 Found 104 hits with Last Name = 'ferla' and Initial = 'sw'
Found 104 hits with Last Name = 'ferla' and Initial = 'sw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171117

(2-(4-(N-(3-bromo-4-(difluoro(phosphono)methyl)benz...)Show SMILES CN(C)S(=O)(=O)c1ccc(CN(Cc2ccc(c(Br)c2)C(F)(F)P(O)(O)=O)S(=O)(=O)c2ccc(OCC(O)=O)cc2)cc1 Show InChI InChI=1S/C25H26BrF2N2O10PS2/c1-29(2)42(36,37)20-8-3-17(4-9-20)14-30(43(38,39)21-10-6-19(7-11-21)40-16-24(31)32)15-18-5-12-22(23(26)13-18)25(27,28)41(33,34)35/h3-13H,14-16H2,1-2H3,(H,31,32)(H2,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171106
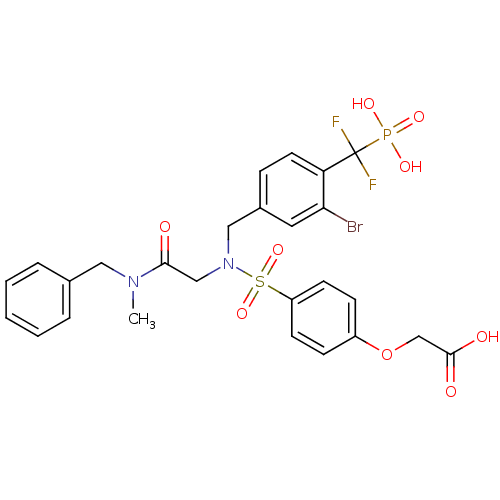
((4-{[(Benzyl-methyl-carbamoyl)-methyl]-[3-bromo-4-...)Show SMILES CN(Cc1ccccc1)C(=O)CN(Cc1ccc(c(Br)c1)C(F)(F)P(O)(O)=O)S(=O)(=O)c1ccc(OCC(O)=O)cc1 Show InChI InChI=1S/C26H26BrF2N2O9PS/c1-30(14-18-5-3-2-4-6-18)24(32)16-31(42(38,39)21-10-8-20(9-11-21)40-17-25(33)34)15-19-7-12-22(23(27)13-19)26(28,29)41(35,36)37/h2-13H,14-17H2,1H3,(H,33,34)(H2,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171096
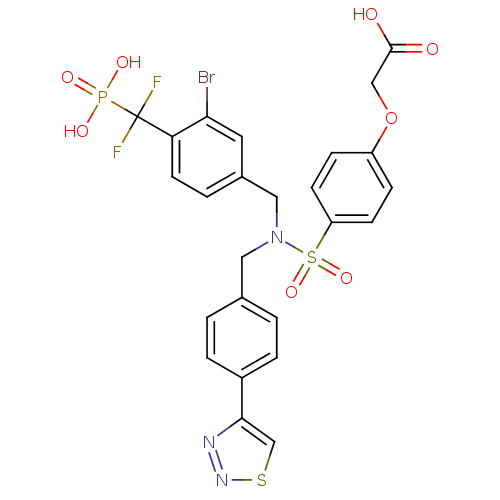
(CHEMBL371929 | {4-[[3-Bromo-4-(difluoro-phosphono-...)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(c(Br)c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C25H21BrF2N3O8PS2/c26-22-11-17(3-10-21(22)25(27,28)40(34,35)36)13-31(12-16-1-4-18(5-2-16)23-15-41-30-29-23)42(37,38)20-8-6-19(7-9-20)39-14-24(32)33/h1-11,15H,12-14H2,(H,32,33)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171100
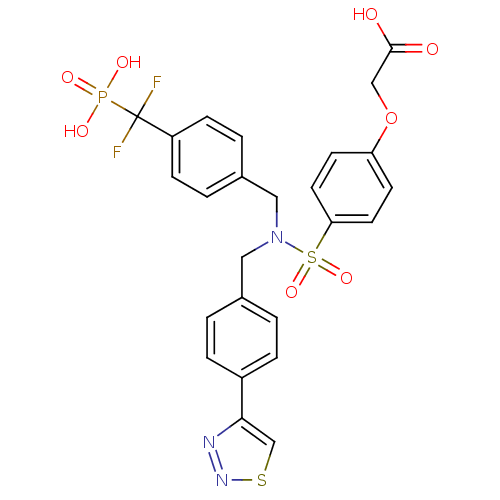
(CHEMBL198882 | {4-[[4-(Difluoro-phosphono-methyl)-...)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C25H22F2N3O8PS2/c26-25(27,39(33,34)35)20-7-3-18(4-8-20)14-30(13-17-1-5-19(6-2-17)23-16-40-29-28-23)41(36,37)22-11-9-21(10-12-22)38-15-24(31)32/h1-12,16H,13-15H2,(H,31,32)(H2,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171099
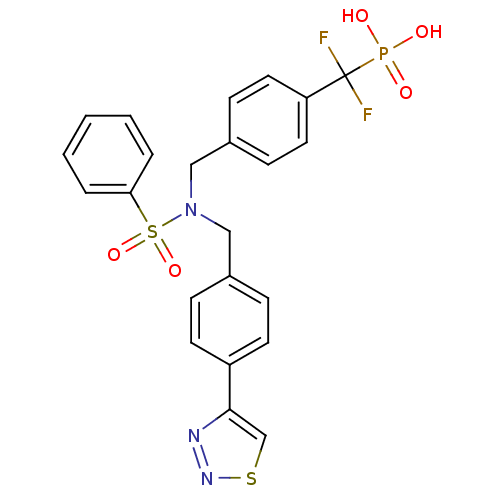
((4-((N-(4-(1,2,3-thiadiazol-4-yl)benzyl)phenylsulf...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CN(Cc2ccc(cc2)-c2csnn2)S(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C23H20F2N3O5PS2/c24-23(25,34(29,30)31)20-12-8-18(9-13-20)15-28(36(32,33)21-4-2-1-3-5-21)14-17-6-10-19(11-7-17)22-16-35-27-26-22/h1-13,16H,14-15H2,(H2,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171118
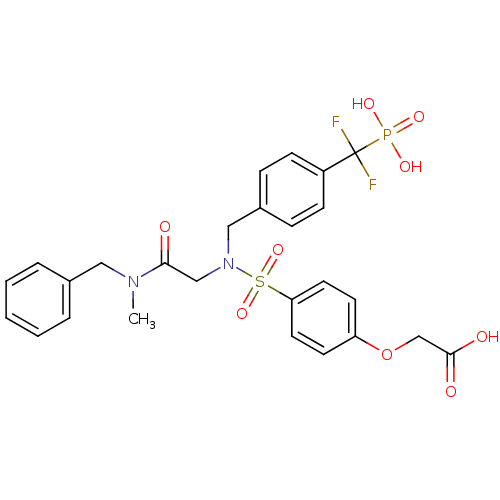
((4-{[(Benzyl-methyl-carbamoyl)-methyl]-[4-(difluor...)Show SMILES CN(Cc1ccccc1)C(=O)CN(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)S(=O)(=O)c1ccc(OCC(O)=O)cc1 Show InChI InChI=1S/C26H27F2N2O9PS/c1-29(15-19-5-3-2-4-6-19)24(31)17-30(16-20-7-9-21(10-8-20)26(27,28)40(34,35)36)41(37,38)23-13-11-22(12-14-23)39-18-25(32)33/h2-14H,15-18H2,1H3,(H,32,33)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171112
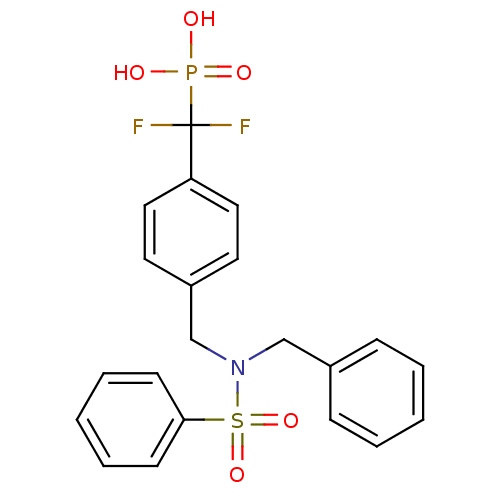
(({4-[(Benzenesulfonyl-benzyl-amino)-methyl]-phenyl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CN(Cc2ccccc2)S(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C21H20F2NO5PS/c22-21(23,30(25,26)27)19-13-11-18(12-14-19)16-24(15-17-7-3-1-4-8-17)31(28,29)20-9-5-2-6-10-20/h1-14H,15-16H2,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171117

