 Found 105 hits with Last Name = 'fernandes' and Initial = 'mx'
Found 105 hits with Last Name = 'fernandes' and Initial = 'mx' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
HIV-1 protease
(Human immunodeficiency virus) | BDBM578

((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...)Show SMILES CC(C)[C@H](N1CCCNC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
| Assay Description
Inhibition assay using HIV protease and Sulfonamide compounds. |
Chem Biol Drug Des 69: 298-313 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00514.x
BindingDB Entry DOI: 10.7270/Q2TQ6011 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
HIV-1 protease
(Human immunodeficiency virus) | BDBM520

(1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
| Assay Description
Inhibition assay using HIV protease and Sulfonamide compounds. |
Chem Biol Drug Des 69: 298-313 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00514.x
BindingDB Entry DOI: 10.7270/Q2TQ6011 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
HIV-1 protease
(Human immunodeficiency virus) | BDBM519

((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
| Assay Description
Inhibition assay using HIV protease and Sulfonamide compounds. |
Chem Biol Drug Des 69: 298-313 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00514.x
BindingDB Entry DOI: 10.7270/Q2TQ6011 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
HIV-1 protease
(Human immunodeficiency virus) | BDBM577

((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
| Assay Description
Inhibition assay using HIV protease and Sulfonamide compounds. |
Chem Biol Drug Des 69: 298-313 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00514.x
BindingDB Entry DOI: 10.7270/Q2TQ6011 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
HIV-1 protease
(Human immunodeficiency virus) | BDBM81680

(IDV)Show SMILES CC(=N[C@@H]1[C@H](O)Cc2ccccc12)[C@H](C[C@H](O)CN1CCN(Cc2cccnc2)C[C@H]1C(=O)NC(C)(C)C)Cc1ccccc1 |r,w:1.0| Show InChI InChI=1S/C37H49N5O3/c1-26(39-35-32-15-9-8-14-29(32)21-34(35)44)30(19-27-11-6-5-7-12-27)20-31(43)24-42-18-17-41(23-28-13-10-16-38-22-28)25-33(42)36(45)40-37(2,3)4/h5-16,22,30-31,33-35,43-44H,17-21,23-25H2,1-4H3,(H,40,45)/t30-,31-,33-,34+,35-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
| Assay Description
Inhibition assay using HIV protease and Sulfonamide compounds. |
Chem Biol Drug Des 69: 298-313 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00514.x
BindingDB Entry DOI: 10.7270/Q2TQ6011 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM81679
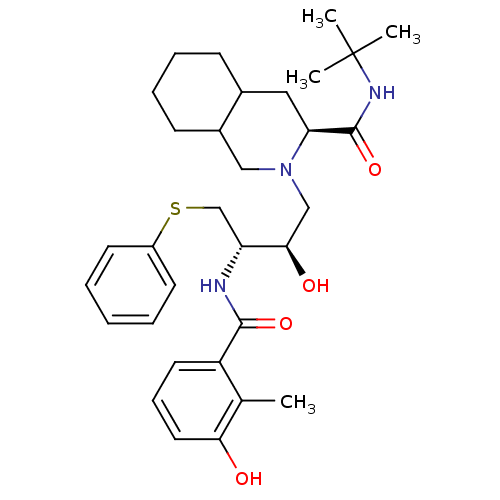
(NFV)Show SMILES Cc1c(O)cccc1C(=O)N[C@H](CSc1ccccc1)[C@H](O)CN1CC2CCCCC2C[C@H]1C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C32H45N3O4S/c1-21-25(15-10-16-28(21)36)30(38)33-26(20-40-24-13-6-5-7-14-24)29(37)19-35-18-23-12-9-8-11-22(23)17-27(35)31(39)34-32(2,3)4/h5-7,10,13-16,22-23,26-27,29,36-37H,8-9,11-12,17-20H2,1-4H3,(H,33,38)(H,34,39)/t22?,23?,26-,27+,29-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
| Assay Description
Inhibition assay using HIV protease and Sulfonamide compounds. |
Chem Biol Drug Des 69: 298-313 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00514.x
BindingDB Entry DOI: 10.7270/Q2TQ6011 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Homo sapiens (Human)) | BDBM50463046
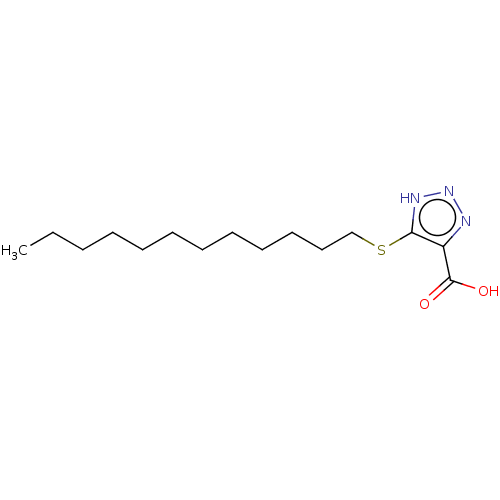
(CHEMBL1229989)Show InChI InChI=1S/C15H27N3O2S/c1-2-3-4-5-6-7-8-9-10-11-12-21-14-13(15(19)20)16-18-17-14/h2-12H2,1H3,(H,19,20)(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged glycolate oxidase expressed in C41(D43) Escherichia coli using glycolate as substrate preincubated for 30 m... |
J Med Chem 61: 7144-7167 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00399
BindingDB Entry DOI: 10.7270/Q23B62SD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
HIV-1 protease
(Human immunodeficiency virus) | BDBM81672

