C@@H](N[C@]2([C@@H]1c1onc(C)c1[N+]([O-])=O)C(=O)Nc1ccccc21)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569156

(CHEMBL4878727)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccc(Cl)c(Cl)c2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569146
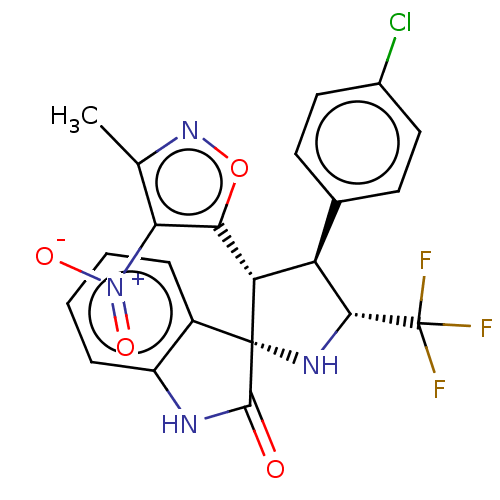
(CHEMBL4870252)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccc(Cl)cc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569148
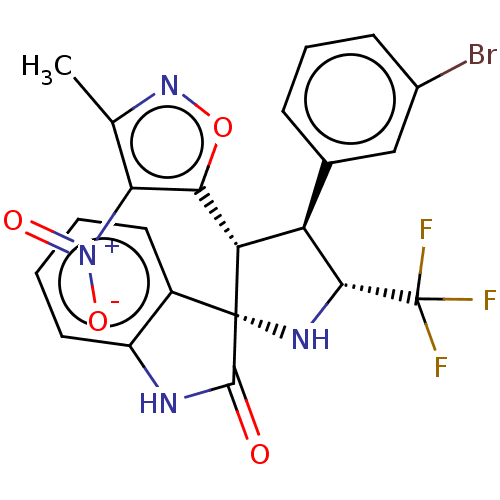
(CHEMBL4848056)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2cccc(Br)c2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569141

(CHEMBL4878828)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccc(cc23)[N+]([O-])=O)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569157
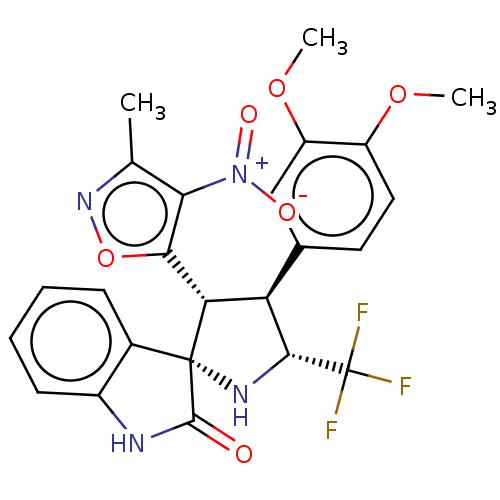
(CHEMBL4855468)Show SMILES COc1ccc(cc1OC)[C@H]1[C@@H](N[C@]2([C@@H]1c1onc(C)c1[N+]([O-])=O)C(=O)Nc1ccccc21)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493364

(CHEMBL2425480)Show InChI InChI=1S/C9H10ClNO4/c10-5-1-2-7(12)6(3-5)8(13)4-9(14)11-15/h1-3,8,12-13,15H,4H2,(H,11,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori urease using urea as substrate assessed as inhibition constant for enzyme-inhibitor complex by non-linear fitting a... |
Bioorg Med Chem 24: 4519-4527 (2016)
Article DOI: 10.1016/j.bmc.2016.07.052
BindingDB Entry DOI: 10.7270/Q24F1V76 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569164
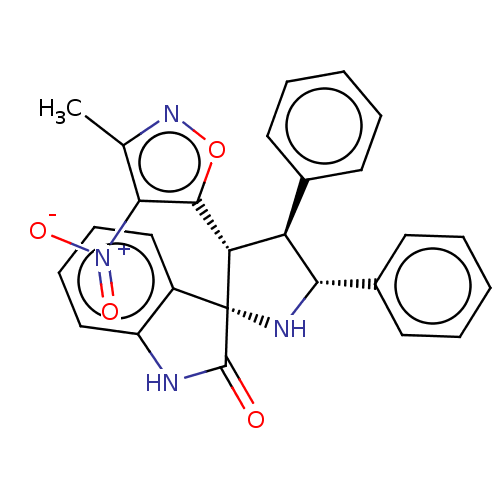
(CHEMBL4874048)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)c2ccccc2)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569150

