 Found 169 hits with Last Name = 'finkelstein' and Initial = 'ja'
Found 169 hits with Last Name = 'finkelstein' and Initial = 'ja' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281205
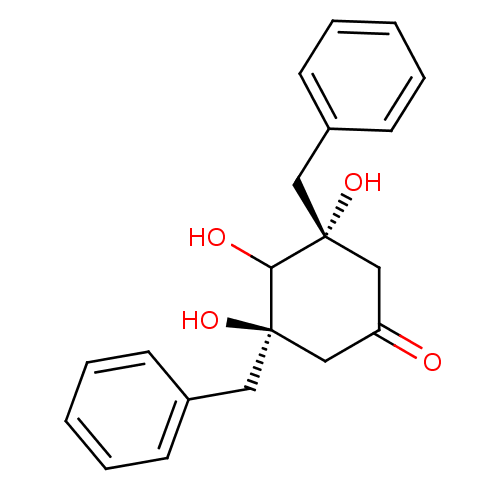
((3R,5R)-3,5-Dibenzyl-3,4,5-trihydroxy-cyclohexanon...)Show SMILES OC1[C@@](O)(Cc2ccccc2)CC(=O)C[C@]1(O)Cc1ccccc1 Show InChI InChI=1S/C20H22O4/c21-17-13-19(23,11-15-7-3-1-4-8-15)18(22)20(24,14-17)12-16-9-5-2-6-10-16/h1-10,18,22-24H,11-14H2/t19-,20-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of recombinant HIV-1 protease at 37 degrees C and pH of 6. |
Bioorg Med Chem Lett 3: 2717-2722 (1993)
Article DOI: 10.1016/S0960-894X(01)80749-6
BindingDB Entry DOI: 10.7270/Q2ZP4623 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281202
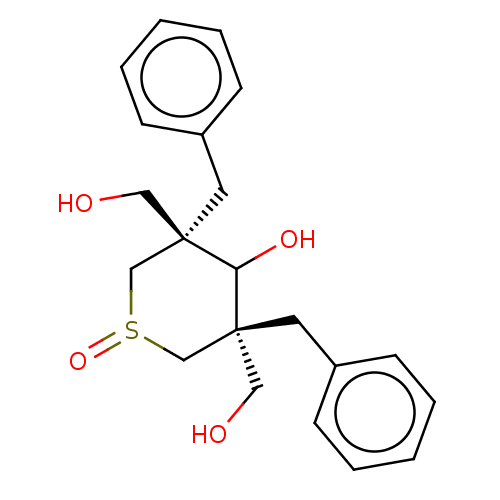
((3S,5S)-3,5-Dibenzyl-3,5-bis-hydroxymethyl-1-oxo-t...)Show SMILES OC[C@]1(Cc2ccccc2)C[S+]([O-])C[C@](CO)(Cc2ccccc2)C1O |r,wU:2.2,14.15,wD:14.17,2.1,(4.21,-7.86,;5.54,-7.08,;6.87,-7.85,;6.89,-9.39,;5.81,-10.49,;6.22,-11.96,;5.14,-13.06,;3.63,-12.69,;3.22,-11.2,;4.3,-10.1,;6.87,-6.31,;8.2,-5.56,;8.19,-4,;9.53,-6.3,;9.53,-7.84,;10.31,-9.17,;9.55,-10.51,;10.86,-7.07,;12.19,-7.84,;12.18,-9.39,;13.51,-10.16,;14.84,-9.39,;14.85,-7.85,;13.52,-7.08,;8.21,-8.62,;8.21,-10.16,)| Show InChI InChI=1S/C21H26O4S/c22-13-20(11-17-7-3-1-4-8-17)15-26(25)16-21(14-23,19(20)24)12-18-9-5-2-6-10-18/h1-10,19,22-24H,11-16H2/t19?,20-,21-,26?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of recombinant HIV-1 protease at 37 degrees C and pH of 6. |
Bioorg Med Chem Lett 3: 2717-2722 (1993)
Article DOI: 10.1016/S0960-894X(01)80749-6
BindingDB Entry DOI: 10.7270/Q2ZP4623 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281202

((3S,5S)-3,5-Dibenzyl-3,5-bis-hydroxymethyl-1-oxo-t...)Show SMILES OC[C@]1(Cc2ccccc2)C[S+]([O-])C[C@](CO)(Cc2ccccc2)C1O |r,wU:2.2,14.15,wD:14.17,2.1,(4.21,-7.86,;5.54,-7.08,;6.87,-7.85,;6.89,-9.39,;5.81,-10.49,;6.22,-11.96,;5.14,-13.06,;3.63,-12.69,;3.22,-11.2,;4.3,-10.1,;6.87,-6.31,;8.2,-5.56,;8.19,-4,;9.53,-6.3,;9.53,-7.84,;10.31,-9.17,;9.55,-10.51,;10.86,-7.07,;12.19,-7.84,;12.18,-9.39,;13.51,-10.16,;14.84,-9.39,;14.85,-7.85,;13.52,-7.08,;8.21,-8.62,;8.21,-10.16,)| Show InChI InChI=1S/C21H26O4S/c22-13-20(11-17-7-3-1-4-8-17)15-26(25)16-21(14-23,19(20)24)12-18-9-5-2-6-10-18/h1-10,19,22-24H,11-16H2/t19?,20-,21-,26?