(CARB-AD37)Show SMILES CNC(=O)CCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC1CC1)S(=O)(=O)c1cccc(OC)c1 |r| Show InChI InChI=1S/C26H35N3O6S/c1-27-25(31)13-14-26(32)28-23(15-19-7-4-3-5-8-19)24(30)18-29(17-20-11-12-20)36(33,34)22-10-6-9-21(16-22)35-2/h3-10,16,20,23-24,30H,11-15,17-18H2,1-2H3,(H,27,31)(H,28,32)/t23-,24+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
| Assay Description
Inhibition assay using HIV protease and Sulfonamide compounds. |
Chem Biol Drug Des 69: 298-313 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00514.x
BindingDB Entry DOI: 10.7270/Q2TQ6011 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
HIV-1 protease
(Human immunodeficiency virus) | BDBM81673

(CARB-KB45)Show SMILES CNC(=O)CCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC1CC1)S(=O)(=O)c1ccco1 |r| Show InChI InChI=1S/C23H31N3O6S/c1-24-21(28)11-12-22(29)25-19(14-17-6-3-2-4-7-17)20(27)16-26(15-18-9-10-18)33(30,31)23-8-5-13-32-23/h2-8,13,18-20,27H,9-12,14-16H2,1H3,(H,24,28)(H,25,29)/t19-,20+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
| Assay Description
Inhibition assay using HIV protease and Sulfonamide compounds. |
Chem Biol Drug Des 69: 298-313 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00514.x
BindingDB Entry DOI: 10.7270/Q2TQ6011 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594018