(CHEMBL4859002)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccc(cc2)[N+]([O-])=O)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569160
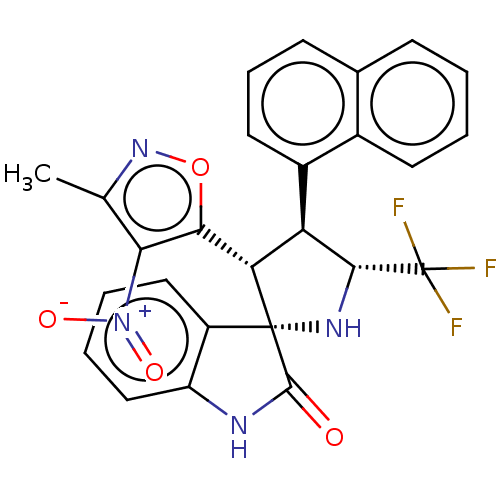
(CHEMBL4848654)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2cccc3ccccc23)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569163

(CHEMBL4874032)Show SMILES CC(=O)N1C(=O)[C@@]2(N[C@H]([C@@H]([C@H]2c2onc(C)c2[N+]([O-])=O)c2ccccc2)C(F)(F)F)c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569162
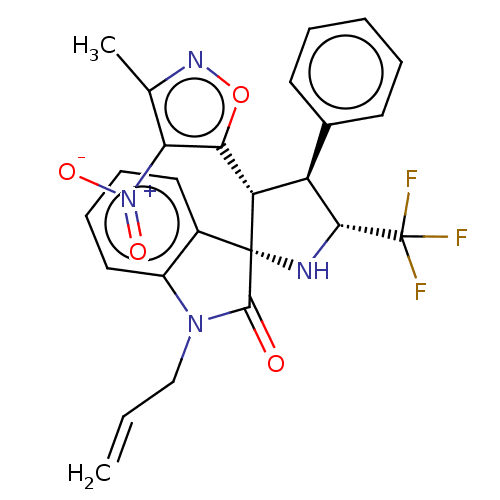
(CHEMBL4849387)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)N(CC=C)c3ccccc23)C(F)(F)F)c2ccccc2)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569161

(CHEMBL4849608)Show SMILES CN1C(=O)[C@@]2(N[C@H]([C@@H]([C@H]2c2onc(C)c2[N+]([O-])=O)c2ccccc2)C(F)(F)F)c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569145

(CHEMBL4864855)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccccc2Cl)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569155
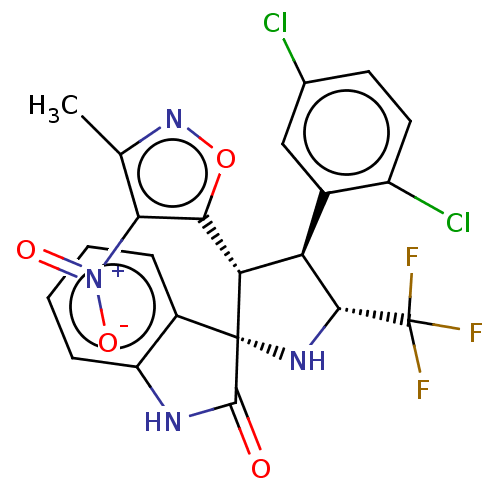
(CHEMBL4869937)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2cc(Cl)ccc2Cl)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569147

(CHEMBL4860063)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccccc2Br)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569151
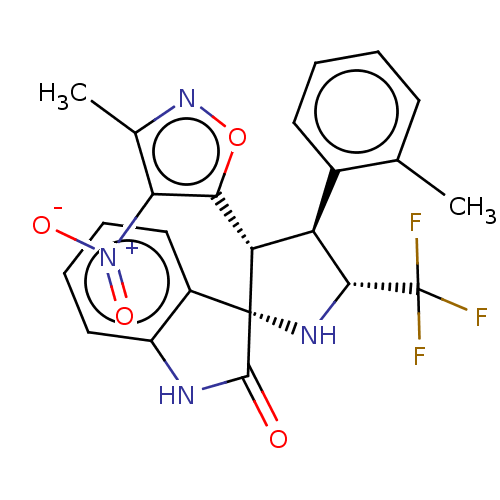
(CHEMBL4873310)Show SMILES Cc1noc([C@@H]2[C@H]([C@@H](N[C@@]22C(=O)Nc3ccccc23)C(F)(F)F)c2ccccc2C)c1[N+]([O-])=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50569153