(2-(4-(N-(3-bromo-4-(difluoro(phosphono)methyl)benz...)Show SMILES CN(C)S(=O)(=O)c1ccc(CN(Cc2ccc(c(Br)c2)C(F)(F)P(O)(O)=O)S(=O)(=O)c2ccc(OCC(O)=O)cc2)cc1 Show InChI InChI=1S/C25H26BrF2N2O10PS2/c1-29(2)42(36,37)20-8-3-17(4-9-20)14-30(43(38,39)21-10-6-19(7-11-21)40-16-24(31)32)15-18-5-12-22(23(26)13-18)25(27,28)41(33,34)35/h3-13H,14-16H2,1-2H3,(H,31,32)(H2,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171106

((4-{[(Benzyl-methyl-carbamoyl)-methyl]-[3-bromo-4-...)Show SMILES CN(Cc1ccccc1)C(=O)CN(Cc1ccc(c(Br)c1)C(F)(F)P(O)(O)=O)S(=O)(=O)c1ccc(OCC(O)=O)cc1 Show InChI InChI=1S/C26H26BrF2N2O9PS/c1-30(14-18-5-3-2-4-6-18)24(32)16-31(42(38,39)21-10-8-20(9-11-21)40-17-25(33)34)15-19-7-12-22(23(27)13-19)26(28,29)41(35,36)37/h2-13H,14-17H2,1H3,(H,33,34)(H2,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171096

(CHEMBL371929 | {4-[[3-Bromo-4-(difluoro-phosphono-...)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(c(Br)c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C25H21BrF2N3O8PS2/c26-22-11-17(3-10-21(22)25(27,28)40(34,35)36)13-31(12-16-1-4-18(5-2-16)23-15-41-30-29-23)42(37,38)20-8-6-19(7-9-20)39-14-24(32)33/h1-11,15H,12-14H2,(H,32,33)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171091
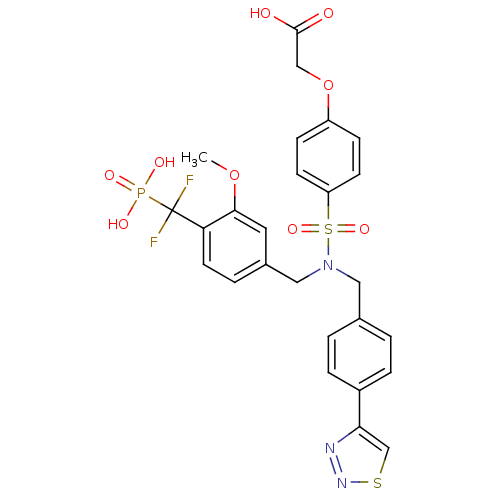
(CHEMBL198939 | {4-[[4-(Difluoro-phosphono-methyl)-...)Show SMILES COc1cc(CN(Cc2ccc(cc2)-c2csnn2)S(=O)(=O)c2ccc(OCC(O)=O)cc2)ccc1C(F)(F)P(O)(O)=O Show InChI InChI=1S/C26H24F2N3O9PS2/c1-39-24-12-18(4-11-22(24)26(27,28)41(34,35)36)14-31(13-17-2-5-19(6-3-17)23-16-42-30-29-23)43(37,38)21-9-7-20(8-10-21)40-15-25(32)33/h2-12,16H,13-15H2,1H3,(H,32,33)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171100

(CHEMBL198882 | {4-[[4-(Difluoro-phosphono-methyl)-...)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C25H22F2N3O8PS2/c26-25(27,39(33,34)35)20-7-3-18(4-8-20)14-30(13-17-1-5-19(6-2-17)23-16-40-29-28-23)41(36,37)22-11-9-21(10-12-22)38-15-24(31)32/h1-12,16H,13-15H2,(H,31,32)(H2,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 18: 2719-24 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.007
BindingDB Entry DOI: 10.7270/Q23J3DTX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171100

(CHEMBL198882 | {4-[[4-(Difluoro-phosphono-methyl)-...)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C25H22F2N3O8PS2/c26-25(27,39(33,34)35)20-7-3-18(4-8-20)14-30(13-17-1-5-19(6-2-17)23-16-40-29-28-23)41(36,37)22-11-9-21(10-12-22)38-15-24(31)32/h1-12,16H,13-15H2,(H,31,32)(H2,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171109