(CHEMBL5180187)Show SMILES N.N.OC(=O)c1cc(ccc1O)-c1ccc(CN(Cc2ccc(o2)-c2ccc(O)c(c2)C(O)=O)Cc2ccccc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM81674
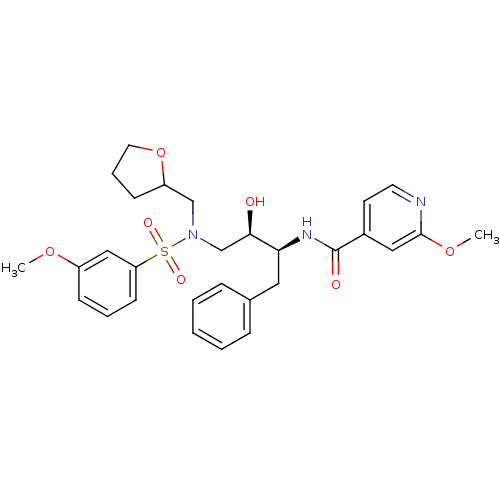
(CARB-AD08)Show SMILES COc1cccc(c1)S(=O)(=O)N(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1ccnc(OC)c1)CC1CCCO1 |r| Show InChI InChI=1S/C29H35N3O7S/c1-37-23-10-6-12-25(18-23)40(35,36)32(19-24-11-7-15-39-24)20-27(33)26(16-21-8-4-3-5-9-21)31-29(34)22-13-14-30-28(17-22)38-2/h3-6,8-10,12-14,17-18,24,26-27,33H,7,11,15-16,19-20H2,1-2H3,(H,31,34)/t24?,26-,27+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
| Assay Description
Inhibition assay using HIV protease and Sulfonamide compounds. |
Chem Biol Drug Des 69: 298-313 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00514.x
BindingDB Entry DOI: 10.7270/Q2TQ6011 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM81675
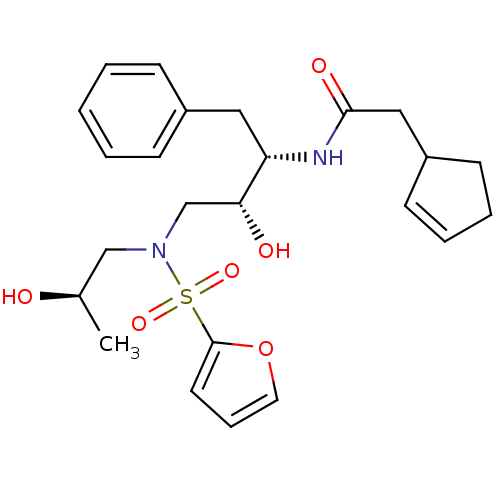
(CARB-KB51)Show SMILES C[C@@H](O)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)CC1CCC=C1)S(=O)(=O)c1ccco1 |r,c:24| Show InChI InChI=1S/C24H32N2O6S/c1-18(27)16-26(33(30,31)24-12-7-13-32-24)17-22(28)21(14-19-8-3-2-4-9-19)25-23(29)15-20-10-5-6-11-20/h2-5,7-10,12-13,18,20-22,27-28H,6,11,14-17H2,1H3,(H,25,29)/t18-,20?,21+,22-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
| Assay Description
Inhibition assay using HIV protease and Sulfonamide compounds. |
Chem Biol Drug Des 69: 298-313 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00514.x
BindingDB Entry DOI: 10.7270/Q2TQ6011 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM81676
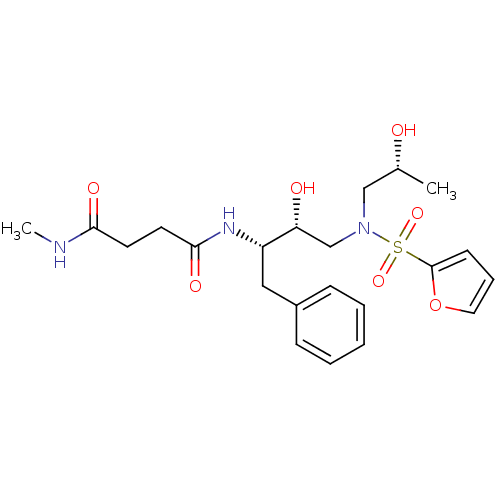
(CARB-KB49)Show SMILES CNC(=O)CCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@@H](C)O)S(=O)(=O)c1ccco1 |r| Show InChI InChI=1S/C22H31N3O7S/c1-16(26)14-25(33(30,31)22-9-6-12-32-22)15-19(27)18(13-17-7-4-3-5-8-17)24-21(29)11-10-20(28)23-2/h3-9,12,16,18-19,26-27H,10-11,13-15H2,1-2H3,(H,23,28)(H,24,29)/t16-,18+,19-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
| Assay Description
Inhibition assay using HIV protease and Sulfonamide compounds. |
Chem Biol Drug Des 69: 298-313 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00514.x
BindingDB Entry DOI: 10.7270/Q2TQ6011 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50594018

(CHEMBL5180187)Show SMILES N.N.OC(=O)c1cc(ccc1O)-c1ccc(CN(Cc2ccc(o2)-c2ccc(O)c(c2)C(O)=O)Cc2ccccc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM81677

