

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50419411 (CHEMBL1915012) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant EP1 receptor expressed in CHO-K1 cells assessed as inhibition of PGE2-mediated intracellular calcium mobiliz... | Bioorg Med Chem Lett 21: 4343-8 (2011) Article DOI: 10.1016/j.bmcl.2011.05.047 BindingDB Entry DOI: 10.7270/Q2RN394N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50419411 (CHEMBL1915012) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant EP3 receptor expressed in CHO-K1 cells assessed as inhibition of PGE2-mediated intracellular calcium mobiliz... | Bioorg Med Chem Lett 21: 4343-8 (2011) Article DOI: 10.1016/j.bmcl.2011.05.047 BindingDB Entry DOI: 10.7270/Q2RN394N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM256877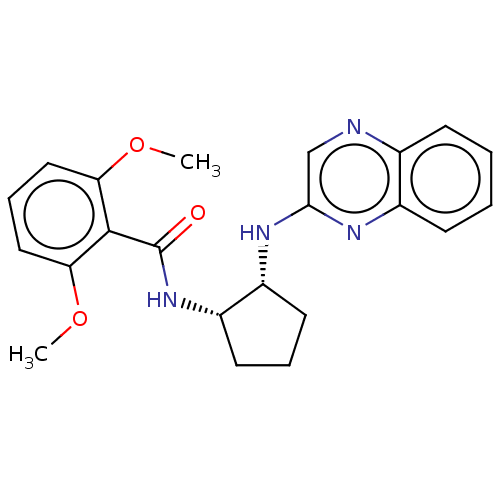 (US9493432, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceuticals Company Limited US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US9493432 (2016) BindingDB Entry DOI: 10.7270/Q2FQ9VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM256953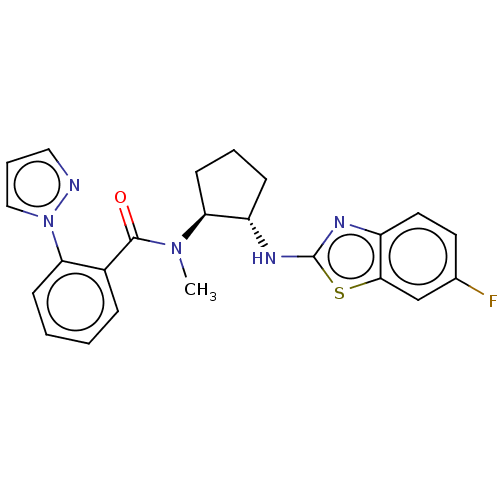 (US9493432, 79) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceuticals Company Limited US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US9493432 (2016) BindingDB Entry DOI: 10.7270/Q2FQ9VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM256957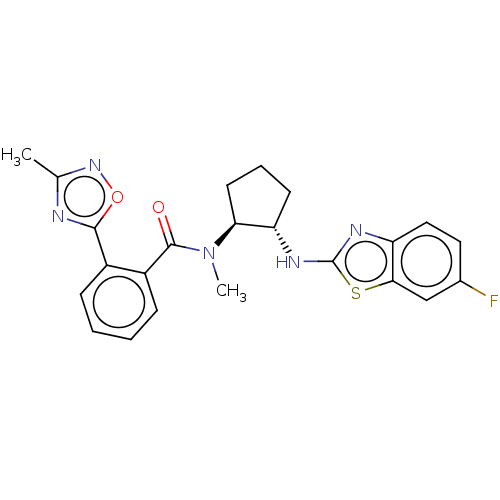 (US9493432, 83) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceuticals Company Limited US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US9493432 (2016) BindingDB Entry DOI: 10.7270/Q2FQ9VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM256955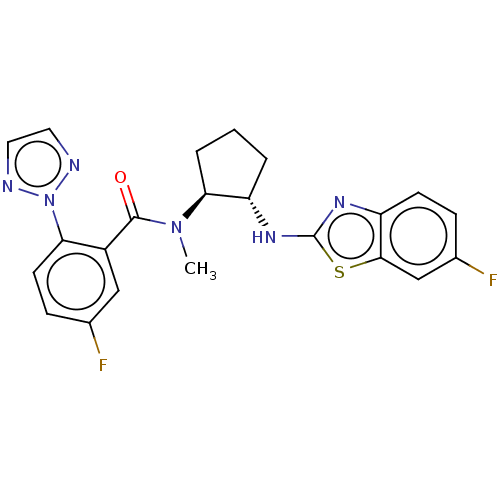 (US9493432, 81) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceuticals Company Limited US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US9493432 (2016) BindingDB Entry DOI: 10.7270/Q2FQ9VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM256954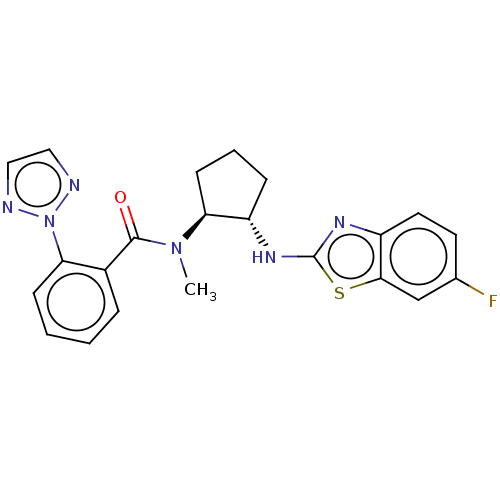 (US9493432, 80) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceuticals Company Limited US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US9493432 (2016) BindingDB Entry