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of recombinant HIV-1 protease at 37 degrees C and pH of 6. |
Bioorg Med Chem Lett 3: 2717-2722 (1993)
Article DOI: 10.1016/S0960-894X(01)80749-6
BindingDB Entry DOI: 10.7270/Q2ZP4623 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281207
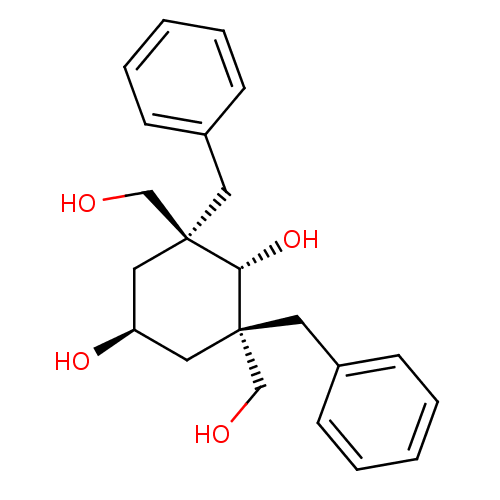
((2S,6S)-2,6-Dibenzyl-2,6-bis-hydroxymethyl-cyclohe...)Show SMILES OC[C@]1(Cc2ccccc2)C[C@H](O)C[C@](CO)(Cc2ccccc2)[C@H]1O |wU:2.2,14.15,24.27,wD:14.17,2.1,11.12,(15.23,-12.28,;13.9,-13.04,;12.57,-12.28,;12.56,-10.74,;13.64,-9.64,;13.23,-8.15,;14.32,-7.05,;15.82,-7.45,;16.23,-8.94,;15.14,-10.04,;12.57,-13.81,;11.24,-14.58,;11.24,-16.12,;9.91,-13.81,;9.91,-12.28,;9.14,-10.94,;9.9,-9.59,;8.58,-13.04,;7.25,-12.25,;7.26,-10.71,;5.93,-9.94,;4.6,-10.69,;4.59,-12.24,;5.92,-13.01,;11.24,-11.5,;11.24,-9.96,)| Show InChI InChI=1S/C22H28O4/c23-15-21(11-17-7-3-1-4-8-17)13-19(25)14-22(16-24,20(21)26)12-18-9-5-2-6-10-18/h1-10,19-20,23-26H,11-16H2/t19-,20-,21-,22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of recombinant HIV-1 protease at 37 degrees C and pH of 6. |
Bioorg Med Chem Lett 3: 2717-2722 (1993)
Article DOI: 10.1016/S0960-894X(01)80749-6
BindingDB Entry DOI: 10.7270/Q2ZP4623 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281204

((3S,5S)-3,5-Dibenzyl-4-hydroxy-3,5-bis-hydroxymeth...)Show SMILES OC[C@]1(Cc2ccccc2)CC(=O)C[C@](CO)(Cc2ccccc2)C1O Show InChI InChI=1S/C22H26O4/c23-15-21(11-17-7-3-1-4-8-17)13-19(25)14-22(16-24,20(21)26)12-18-9-5-2-6-10-18/h1-10,20,23-24,26H,11-16H2/t21-,22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of recombinant HIV-1 protease at 37 degrees C and pH of 6. |
Bioorg Med Chem Lett 3: 2717-2722 (1993)
Article DOI: 10.1016/S0960-894X(01)80749-6
BindingDB Entry DOI: 10.7270/Q2ZP4623 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281206