(CHEMBL4852407)Show SMILES COc1ccccc1[C@H]1[C@@H](N[C@]2([C@@H]1c1onc(C)c1[N+]([O-])=O)C(=O)Nc1ccccc21)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MDM2 (unknown origin) using peptide as substrate incubated for 30 mins by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113359
BindingDB Entry DOI: 10.7270/Q20005VW |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM24961
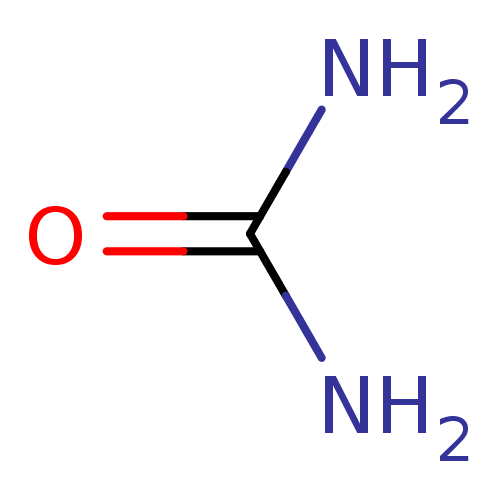
(Urea) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 6.47E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Substrate inhibition of Helicobacter pylori urease in presence of >4 mM urea by indophenol method |
Bioorg Med Chem 24: 4519-4527 (2016)
Article DOI: 10.1016/j.bmc.2016.07.052
BindingDB Entry DOI: 10.7270/Q24F1V76 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437403

(CHEMBL2408771)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C26H32N6O3/c1-17(2)11-13-31-22-23(28-25(31)30-14-19-10-7-12-27-20(19)15-30)29(3)26(35)32(24(22)34)16-21(33)18-8-5-4-6-9-18/h4-6,8-9,11,19-20,27H,7,10,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427749
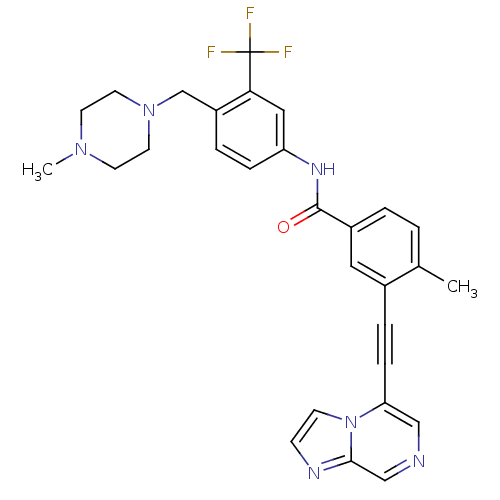
(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437403