DOI: 10.7270/Q2FQ9VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50419410 (CHEMBL1915252) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting | Bioorg Med Chem Lett 21: 4343-8 (2011) Article DOI: 10.1016/j.bmcl.2011.05.047 BindingDB Entry DOI: 10.7270/Q2RN394N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM210775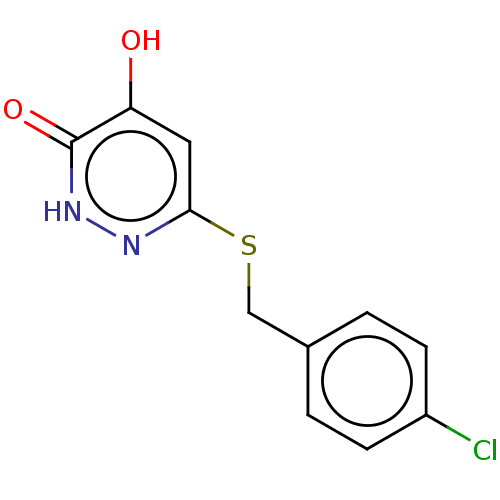 (US10463663, Example 4 | US11129828, Example 4 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The functional activity of compounds inhibiting the DAAO enzyme was determined by utilizing the co-product of the catalysis of D-Serine, H2O2 which c... | US Patent US10463663 (2019) BindingDB Entry DOI: 10.7270/Q20K2BXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM210775 (US10463663, Example 4 | US11129828, Example 4 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Human DAAO enzyme was supplied by the Takeda Pharmaceutical Company (Osaka) and each batch was tested and used at concentrations giving comparable le... | US Patent US9290456 (2016) BindingDB Entry DOI: 10.7270/Q2RF5SW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM210775 (US10463663, Example 4 | US11129828, Example 4 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Human DAAO enzyme was supplied by the Takeda Pharmaceutical Company (Osaka) and each batch was tested and used at concentrations giving comparable le... | Citation and Details BindingDB Entry DOI: 10.7270/Q27W6GCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM210775 (US10463663, Example 4 | US11129828, Example 4 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Human DAAO enzyme was supplied by the Takeda Pharmaceutical Company (Osaka) and each batch was tested and used at concentrations giving comparable le... | Bioorg Med Chem Lett 18: 4282-6 (2008) BindingDB Entry DOI: 10.7270/Q2F76FWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50419415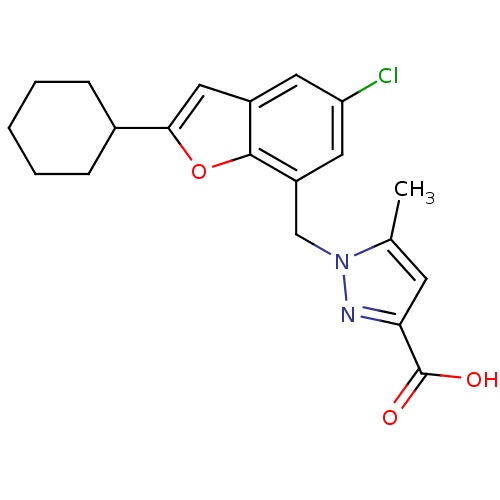 (CHEMBL1915015) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting | Bioorg Med Chem Lett 21: 4343-8 (2011) Article DOI: 10.1016/j.bmcl.2011.05.047 BindingDB Entry DOI: 10.7270/Q2RN394N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM256879 (US9493432, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceuticals Company Limited US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US9493432 (2016) BindingDB Entry DOI: 10.7270/Q2FQ9VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM256910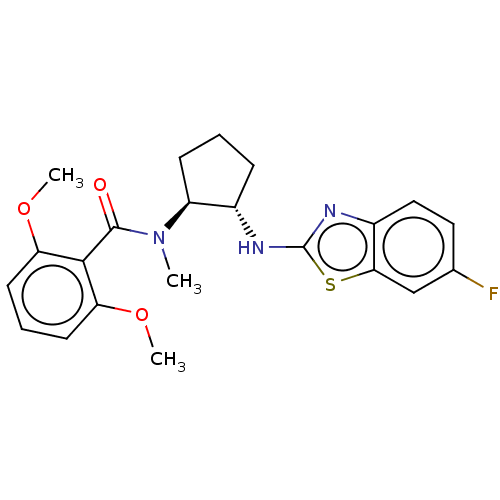 (US9493432, 34) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceuticals Company Limited US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US9493432 (2016) BindingDB Entry DOI: 10.7270/Q2FQ9VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185336 (US10011588, Example 158 | US10689373, Example 158 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | J Med Chem 52: 6362-8 (2009) BindingDB Entry DOI: 10.7270/Q2891871 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM256949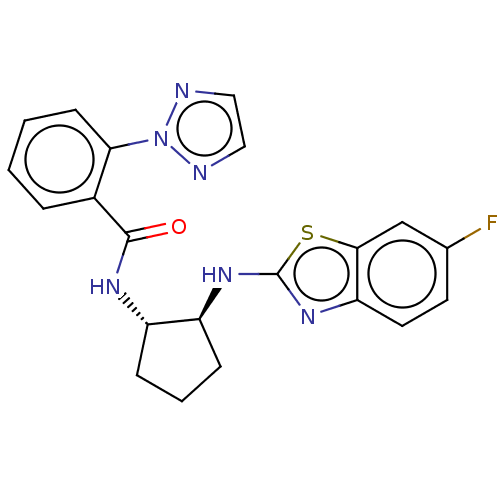 (US9493432, 75) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceuticals Company Limited US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US9493432 (2016) BindingDB Entry DOI: 10.7270/Q2FQ9VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185336 (US10011588, Example 158 | US10689373, Example 158 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | 37 |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca.sup.2+ sensitive fluorescent dye. The chang... | US Patent US9156829 (2015) BindingDB Entry DOI: 10.7270/Q2X9293X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185336 (US10011588, Example 158 | US10689373, Example 158 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US10689373 (2020) BindingDB Entry DOI: 10.7270/Q2DR2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50419411 (CHEMBL1915012) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human prostanoid EP1 receptor expressed in CHO-K1 cells after 30 mins by topcount liquid scintillation counting | Bioorg Med Chem Lett 21: 4343-8 (2011) Article DOI: 10.1016/j.bmcl.2011.05.047 BindingDB Entry DOI: 10.7270/Q2RN394N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50419403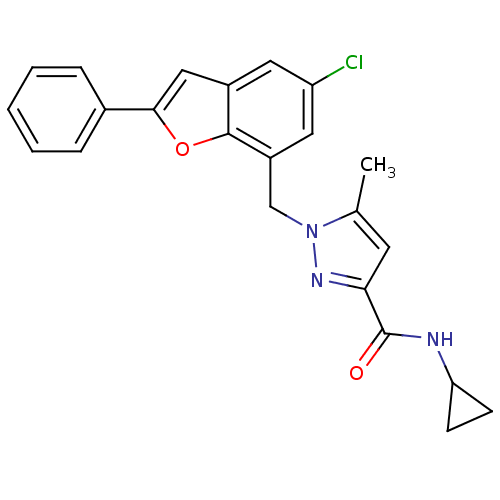 (CHEMBL1914467) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting | Bioorg Med Chem Lett 21: 4343-8 (2011) Article DOI: 10.1016/j.bmcl.2011.05.047 BindingDB Entry DOI: 