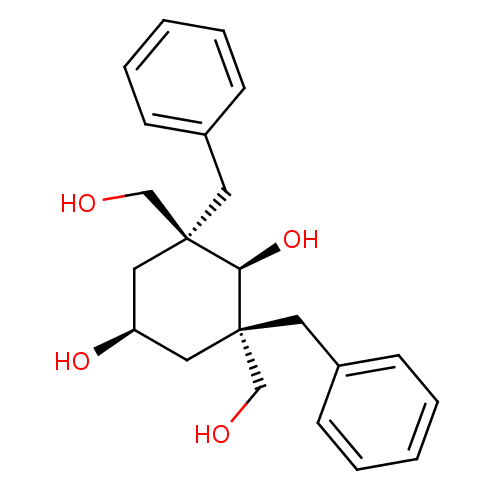
((2S,6S)-2,6-Dibenzyl-2,6-bis-hydroxymethyl-cyclohe...)Show SMILES OC[C@]1(Cc2ccccc2)C[C@H](O)C[C@](CO)(Cc2ccccc2)[C@@H]1O |wU:14.17,2.1,24.27,11.12,wD:2.2,14.15,(9.9,-9.59,;9.14,-10.94,;9.91,-12.28,;8.58,-13.04,;7.25,-12.25,;7.26,-10.71,;5.93,-9.94,;4.6,-10.69,;4.59,-12.24,;5.92,-13.01,;9.91,-13.81,;11.24,-14.58,;11.24,-16.12,;12.57,-13.81,;12.57,-12.28,;13.9,-13.04,;15.23,-12.28,;12.56,-10.74,;13.64,-9.64,;13.23,-8.15,;14.32,-7.05,;15.82,-7.45,;16.23,-8.94,;15.14,-10.04,;11.24,-11.5,;11.24,-9.96,)| Show InChI InChI=1S/C22H28O4/c23-15-21(11-17-7-3-1-4-8-17)13-19(25)14-22(16-24,20(21)26)12-18-9-5-2-6-10-18/h1-10,19-20,23-26H,11-16H2/t19-,20+,21-,22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 8.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of recombinant HIV-1 protease at 37 degrees C and pH of 6. |
Bioorg Med Chem Lett 3: 2717-2722 (1993)
Article DOI: 10.1016/S0960-894X(01)80749-6
BindingDB Entry DOI: 10.7270/Q2ZP4623 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281203
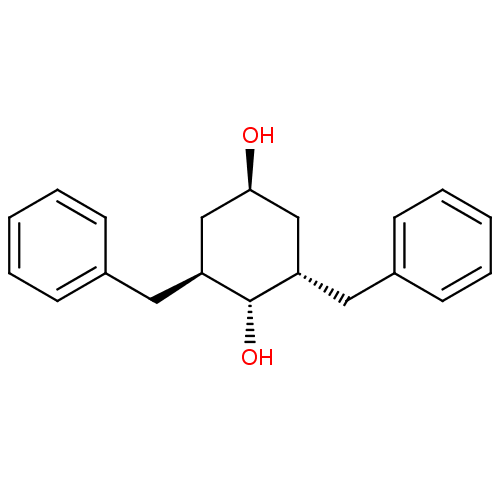
((2S,6S)-2,6-Dibenzyl-cyclohexane-1,4-diol | CHEMBL...)Show SMILES O[C@H]1C[C@H](Cc2ccccc2)[C@H](O)[C@@H](Cc2ccccc2)C1 |wU:3.3,11.12,wD:13.14,1.0,(11.67,-10.37,;11.67,-8.83,;13,-8.06,;13,-6.53,;13,-4.99,;14.08,-3.9,;13.67,-2.41,;14.75,-1.31,;16.26,-1.7,;16.67,-3.2,;15.57,-4.29,;11.67,-5.75,;11.67,-4.21,;10.34,-6.53,;9.01,-7.29,;7.68,-6.52,;7.68,-4.97,;6.37,-4.18,;5.02,-4.95,;5.02,-6.49,;6.35,-7.26,;10.34,-8.06,)| Show InChI InChI=1S/C20H24O2/c21-19-13-17(11-15-7-3-1-4-8-15)20(22)18(14-19)12-16-9-5-2-6-10-16/h1-10,17-22H,11-14H2/t17-,18-,19-,20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of recombinant HIV-1 protease at 37 degrees C and pH of 6. |
Bioorg Med Chem Lett 3: 2717-2722 (1993)
Article DOI: 10.1016/S0960-894X(01)80749-6
BindingDB Entry DOI: 10.7270/Q2ZP4623 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281208
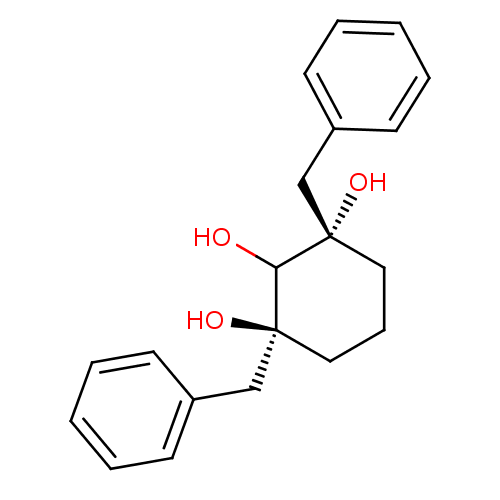