(CARB-KB32)Show SMILES CNC(=O)CCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@@H](C)O)S(=O)(=O)c1cccc(OC)c1 |r| Show InChI InChI=1S/C25H35N3O7S/c1-18(29)16-28(36(33,34)21-11-7-10-20(15-21)35-3)17-23(30)22(14-19-8-5-4-6-9-19)27-25(32)13-12-24(31)26-2/h4-11,15,18,22-23,29-30H,12-14,16-17H2,1-3H3,(H,26,31)(H,27,32)/t18-,22+,23-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
| Assay Description
Inhibition assay using HIV protease and Sulfonamide compounds. |
Chem Biol Drug Des 69: 298-313 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00514.x
BindingDB Entry DOI: 10.7270/Q2TQ6011 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594014
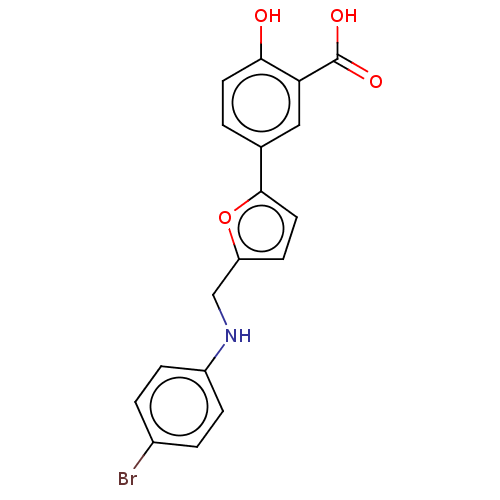
(CHEMBL5182027)Show SMILES N.OC(=O)c1cc(ccc1O)-c1ccc(CNc2ccc(Br)cc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM81678

(CARB-AC97)Show SMILES COc1cc(ccn1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1CCCO1)S(=O)(=O)c1cn(C)cn1 |r| Show InChI InChI=1S/C26H33N5O6S/c1-30-17-25(28-18-30)38(34,35)31(15-21-9-6-12-37-21)16-23(32)22(13-19-7-4-3-5-8-19)29-26(33)20-10-11-27-24(14-20)36-2/h3-5,7-8,10-11,14,17-18,21-23,32H,6,9,12-13,15-16H2,1-2H3,(H,29,33)/t21-,22+,23-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
| Assay Description
Inhibition assay using HIV protease and Sulfonamide compounds. |
Chem Biol Drug Des 69: 298-313 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00514.x
BindingDB Entry DOI: 10.7270/Q2TQ6011 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Homo sapiens (Human)) | BDBM50594014

(CHEMBL5182027)Show SMILES N.OC(=O)c1cc(ccc1O)-c1ccc(CNc2ccc(Br)cc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50463040

(CHEMBL4247590)Show InChI InChI=1S/C12H8O5/c13-6-8-2-4-11(17-8)7-1-3-10(14)9(5-7)12(15)16/h1-6,14H,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594017
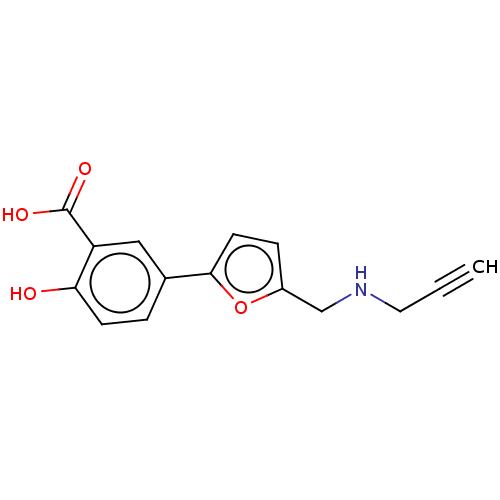
(CHEMBL5190607) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50594017

(CHEMBL5190607) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Homo sapiens (Human)) | BDBM50594018

(CHEMBL5180187)Show SMILES N.N.OC(=O)c1cc(ccc1O)-c1ccc(CN(Cc2ccc(o2)-c2ccc(O)c(c2)C(O)=O)Cc2ccccc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50594017

(CHEMBL5190607) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594017

(CHEMBL5190607) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Mus musculus) | BDBM50463040

(CHEMBL4247590)Show InChI InChI=1S/C12H8O5/c13-6-8-2-4-11(17-8)7-1-3-10(14)9(5-7)12(15)16/h1-6,14H,(H,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Mus musculus) | BDBM50594017

(CHEMBL5190607) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50463040

(CHEMBL4247590)Show InChI InChI=1S/C12H8O5/c13-6-8-2-4-11(17-8)7-1-3-10(14)9(5-7)12(15)16/h1-6,14H,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Homo sapiens (Human)) | BDBM50463040

(CHEMBL4247590)Show InChI InChI=1S/C12H8O5/c13-6-8-2-4-11(17-8)7-1-3-10(14)9(5-7)12(15)16/h1-6,14H,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Mus musculus) | BDBM50463047