10.7270/Q2RN394N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50419417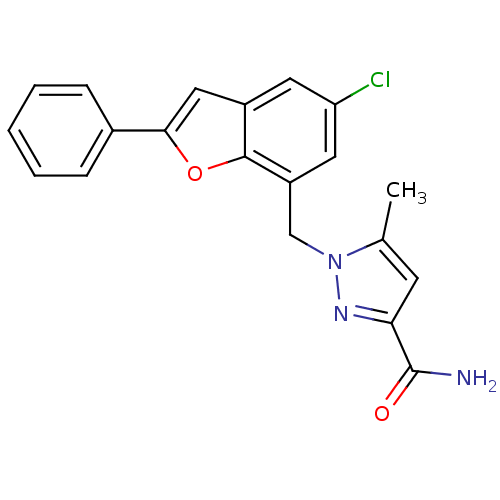 (CHEMBL1915254) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting | Bioorg Med Chem Lett 21: 4343-8 (2011) Article DOI: 10.1016/j.bmcl.2011.05.047 BindingDB Entry DOI: 10.7270/Q2RN394N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM210789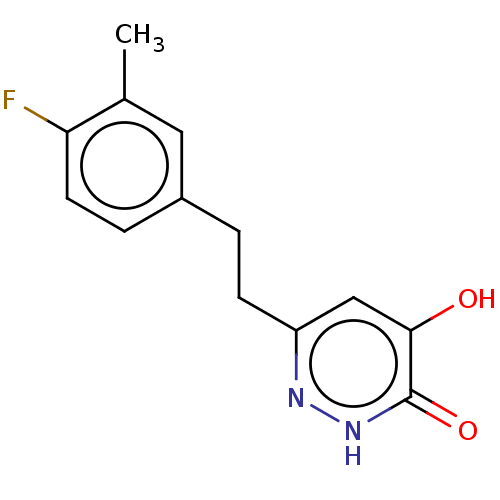 (US10463663, Example 24 | US11129828, Example 24 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The functional activity of compounds inhibiting the DAAO enzyme was determined by utilizing the co-product of the catalysis of D-Serine, H2O2 which c... | US Patent US10463663 (2019) BindingDB Entry DOI: 10.7270/Q20K2BXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM210789 (US10463663, Example 24 | US11129828, Example 24 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Human DAAO enzyme was supplied by the Takeda Pharmaceutical Company (Osaka) and each batch was tested and used at concentrations giving comparable le... | US Patent US9290456 (2016) BindingDB Entry DOI: 10.7270/Q2RF5SW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185275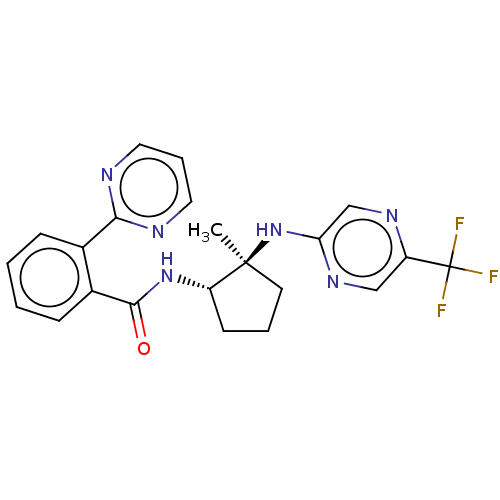 (US10011588, Example 91 | US10689373, Example 91 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 37 |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca.sup.2+ sensitive fluorescent dye. The chang... | US Patent US9156829 (2015) BindingDB Entry DOI: 10.7270/Q2X9293X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM210789 (US10463663, Example 24 | US11129828, Example 24 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Human DAAO enzyme was supplied by the Takeda Pharmaceutical Company (Osaka) and each batch was tested and used at concentrations giving comparable le... | Bioorg Med Chem Lett 18: 4282-6 (2008) BindingDB Entry DOI: 10.7270/Q2F76FWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM210789 (US10463663, Example 24 | US11129828, Example 24 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Human DAAO enzyme was supplied by the Takeda Pharmaceutical Company (Osaka) and each batch was tested and used at concentrations giving comparable le... | Citation and Details BindingDB Entry