((1S,3S)-1,3-Dibenzyl-cyclohexane-1,2,3-triol | CHE...)Show InChI InChI=1S/C20H24O3/c21-18-19(22,14-16-8-3-1-4-9-16)12-7-13-20(18,23)15-17-10-5-2-6-11-17/h1-6,8-11,18,21-23H,7,12-15H2/t19-,20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of recombinant HIV-1 protease at 37 degrees C and pH of 6. |
Bioorg Med Chem Lett 3: 2717-2722 (1993)
Article DOI: 10.1016/S0960-894X(01)80749-6
BindingDB Entry DOI: 10.7270/Q2ZP4623 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50011972

(CHEMBL385189 | Sar-Arg-Val-Tyr-Ile-His-Pro-Thi-OH)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1cccs1)C(O)=O Show InChI InChI=1S/C47H69N13O10S/c1-6-27(4)39(44(67)56-34(21-29-23-51-25-53-29)45(68)60-18-8-12-36(60)42(65)57-35(46(69)70)22-31-10-9-19-71-31)59-41(64)33(20-28-13-15-30(61)16-14-28)55-43(66)38(26(2)3)58-40(63)32(54-37(62)24-50-5)11-7-17-52-47(48)49/h9-10,13-16,19,23,25-27,32-36,38-39,50,61H,6-8,11-12,17-18,20-22,24H2,1-5H3,(H,51,53)(H,54,62)(H,55,66)(H,56,67)(H,57,65)(H,58,63)(H,59,64)(H,69,70)(H4,48,49,52)/t27-,32-,33-,34-,35-,36-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of [125I]-AII specific binding towards angiotensin II receptor in rat mesenteric membranes. |
J Med Chem 34: 1514-7 (1991)
BindingDB Entry DOI: 10.7270/Q2TD9WBV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230790

(CHEMBL292892)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1[N+]([O-])=O)C(O)=O Show InChI InChI=1S/C23H23N3O6S/c1-2-3-6-21-24-13-18(10-17(23(29)30)11-19-5-4-9-33-19)25(21)14-16-8-7-15(22(27)28)12-20(16)26(31)32/h4-5,7-10,12-13H,2-3,6,11,14H2,1H3,(H,27,28)(H,29,30)/b17-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230779

(CHEMBL294686)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)c2nn[nH]n2)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N6O2S/c1-2-3-6-21-24-14-19(29(21)15-16-7-9-17(10-8-16)23(30)31)12-18(22-25-27-28-26-22)13-20-5-4-11-32-20/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,30,31)(H,25,26,27,28)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230811
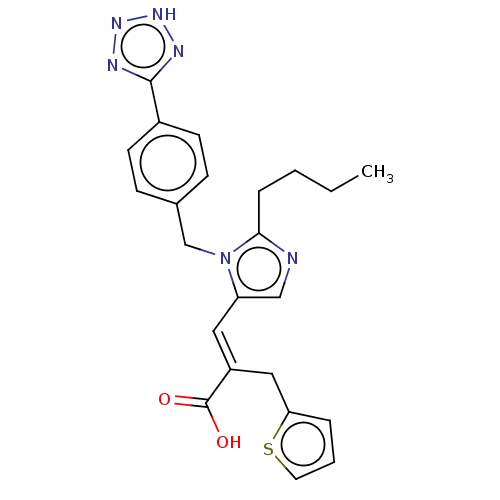
(CHEMBL293091)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)-c1nn[nH]n1 Show InChI InChI=1S/C23H24N6O2S/c1-2-3-6-21-24-14-19(12-18(23(30)31)13-20-5-4-11-32-20)29(21)15-16-7-9-17(10-8-16)22-25-27-28-26-22/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,30,31)(H,25,26,27,28)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230778