(CHEMBL196268 | {4-[[4-(Difluoro-phosphono-methyl)-...)Show SMILES CS(=O)(=O)Nc1ccc(CN(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)S(=O)(=O)c2ccc(OCC(O)=O)cc2)cc1 Show InChI InChI=1S/C24H25F2N2O10PS2/c1-40(34,35)27-20-8-4-18(5-9-20)15-28(14-17-2-6-19(7-3-17)24(25,26)39(31,32)33)41(36,37)22-12-10-21(11-13-22)38-16-23(29)30/h2-13,27H,14-16H2,1H3,(H,29,30)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171099

((4-((N-(4-(1,2,3-thiadiazol-4-yl)benzyl)phenylsulf...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CN(Cc2ccc(cc2)-c2csnn2)S(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C23H20F2N3O5PS2/c24-23(25,34(29,30)31)20-12-8-18(9-13-20)15-28(36(32,33)21-4-2-1-3-5-21)14-17-6-10-19(11-7-17)22-16-35-27-26-22/h1-13,16H,14-15H2,(H2,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 18: 2719-24 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.007
BindingDB Entry DOI: 10.7270/Q23J3DTX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171099

((4-((N-(4-(1,2,3-thiadiazol-4-yl)benzyl)phenylsulf...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CN(Cc2ccc(cc2)-c2csnn2)S(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C23H20F2N3O5PS2/c24-23(25,34(29,30)31)20-12-8-18(9-13-20)15-28(36(32,33)21-4-2-1-3-5-21)14-17-6-10-19(11-7-17)22-16-35-27-26-22/h1-13,16H,14-15H2,(H2,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171097

(CHEMBL197681 | {4-[[4-(Difluoro-phosphono-methyl)-...)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)c1ccc(cc1)-c1csnn1 Show InChI InChI=1S/C24H20F2N3O8PS2/c25-24(26,38(32,33)34)18-5-1-16(2-6-18)13-29(19-7-3-17(4-8-19)22-15-39-28-27-22)40(35,36)21-11-9-20(10-12-21)37-14-23(30)31/h1-12,15H,13-14H2,(H,30,31)(H2,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171101
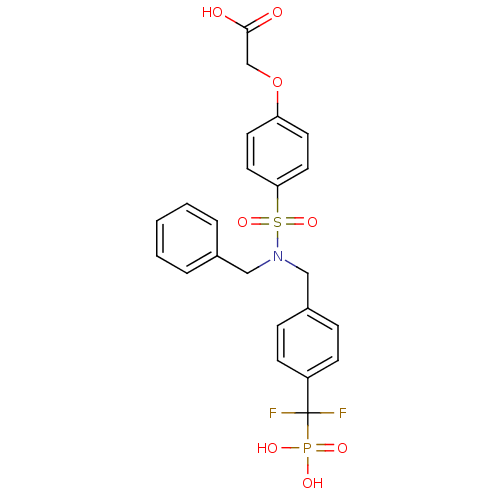
((4-{Benzyl-[4-(difluoro-phosphono-methyl)-benzyl]-...)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C23H22F2NO8PS/c24-23(25,35(29,30)31)19-8-6-18(7-9-19)15-26(14-17-4-2-1-3-5-17)36(32,33)21-12-10-20(11-13-21)34-16-22(27)28/h1-13H,14-16H2,(H,27,28)(H2,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171118

((4-{[(Benzyl-methyl-carbamoyl)-methyl]-[4-(difluor...)Show SMILES CN(Cc1ccccc1)C(=O)CN(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)S(=O)(=O)c1ccc(OCC(O)=O)cc1 Show InChI InChI=1S/C26H27F2N2O9PS/c1-29(15-19-5-3-2-4-6-19)24(31)17-30(16-20-7-9-21(10-8-20)26(27,28)40(34,35)36)41(37,38)23-13-11-22(12-14-23)39-18-25(32)33/h2-14H,15-18H2,1H3,(H,32,33)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171098