DOI: 10.7270/Q27W6GCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185275 (US10011588, Example 91 | US10689373, Example 91 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | J Med Chem 52: 6362-8 (2009) BindingDB Entry DOI: 10.7270/Q2891871 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185275 (US10011588, Example 91 | US10689373, Example 91 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US10689373 (2020) BindingDB Entry DOI: 10.7270/Q2DR2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50419419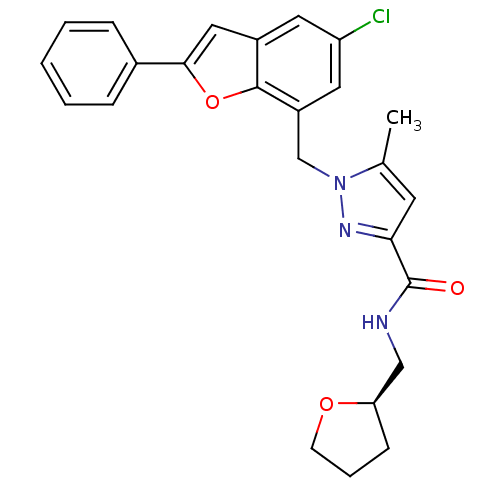 (CHEMBL1915262) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting | Bioorg Med Chem Lett 21: 4343-8 (2011) Article DOI: 10.1016/j.bmcl.2011.05.047 BindingDB Entry DOI: 10.7270/Q2RN394N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM256911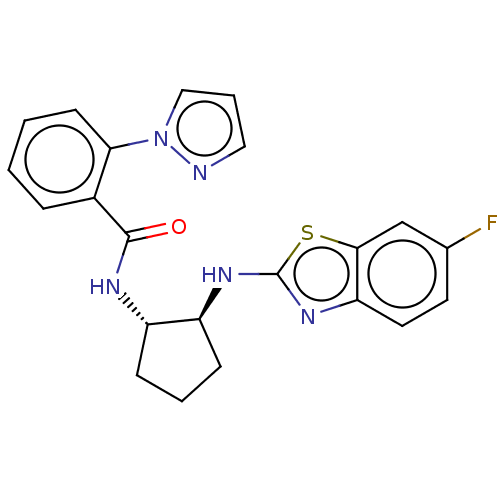 (US9493432, 35) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceuticals Company Limited US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US9493432 (2016) BindingDB Entry DOI: 10.7270/Q2FQ9VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185325 (US10011588, Example 146 | US10689373, Example 146 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | 37 |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca.sup.2+ sensitive fluorescent dye. The chang... | US Patent US9156829 (2015) BindingDB Entry DOI: 10.7270/Q2X9293X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185265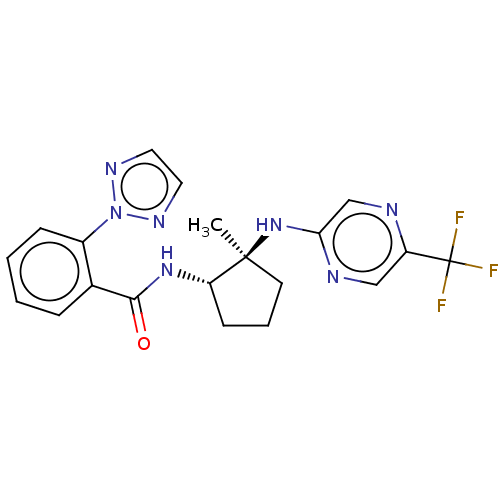 (US10011588, Example 81 | US10689373, Example 81 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | 37 |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca.sup.2+ sensitive fluorescent dye. The chang... | US Patent US9156829 (2015) BindingDB Entry DOI: 10.7270/Q2X9293X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM256979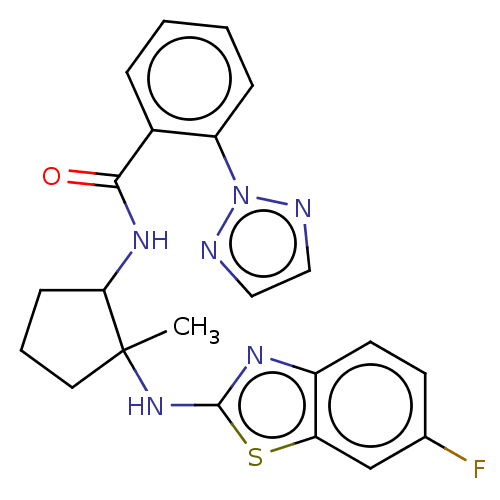 (US9493432, 107) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceuticals Company Limited US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US9493432 (2016) BindingDB Entry DOI: 10.7270/Q2FQ9VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185265 (US10011588, Example 81 | US10689373, Example 81 