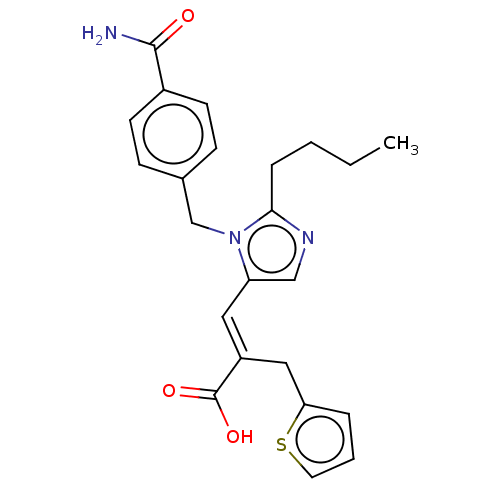
(CHEMBL56211)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(N)=O Show InChI InChI=1S/C23H25N3O3S/c1-2-3-6-21-25-14-19(12-18(23(28)29)13-20-5-4-11-30-20)26(21)15-16-7-9-17(10-8-16)22(24)27/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H2,24,27)(H,28,29)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230771
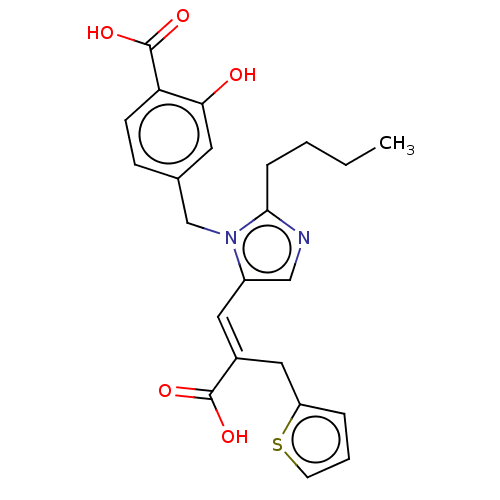
(CHEMBL55510)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(O)c1 Show InChI InChI=1S/C23H24N2O5S/c1-2-3-6-21-24-13-17(11-16(22(27)28)12-18-5-4-9-31-18)25(21)14-15-7-8-19(23(29)30)20(26)10-15/h4-5,7-11,13,26H,2-3,6,12,14H2,1H3,(H,27,28)(H,29,30)/b16-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230806

(CHEMBL294415)Show SMILES CCCCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H28N2O4S/c1-2-3-4-5-8-23-26-16-21(14-20(25(30)31)15-22-7-6-13-32-22)27(23)17-18-9-11-19(12-10-18)24(28)29/h6-7,9-14,16H,2-5,8,15,17H2,1H3,(H,28,29)(H,30,31)/b20-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50011977
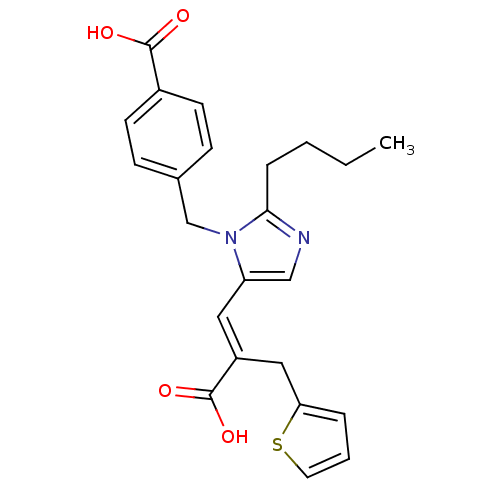
((E)-2-butyl-1-(p-carboxybenzyl)-alpha-2-thenylimid...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O4S/c1-2-3-6-21-24-14-19(12-18(23(28)29)13-20-5-4-11-30-20)25(21)15-16-7-9-17(10-8-16)22(26)27/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,26,27)(H,28,29)/b18-12+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of [125I]-AII specific binding towards Angiotensin II receptor in rat mesenteric membranes. |
J Med Chem 34: 1514-7 (1991)
BindingDB Entry DOI: 10.7270/Q2TD9WBV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50048078

(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(Cl)c1 Show InChI InChI=1S/C23H23ClN2O4S/c1-2-3-6-21-25-13-17(11-16(22(27)28)12-18-5-4-9-31-18)26(21)14-15-7-8-19(23(29)30)20(24)10-15/h4-5,7-11,13H,2-3,6,12,14H2,1H3,(H,27,28)(H,29,30)/b16-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50011973