(CHEMBL2408771)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C26H32N6O3/c1-17(2)11-13-31-22-23(28-25(31)30-14-19-10-7-12-27-20(19)15-30)29(3)26(35)32(24(22)34)16-21(33)18-8-5-4-6-9-18/h4-6,8-9,11,19-20,27H,7,10,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM172038
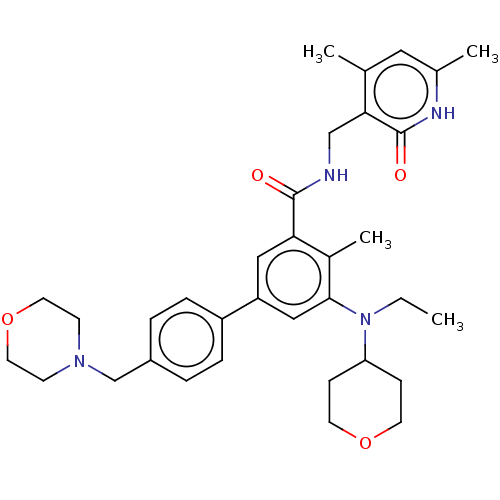
(US10155002, Compound 44 | US10647700, Compound EPZ...)Show SMILES CCN(C1CCOCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C34H44N4O4/c1-5-38(29-10-14-41-15-11-29)32-20-28(27-8-6-26(7-9-27)22-37-12-16-42-17-13-37)19-30(25(32)4)33(39)35-21-31-23(2)18-24(3)36-34(31)40/h6-9,18-20,29H,5,10-17,21-22H2,1-4H3,(H,35,39)(H,36,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) by AlphaLISA assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126957
BindingDB Entry DOI: 10.7270/Q28P644Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427748
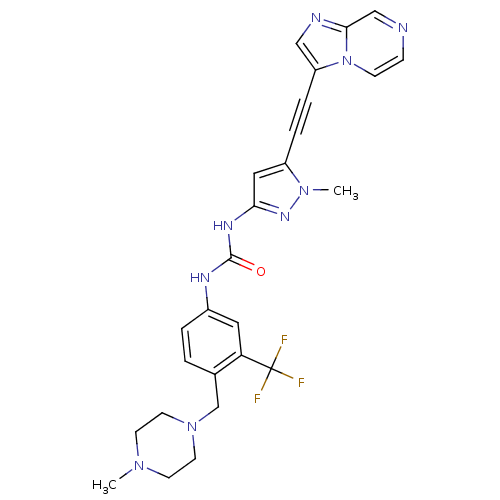
(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM50520955

(CHEMBL4445123)Show SMILES C[C@@H](CN)c1ccc(cc1)-c1c(O)cc(C)c2[nH]c(=O)c3ccccc3c12 |r| Show InChI InChI=1S/C23H22N2O2/c1-13-11-19(26)20(16-9-7-15(8-10-16)14(2)12-24)21-17-5-3-4-6-18(17)23(27)25-22(13)21/h3-11,14,26H,12,24H2,1-2H3,(H,25,27)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human TOPK using MBP as substrate after 2 hrs in presence of [gamma-33P]-ATP by filter-binding method |
Eur J Med Chem 162: 407-422 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.007
BindingDB Entry DOI: 10.7270/Q28K7DGV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50594756

(CHEMBL5201486)Show SMILES CN(C(C)=N)c1ccc(cc1)C(=O)Nc1ccc(C)cc1C(=O)Nc1ccc(Cl)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114035
BindingDB Entry DOI: 10.7270/Q2Q52TMM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427749

(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type Abl1 kinase (unknown origin) |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50228407

(8-((R)-3-amino-piperidin-1-yl)-3-methyl-7-(3-methy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-1 Show InChI InChI=1S/C24H30N6O3/c1-16(2)11-13-29-20-21(26-23(29)28-12-7-10-18(25)14-28)27(3)24(33)30(22(20)32)15-19(31)17-8-5-4-6-9-17/h4-6,8-9,11,18H,7,10,12-15,25H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228407

(8-((R)-3-amino-piperidin-1-yl)-3-methyl-7-(3-methy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-1 Show InChI InChI=1S/C24H30N6O3/c1-16(2)11-13-29-20-21(26-23(29)28-12-7-10-18(25)14-28)27(3)24(33)30(22(20)32)15-19(31)17-8-5-4-6-9-17/h4-6,8-9,11,18H,7,10,12-15,25H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437404
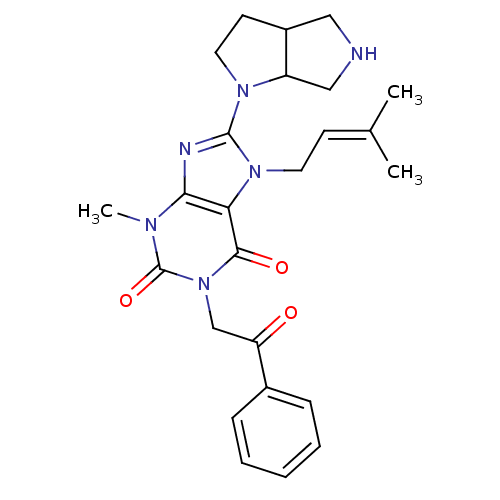
(CHEMBL2408655)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C25H30N6O3/c1-16(2)9-11-30-21-22(27-24(30)29-12-10-18-13-26-14-19(18)29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-9,18-19,26H,10-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427749

(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50427748

(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type Abl1 kinase (unknown origin) |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data