((4-{(4-Benzoylamino-benzyl)-[4-(difluoro-phosphono...)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(NC(=O)c2ccccc2)cc1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C30H27F2N2O9PS/c31-30(32,44(38,39)40)24-10-6-21(7-11-24)18-34(45(41,42)27-16-14-26(15-17-27)43-20-28(35)36)19-22-8-12-25(13-9-22)33-29(37)23-4-2-1-3-5-23/h1-17H,18-20H2,(H,33,37)(H,35,36)(H2,38,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171103
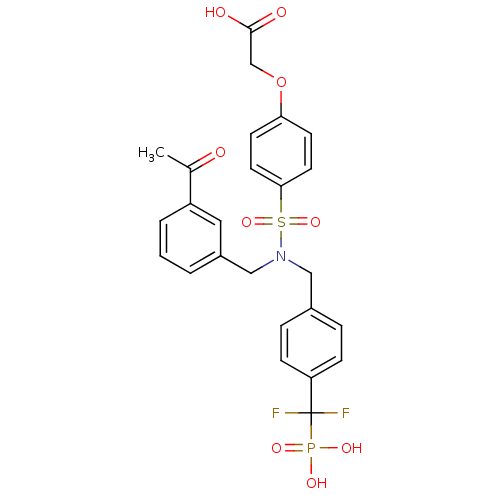
((4-{(3-Acetyl-benzyl)-[4-(difluoro-phosphono-methy...)Show SMILES CC(=O)c1cccc(CN(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)S(=O)(=O)c2ccc(OCC(O)=O)cc2)c1 Show InChI InChI=1S/C25H24F2NO9PS/c1-17(29)20-4-2-3-19(13-20)15-28(14-18-5-7-21(8-6-18)25(26,27)38(32,33)34)39(35,36)23-11-9-22(10-12-23)37-16-24(30)31/h2-13H,14-16H2,1H3,(H,30,31)(H2,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171105
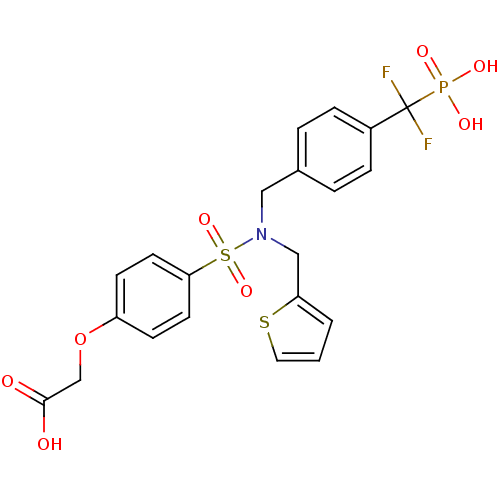
((4-{[4-(Difluoro-phosphono-methyl)-benzyl]-thiophe...)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1cccs1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C21H20F2NO8PS2/c22-21(23,33(27,28)29)16-5-3-15(4-6-16)12-24(13-18-2-1-11-34-18)35(30,31)19-9-7-17(8-10-19)32-14-20(25)26/h1-11H,12-14H2,(H,25,26)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171094
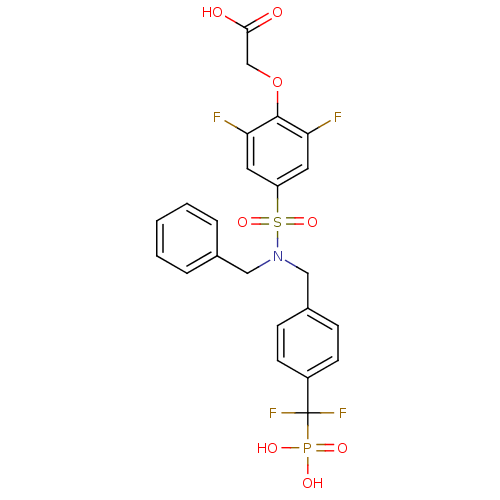
((4-{Benzyl-[4-(difluoro-phosphono-methyl)-benzyl]-...)Show SMILES OC(=O)COc1c(F)cc(cc1F)S(=O)(=O)N(Cc1ccccc1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C23H20F4NO8PS/c24-19-10-18(11-20(25)22(19)36-14-21(29)30)38(34,35)28(12-15-4-2-1-3-5-15)13-16-6-8-17(9-7-16)23(26,27)37(31,32)33/h1-11H,12-14H2,(H,29,30)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171124
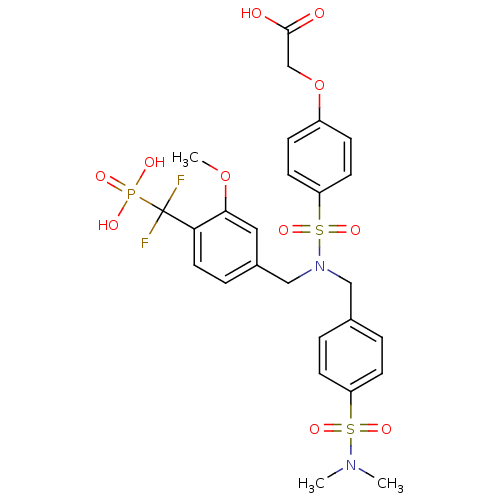
(CHEMBL383163 | {4-[[4-(Difluoro-phosphono-methyl)-...)Show SMILES COc1cc(CN(Cc2ccc(cc2)S(=O)(=O)N(C)C)S(=O)(=O)c2ccc(OCC(O)=O)cc2)ccc1C(F)(F)P(O)(O)=O Show InChI InChI=1S/C26H29F2N2O11PS2/c1-29(2)43(36,37)21-9-4-18(5-10-21)15-30(44(38,39)22-11-7-20(8-12-22)41-17-25(31)32)16-19-6-13-23(24(14-19)40-3)26(27,28)42(33,34)35/h4-14H,15-17H2,1-3H3,(H,31,32)(H2,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171123