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US10689373 (2020) BindingDB Entry DOI: 10.7270/Q2DR2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185325 (US10011588, Example 146 | US10689373, Example 146 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | J Med Chem 52: 6362-8 (2009) BindingDB Entry DOI: 10.7270/Q2891871 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185325 (US10011588, Example 146 | US10689373, Example 146 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US10689373 (2020) BindingDB Entry DOI: 10.7270/Q2DR2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185265 (US10011588, Example 81 | US10689373, Example 81 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | J Med Chem 52: 6362-8 (2009) BindingDB Entry DOI: 10.7270/Q2891871 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50419408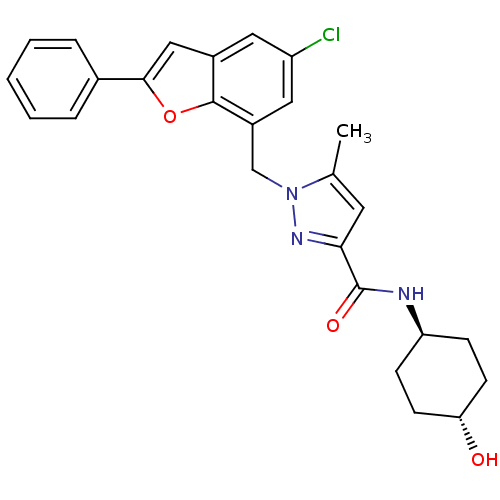 (CHEMBL1915261) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]GW875240X from human prostanoid EP1 receptor expressed in CHO-K1 cells after 45 mins by topcount liquid scintillation counting | Bioorg Med Chem Lett 21: 4343-8 (2011) Article DOI: 10.1016/j.bmcl.2011.05.047 BindingDB Entry DOI: 10.7270/Q2RN394N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185332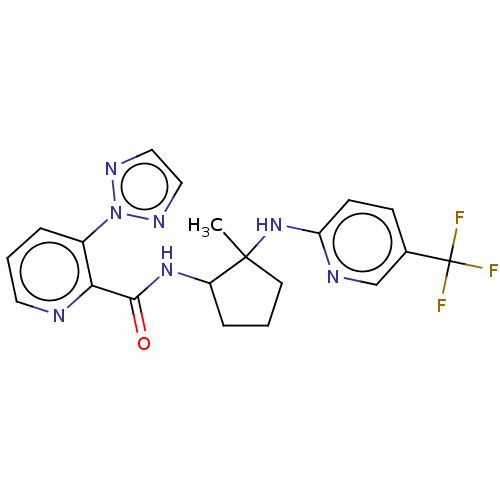 (US10011588, Example 155 | US10689373, Example 155 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | 37 |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca.sup.2+ sensitive fluorescent dye. The chang... | US Patent US9156829 (2015) BindingDB Entry DOI: 10.7270/Q2X9293X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185287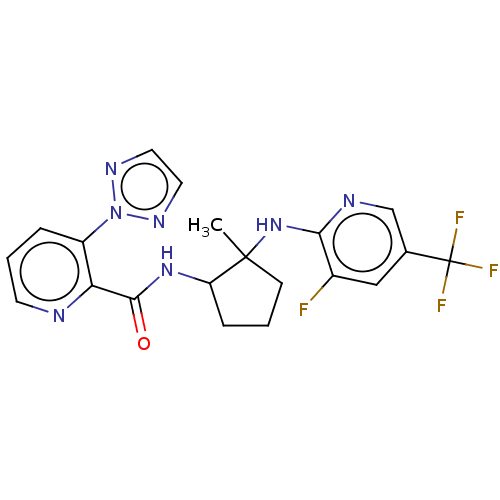 (US10011588, Example 103 | US10689373, Example 103 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | J Med Chem 52: 6362-8 (2009) BindingDB Entry DOI: 10.7270/Q2891871 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185276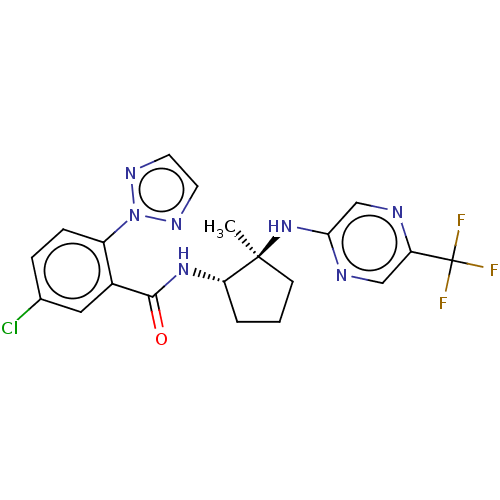 (US10011588, Example 92 | US10689373, Example 92 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | J Med Chem 52: 6362-8 (2009) BindingDB Entry DOI: 10.7270/Q2891871 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185271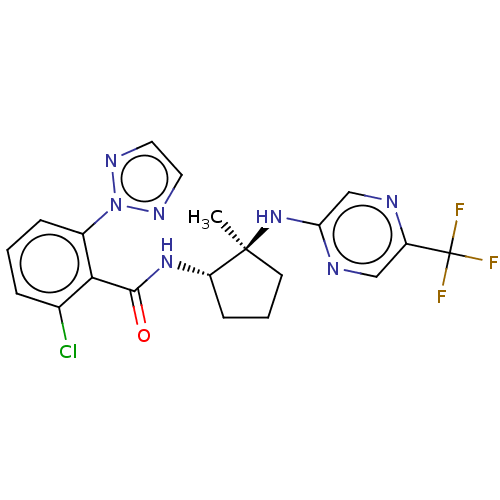 (US10011588, Example 