(CHEMBL1794748)Show InChI InChI=1S/C9H5ClN2O2S2/c10-5-1-3-6(4-2-5)15-9-7(8(13)14)11-12-16-9/h1-4H,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of mouse N-terminal His-tagged glycolate oxidase expressed in BL21(DE3) Escherichia coli using glycolate as substrate by Cornish-Bowden pl... |
J Med Chem 61: 7144-7167 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00399
BindingDB Entry DOI: 10.7270/Q23B62SD |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Homo sapiens (Human)) | BDBM50594017

(CHEMBL5190607) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Mus musculus) | BDBM50463040

(CHEMBL4247590)Show InChI InChI=1S/C12H8O5/c13-6-8-2-4-11(17-8)7-1-3-10(14)9(5-7)12(15)16/h1-6,14H,(H,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant glycolate oxidase expressed in Escherichia coli using glycolate as substrate by Dixon plot and Cornish-Bowden plot an... |
J Med Chem 61: 7144-7167 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00399
BindingDB Entry DOI: 10.7270/Q23B62SD |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50594018

(CHEMBL5180187)Show SMILES N.N.OC(=O)c1cc(ccc1O)-c1ccc(CN(Cc2ccc(o2)-c2ccc(O)c(c2)C(O)=O)Cc2ccccc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50594016
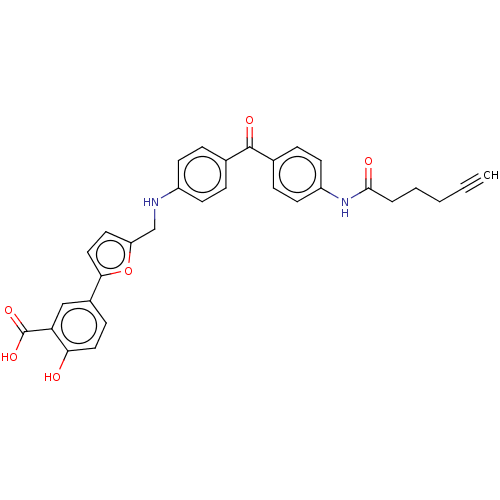
(CHEMBL5204083)Show SMILES N.OC(=O)c1cc(ccc1O)-c1ccc(CNc2ccc(cc2)C(=O)c2ccc(NC(=O)CCCC#C)cc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594018

(CHEMBL5180187)Show SMILES N.N.OC(=O)c1cc(ccc1O)-c1ccc(CN(Cc2ccc(o2)-c2ccc(O)c(c2)C(O)=O)Cc2ccccc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594016

(CHEMBL5204083)Show SMILES N.OC(=O)c1cc(ccc1O)-c1ccc(CNc2ccc(cc2)C(=O)c2ccc(NC(=O)CCCC#C)cc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594014

(CHEMBL5182027)Show SMILES N.OC(=O)c1cc(ccc1O)-c1ccc(CNc2ccc(Br)cc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Mus musculus) | BDBM50594019

(CHEMBL5170581)Show SMILES N.N.OC(=O)c1cc(ccc1O)-c1ccc(CN(CC#C)Cc2ccc(o2)-c2ccc(O)c(c2)C(O)=O)o1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50594014

(CHEMBL5182027)Show SMILES N.OC(=O)c1cc(ccc1O)-c1ccc(CNc2ccc(Br)cc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Mus musculus) | BDBM50594016

(CHEMBL5204083)Show SMILES N.OC(=O)c1cc(ccc1O)-c1ccc(CNc2ccc(cc2)C(=O)c2ccc(NC(=O)CCCC#C)cc2)o1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594015

(CHEMBL5197026)Show SMILES N.OC(=O)c1cc(ccc1O)-c1ccc(CNc2ccc(cc2)[N+]([O-])=O)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Homo sapiens (Human)) | BDBM50594018

(CHEMBL5180187)Show SMILES N.N.OC(=O)c1cc(ccc1O)-c1ccc(CN(Cc2ccc(o2)-c2ccc(O)c(c2)C(O)=O)Cc2ccccc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594019

(CHEMBL5170581)Show SMILES N.N.OC(=O)c1cc(ccc1O)-c1ccc(CN(CC#C)Cc2ccc(o2)-c2ccc(O)c(c2)C(O)=O)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Mus musculus) | BDBM50594018