(CHEMBL197720 | {4-[[4-(Difluoro-phosphono-methyl)-...)Show SMILES CN(C)S(=O)(=O)c1ccc(CN(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)S(=O)(=O)c2ccc(OCC(O)=O)cc2)cc1 Show InChI InChI=1S/C25H27F2N2O10PS2/c1-28(2)41(35,36)22-11-5-19(6-12-22)16-29(15-18-3-7-20(8-4-18)25(26,27)40(32,33)34)42(37,38)23-13-9-21(10-14-23)39-17-24(30)31/h3-14H,15-17H2,1-2H3,(H,30,31)(H2,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50376607
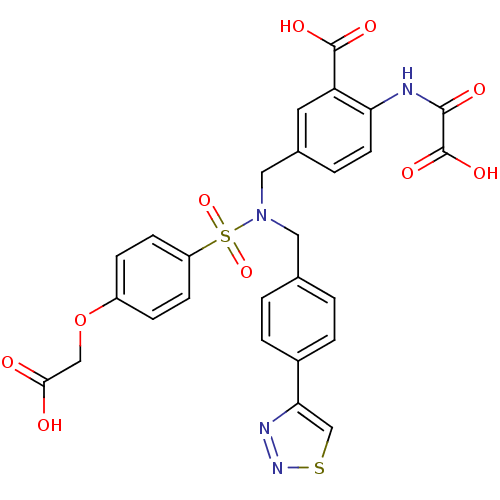
(CHEMBL445680)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(NC(=O)C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C27H22N4O10S2/c32-24(33)14-41-19-6-8-20(9-7-19)43(39,40)31(12-16-1-4-18(5-2-16)23-15-42-30-29-23)13-17-3-10-22(21(11-17)26(35)36)28-25(34)27(37)38/h1-11,15H,12-14H2,(H,28,34)(H,32,33)(H,35,36)(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 18: 2719-24 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.007
BindingDB Entry DOI: 10.7270/Q23J3DTX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171115
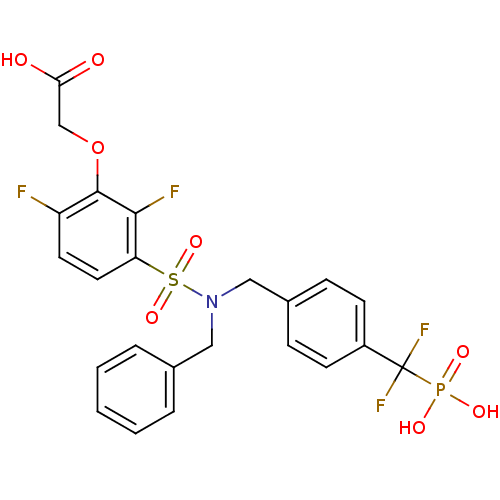
((3-{Benzyl-[4-(difluoro-phosphono-methyl)-benzyl]-...)Show SMILES OC(=O)COc1c(F)ccc(c1F)S(=O)(=O)N(Cc1ccccc1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C23H20F4NO8PS/c24-18-10-11-19(21(25)22(18)36-14-20(29)30)38(34,35)28(12-15-4-2-1-3-5-15)13-16-6-8-17(9-7-16)23(26,27)37(31,32)33/h1-11H,12-14H2,(H,29,30)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171120
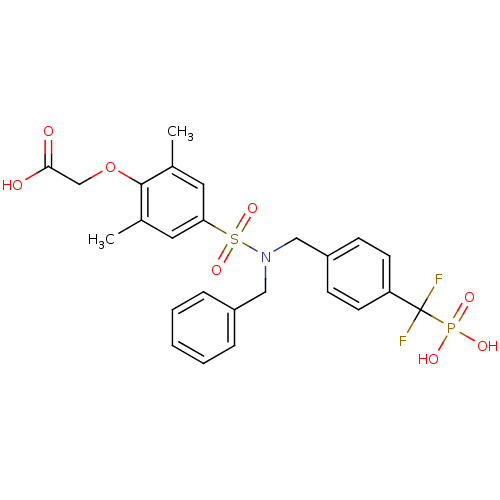
((4-{Benzyl-[4-(difluoro-phosphono-methyl)-benzyl]-...)Show SMILES Cc1cc(cc(C)c1OCC(O)=O)S(=O)(=O)N(Cc1ccccc1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C25H26F2NO8PS/c1-17-12-22(13-18(2)24(17)36-16-23(29)30)38(34,35)28(14-19-6-4-3-5-7-19)15-20-8-10-21(11-9-20)25(26,27)37(31,32)33/h3-13H,14-16H2,1-2H3,(H,29,30)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171121
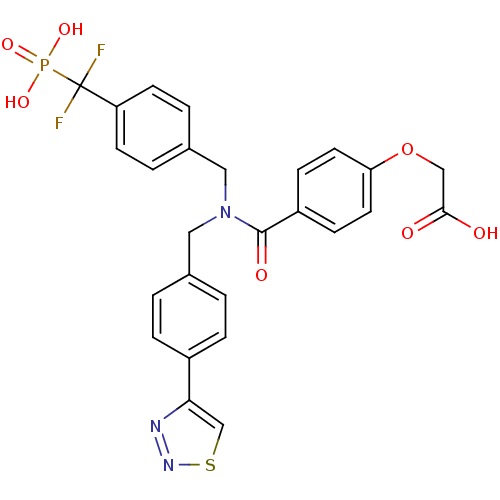
(CHEMBL197827 | {4-[[4-(Difluoro-phosphono-methyl)-...)Show SMILES OC(=O)COc1ccc(cc1)C(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C26H22F2N3O7PS/c27-26(28,39(35,36)37)21-9-3-18(4-10-21)14-31(13-17-1-5-19(6-2-17)23-16-40-30-29-23)25(34)20-7-11-22(12-8-20)38-15-24(32)33/h1-12,16H,13-15H2,(H,32,33)(H2,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171114
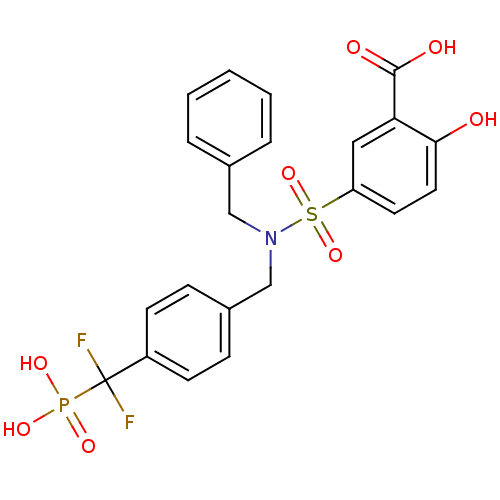