87 | US10689373, Example 87 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | J Med Chem 52: 6362-8 (2009) BindingDB Entry DOI: 10.7270/Q2891871 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185332 (US10011588, Example 155 | US10689373, Example 155 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US10689373 (2020) BindingDB Entry DOI: 10.7270/Q2DR2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185332 (US10011588, Example 155 | US10689373, Example 155 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | J Med Chem 52: 6362-8 (2009) BindingDB Entry DOI: 10.7270/Q2891871 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185271 (US10011588, Example 87 | US10689373, Example 87 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | 37 |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca.sup.2+ sensitive fluorescent dye. The chang... | US Patent US9156829 (2015) BindingDB Entry DOI: 10.7270/Q2X9293X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185276 (US10011588, Example 92 | US10689373, Example 92 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | 37 |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca.sup.2+ sensitive fluorescent dye. The chang... | US Patent US9156829 (2015) BindingDB Entry DOI: 10.7270/Q2X9293X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185287 (US10011588, Example 103 | US10689373, Example 103 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | 37 |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca.sup.2+ sensitive fluorescent dye. The chang... | US Patent US9156829 (2015) BindingDB Entry DOI: 10.7270/Q2X9293X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185271 (US10011588, Example 87 | US10689373, Example 87 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US10689373 (2020) BindingDB Entry DOI: 10.7270/Q2DR2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM185276 (US10011588, Example 92 | US10689373, Example 92 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TAKEDA PHARMACEUTICAL COMPANY LIMITED US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US10689373 (2020) BindingDB Entry DOI: 10.7270/Q2DR2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1547 total ) | Next | Last >> |