(CHEMBL5180187)Show SMILES N.N.OC(=O)c1cc(ccc1O)-c1ccc(CN(Cc2ccc(o2)-c2ccc(O)c(c2)C(O)=O)Cc2ccccc2)o1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594017

(CHEMBL5190607) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Homo sapiens (Human)) | BDBM50594019

(CHEMBL5170581)Show SMILES N.N.OC(=O)c1cc(ccc1O)-c1ccc(CN(CC#C)Cc2ccc(o2)-c2ccc(O)c(c2)C(O)=O)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50594019

(CHEMBL5170581)Show SMILES N.N.OC(=O)c1cc(ccc1O)-c1ccc(CN(CC#C)Cc2ccc(o2)-c2ccc(O)c(c2)C(O)=O)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594020
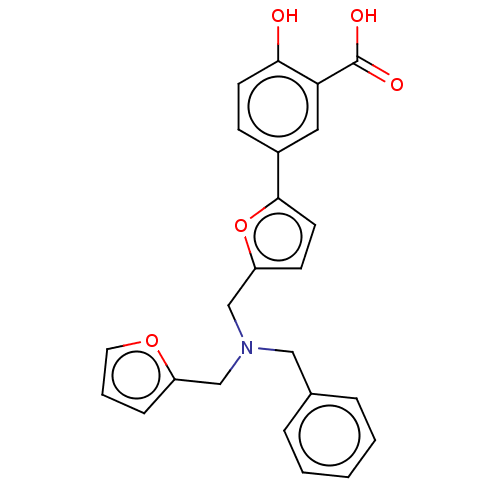
(CHEMBL5177237)Show SMILES CCN(CC)CC.OC(=O)c1cc(ccc1O)-c1ccc(CN(Cc2ccco2)Cc2ccccc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Homo sapiens (Human)) | BDBM50594016

(CHEMBL5204083)Show SMILES N.OC(=O)c1cc(ccc1O)-c1ccc(CNc2ccc(cc2)C(=O)c2ccc(NC(=O)CCCC#C)cc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594021
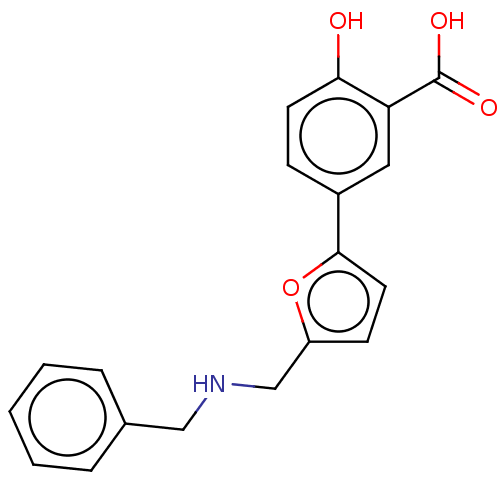
(CHEMBL5189842) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Mus musculus) | BDBM50463037
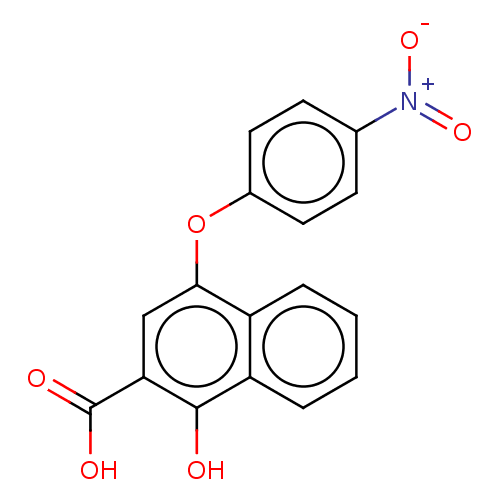
(CHEMBL4245703)Show SMILES OC(=O)c1cc(Oc2ccc(cc2)[N+]([O-])=O)c2ccccc2c1O Show InChI InChI=1S/C17H11NO6/c19-16-13-4-2-1-3-12(13)15(9-14(16)17(20)21)24-11-7-5-10(6-8-11)18(22)23/h1-9,19H,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant glycolate oxidase expressed in Escherichia coli using glycolate as substrate by sulfonated DCIP dye-based assay |
J Med Chem 61: 7144-7167 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00399
BindingDB Entry DOI: 10.7270/Q23B62SD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data