(5-{Benzyl-[4-(difluoro-phosphono-methyl)-benzyl]-s...)Show SMILES OC(=O)c1cc(ccc1O)S(=O)(=O)N(Cc1ccccc1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C22H20F2NO8PS/c23-22(24,34(29,30)31)17-8-6-16(7-9-17)14-25(13-15-4-2-1-3-5-15)35(32,33)18-10-11-20(26)19(12-18)21(27)28/h1-12,26H,13-14H2,(H,27,28)(H2,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171116
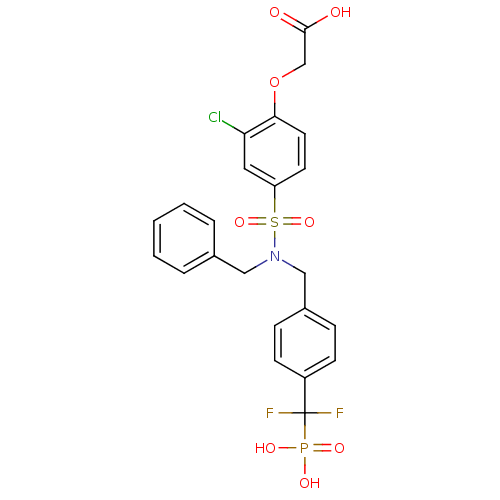
((4-{Benzyl-[4-(difluoro-phosphono-methyl)-benzyl]-...)Show SMILES OC(=O)COc1ccc(cc1Cl)S(=O)(=O)N(Cc1ccccc1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C23H21ClF2NO8PS/c24-20-12-19(10-11-21(20)35-15-22(28)29)37(33,34)27(13-16-4-2-1-3-5-16)14-17-6-8-18(9-7-17)23(25,26)36(30,31)32/h1-12H,13-15H2,(H,28,29)(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171104
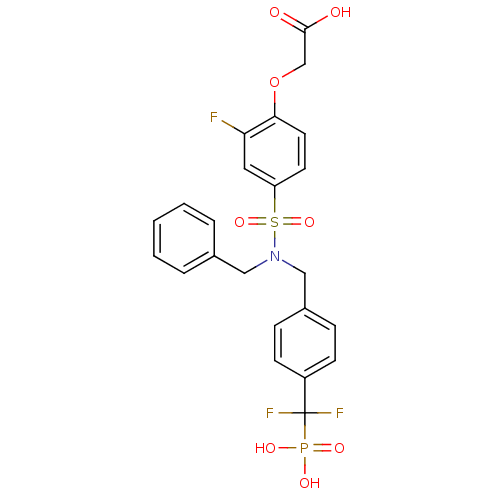
((4-{Benzyl-[4-(difluoro-phosphono-methyl)-benzyl]-...)Show SMILES OC(=O)COc1ccc(cc1F)S(=O)(=O)N(Cc1ccccc1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C23H21F3NO8PS/c24-20-12-19(10-11-21(20)35-15-22(28)29)37(33,34)27(13-16-4-2-1-3-5-16)14-17-6-8-18(9-7-17)23(25,26)36(30,31)32/h1-12H,13-15H2,(H,28,29)(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171122
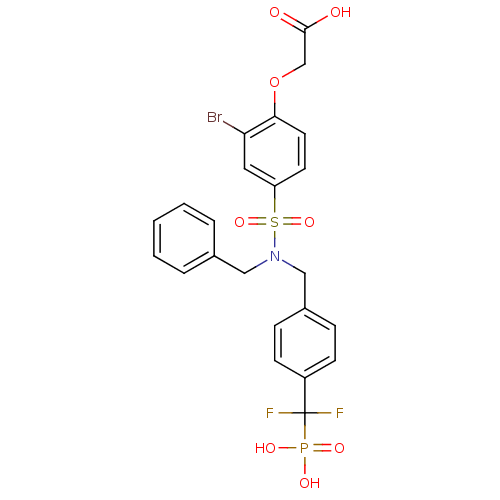
((4-{Benzyl-[4-(difluoro-phosphono-methyl)-benzyl]-...)Show SMILES OC(=O)COc1ccc(cc1Br)S(=O)(=O)N(Cc1ccccc1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C23H21BrF2NO8PS/c24-20-12-19(10-11-21(20)35-15-22(28)29)37(33,34)27(13-16-4-2-1-3-5-16)14-17-6-8-18(9-7-17)23(25,26)36(30,31)32/h1-12H,13-15H2,(H,28,29)(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171093
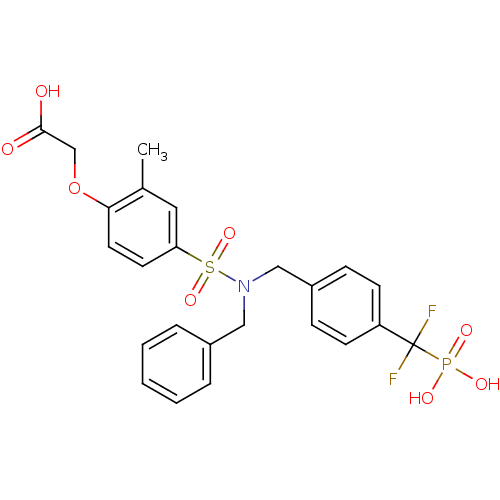
((4-{Benzyl-[4-(difluoro-phosphono-methyl)-benzyl]-...)Show SMILES Cc1cc(ccc1OCC(O)=O)S(=O)(=O)N(Cc1ccccc1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C24H24F2NO8PS/c1-17-13-21(11-12-22(17)35-16-23(28)29)37(33,34)27(14-18-5-3-2-4-6-18)15-19-7-9-20(10-8-19)24(25,26)36(30,31)32/h2-13H,14-16H2,1H3,(H,28,29)(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171108
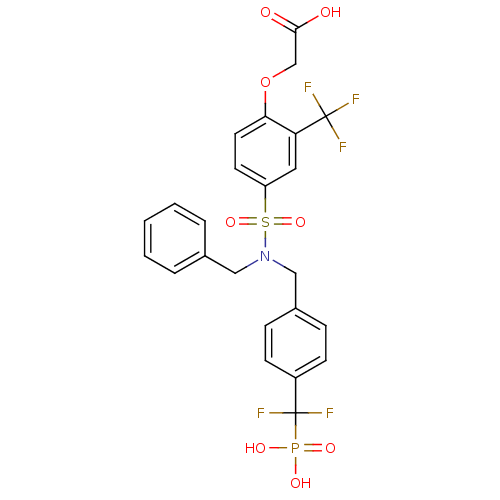
((4-{Benzyl-[4-(difluoro-phosphono-methyl)-benzyl]-...)Show SMILES OC(=O)COc1ccc(cc1C(F)(F)F)S(=O)(=O)N(Cc1ccccc1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C24H21F5NO8PS/c25-23(26,27)20-12-19(10-11-21(20)38-15-22(31)32)40(36,37)30(13-16-4-2-1-3-5-16)14-17-6-8-18(9-7-17)24(28,29)39(33,34)35/h1-12H,13-15H2,(H,31,32)(H2,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50376617

(CHEMBL407722)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(OCC(O)=O)c(Br)c1 Show InChI InChI=1S/C26H22BrN3O8S2/c27-22-11-18(3-10-24(22)38-15-26(33)34)13-30(12-17-1-4-19(5-2-17)23-16-39-29-28-23)40(35,36)21-8-6-20(7-9-21)37-14-25(31)32/h1-11,16H,12-15H2,(H,31,32)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 18: 2719-24 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.007
BindingDB Entry DOI: 10.7270/Q23J3DTX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171112

(({4-[(Benzenesulfonyl-benzyl-amino)-methyl]-phenyl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CN(Cc2ccccc2)S(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C21H20F2NO5PS/c22-21(23,30(25,26)27)19-13-11-18(12-14-19)16-24(15-17-7-3-1-4-8-17)31(28,29)20-9-5-2-6-10-20/h1-14H,15-16H2,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50376614

(CHEMBL259456)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(OCC(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C27H23N3O10S2/c31-25(32)14-39-20-6-8-21(9-7-20)42(37,38)30(12-17-1-4-19(5-2-17)23-16-41-29-28-23)13-18-3-10-24(40-15-26(33)34)22(11-18)27(35)36/h1-11,16H,12-15H2,(H,31,32)(H,33,34)(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 18: 2719-24 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.007
BindingDB Entry DOI: 10.7270/Q23J3DTX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171095
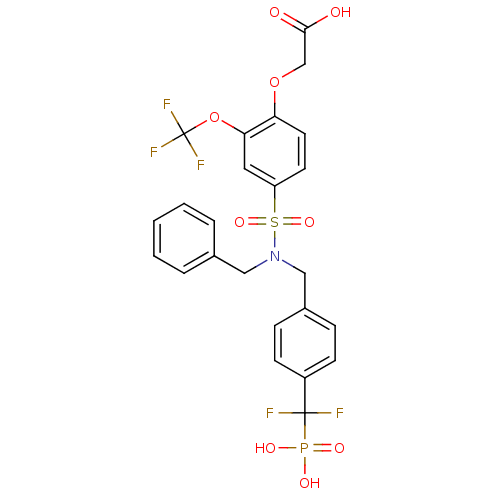
((4-{Benzyl-[4-(difluoro-phosphono-methyl)-benzyl]-...)Show SMILES OC(=O)COc1ccc(cc1OC(F)(F)F)S(=O)(=O)N(Cc1ccccc1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C24H21F5NO9PS/c25-23(26,40(33,34)35)18-8-6-17(7-9-18)14-30(13-16-4-2-1-3-5-16)41(36,37)19-10-11-20(38-15-22(31)32)21(12-19)39-24(27,28)29/h1-12H,13-15H2,(H,31,32)(H2,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171092
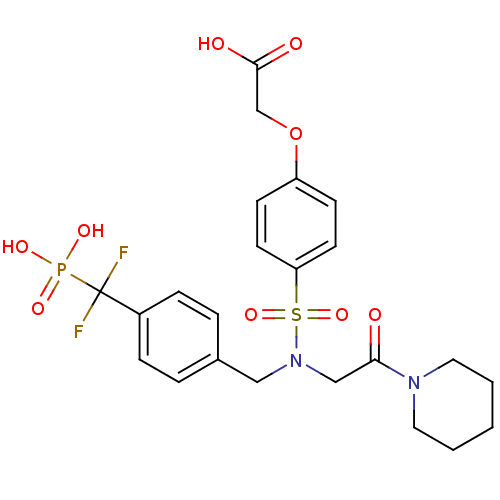
(CHEMBL371083 | {4-[[4-(Difluoro-phosphono-methyl)-...)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(CC(=O)N1CCCCC1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C23H27F2N2O9PS/c24-23(25,37(31,32)33)18-6-4-17(5-7-18)14-27(15-21(28)26-12-2-1-3-13-26)38(34,35)20-10-8-19(9-11-20)36-16-22(29)30/h4-11H,1-3,12-16H2,(H,29,30)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171110
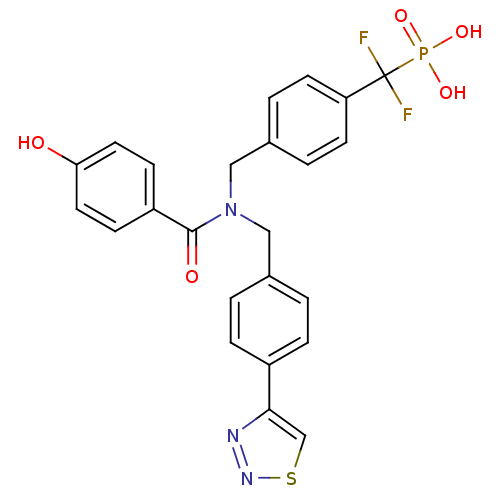
(CHEMBL424840 | [Difluoro-(4-{[(4-hydroxy-benzoyl)-...)Show SMILES Oc1ccc(cc1)C(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(cc1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C24H20F2N3O5PS/c25-24(26,35(32,33)34)20-9-3-17(4-10-20)14-29(23(31)19-7-11-21(30)12-8-19)13-16-1-5-18(6-2-16)22-15-36-28-27-22/h1-12,15,30H,13-14H2,(H2,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171111
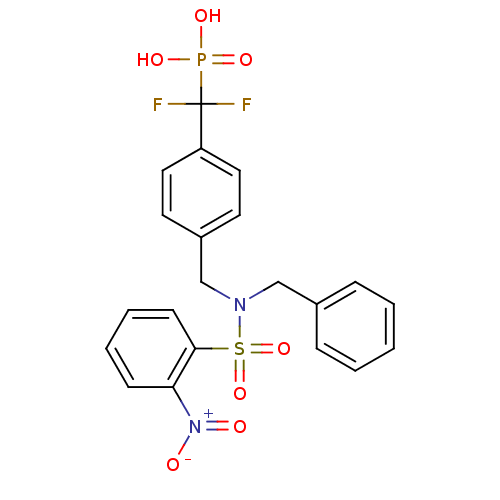
(CHEMBL199124 | [(4-{[Benzyl-(2-nitro-benzenesulfon...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CN(Cc2ccccc2)S(=O)(=O)c2ccccc2[N+]([O-])=O)cc1 Show InChI InChI=1S/C21H19F2N2O7PS/c22-21(23,33(28,29)30)18-12-10-17(11-13-18)15-24(14-16-6-2-1-3-7-16)34(31,32)20-9-5-4-8-19(20)25(26)27/h1-13H,14-15H2,(H2,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
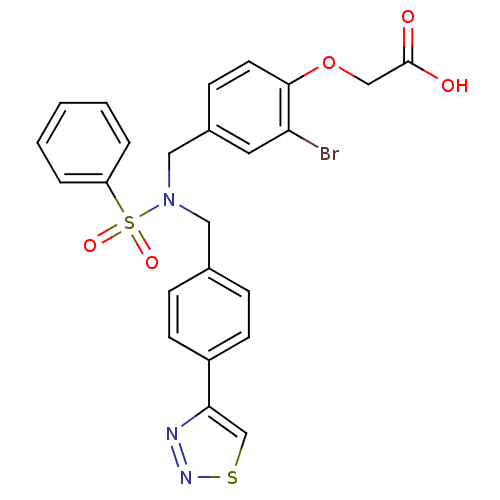
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50376616
(CHEMBL410483)Show SMILES OC(=O)COc1ccc(CN(Cc2ccc(cc2)-c2csnn2)S(=O)(=O)c2ccccc2)cc1Br Show InChI InChI=1S/C24H20BrN3O5S2/c25-21-12-18(8-11-23(21)33-15-24(29)30)14-28(35(31,32)20-4-2-1-3-5-20)13-17-6-9-19(10-7-17)22-16-34-27-26-22/h1-12,16H,13-15H2,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 18: 2719-24 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.007
BindingDB Entry DOI: 10.7270/Q23J3DTX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171107

((4-{(2-Acetylamino-ethyl)-[4-(difluoro-phosphono-m...)Show SMILES CC(=O)NCCN(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)S(=O)(=O)c1ccc(OCC(O)=O)cc1 Show InChI InChI=1S/C20H23F2N2O9PS/c1-14(25)23-10-11-24(12-15-2-4-16(5-3-15)20(21,22)34(28,29)30)35(31,32)18-8-6-17(7-9-18)33-13-19(26)27/h2-9H,10-13H2,1H3,(H,23,25)(H,26,27)(H2,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
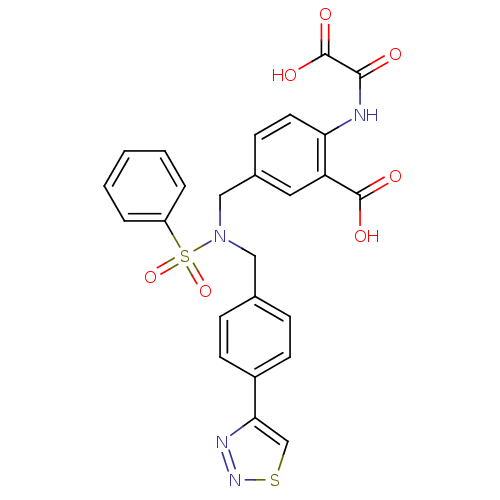
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50376606
(CHEMBL259505)Show SMILES OC(=O)C(=O)Nc1ccc(CN(Cc2ccc(cc2)-c2csnn2)S(=O)(=O)c2ccccc2)cc1C(O)=O Show InChI InChI=1S/C25H20N4O7S2/c30-23(25(33)34)26-21-11-8-17(12-20(21)24(31)32)14-29(38(35,36)19-4-2-1-3-5-19)13-16-6-9-18(10-7-16)22-15-37-28-27-22/h1-12,15H,13-14H2,(H,26,30)(H,31,32)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 18: 2719-24 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.007
BindingDB Entry DOI: 10.7270/Q23J3DTX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171113

(CHEMBL199159 | [2-(4-{[Benzyl-(2-nitro-benzenesulf...)Show SMILES OP(O)(=O)C(F)(F)C(=O)c1ccc(CN(Cc2ccccc2)S(=O)(=O)c2ccccc2[N+]([O-])=O)cc1 Show InChI InChI=1S/C22H19F2N2O8PS/c23-22(24,35(30,31)32)21(27)18-12-10-17(11-13-18)15-25(14-16-6-2-1-3-7-16)36(33,34)20-9-5-4-8-19(20)26(28)29/h1-13H,14-15H2,(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50376613
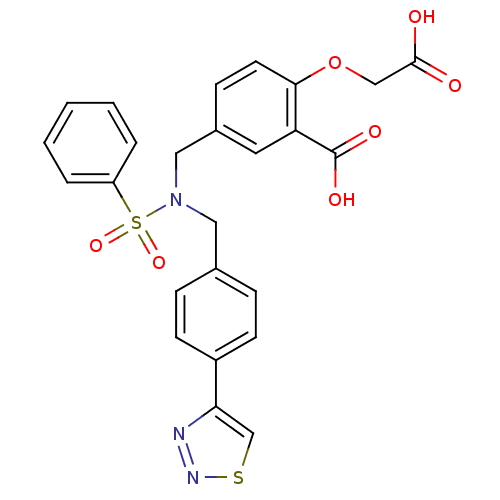
(CHEMBL406646)Show SMILES OC(=O)COc1ccc(CN(Cc2ccc(cc2)-c2csnn2)S(=O)(=O)c2ccccc2)cc1C(O)=O Show InChI InChI=1S/C25H21N3O7S2/c29-24(30)15-35-23-11-8-18(12-21(23)25(31)32)14-28(37(33,34)20-4-2-1-3-5-20)13-17-6-9-19(10-7-17)22-16-36-27-26-22/h1-12,16H,13-15H2,(H,29,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 18: 2719-24 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.007
BindingDB Entry DOI: 10.7270/Q23J3DTX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171119
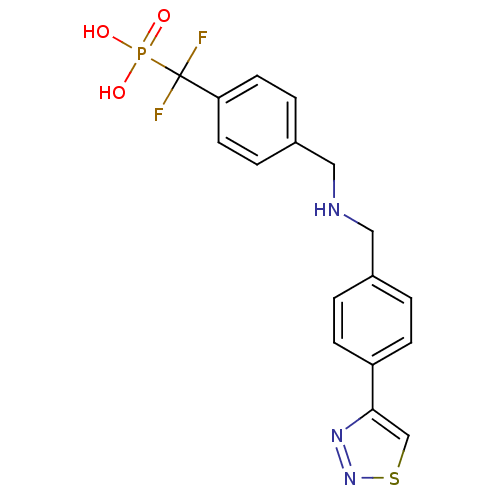
((Difluoro-{4-[(4-[1,2,3]thiadiazol-4-yl-benzylamin...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CNCc2ccc(cc2)-c2csnn2)cc1 Show InChI InChI=1S/C17H16F2N3O3PS/c18-17(19,26(23,24)25)15-7-3-13(4-8-15)10-20-9-12-1-5-14(6-2-12)16-11-27-22-21-16/h1-8,11,20H,9-10H2,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B using O-methyl fluorescein monophosphate |
Bioorg Med Chem Lett 15: 4336-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.061
BindingDB Entry DOI: 10.7270/Q2M61JSK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50376605

(CHEMBL409311)Show SMILES OC(=O)C(=O)Nc1ccc(CN(Cc2ccc(cc2)-c2csnn2)S(=O)(=O)c2ccccc2)cc1Br Show InChI InChI=1S/C24H19BrN4O5S2/c25-20-12-17(8-11-21(20)26-23(30)24(31)32)14-29(36(33,34)19-4-2-1-3-5-19)13-16-6-9-18(10-7-16)22-15-35-28-27-22/h1-12,15H,13-14H2,(H,26,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 18: 2719-24 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.007
BindingDB Entry DOI: 10.7270/Q23J3DTX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50376603
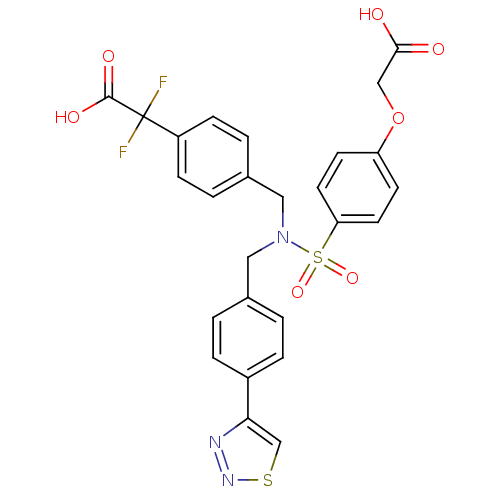
(CHEMBL259284)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(cc1)C(F)(F)C(O)=O Show InChI InChI=1S/C26H21F2N3O7S2/c27-26(28,25(34)35)20-7-3-18(4-8-20)14-31(13-17-1-5-19(6-2-17)23-16-39-30-29-23)40(36,37)22-11-9-21(10-12-22)38-15-24(32)33/h1-12,16H,13-15H2,(H,32,33)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 18: 2719-24 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.007
BindingDB Entry DOI: 10.7270/Q23J3DTX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data