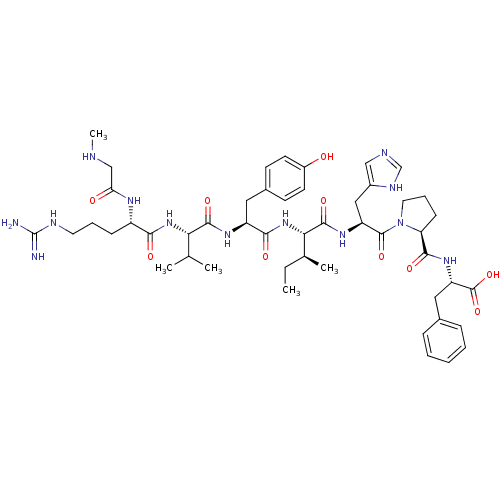
(CHEMBL216061 | Sar-Arg-Val-Tyr-Ile-His-Pro-Phe | S...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C49H71N13O10/c1-6-29(4)41(46(69)58-36(24-32-25-53-27-55-32)47(70)62-21-11-15-38(62)44(67)59-37(48(71)72)23-30-12-8-7-9-13-30)61-43(66)35(22-31-16-18-33(63)19-17-31)57-45(68)40(28(2)3)60-42(65)34(56-39(64)26-52-5)14-10-20-54-49(50)51/h7-9,12-13,16-19,25,27-29,34-38,40-41,52,63H,6,10-11,14-15,20-24,26H2,1-5H3,(H,53,55)(H,56,64)(H,57,68)(H,58,69)(H,59,67)(H,60,65)(H,61,66)(H,71,72)(H4,50,51,54)/t29-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of [125I]-AII specific binding towards Angiotensin II receptor in rat mesenteric membranes. |
J Med Chem 34: 1514-7 (1991)
BindingDB Entry DOI: 10.7270/Q2TD9WBV |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50011975

(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C23H23ClN2O4S/c1-2-3-6-21-25-13-18(10-17(23(29)30)11-19-5-4-9-31-19)26(21)14-16-8-7-15(22(27)28)12-20(16)24/h4-5,7-10,12-13H,2-3,6,11,14H2,1H3,(H,27,28)(H,29,30)/b17-10+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of [125I]-AII specific binding towards angiotensin II receptor in rat mesenteric membranes. |
J Med Chem 34: 1514-7 (1991)
BindingDB Entry DOI: 10.7270/Q2TD9WBV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50011975

(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C23H23ClN2O4S/c1-2-3-6-21-25-13-18(10-17(23(29)30)11-19-5-4-9-31-19)26(21)14-16-8-7-15(22(27)28)12-20(16)24/h4-5,7-10,12-13H,2-3,6,11,14H2,1H3,(H,27,28)(H,29,30)/b17-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230787

(CHEMBL54421)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(c1)-c1ccccc1 Show InChI InChI=1S/C29H28N2O4S/c1-2-3-11-27-30-18-23(16-22(28(32)33)17-24-10-7-14-36-24)31(27)19-20-12-13-25(29(34)35)26(15-20)21-8-5-4-6-9-21/h4-10,12-16,18H,2-3,11,17,19H2,1H3,(H,32,33)(H,34,35)/b22-16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230799
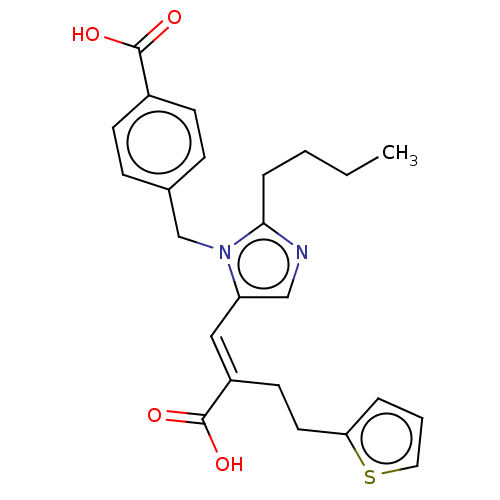
(CHEMBL56333)Show SMILES CCCCc1ncc(\C=C(/CCc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H26N2O4S/c1-2-3-6-22-25-15-20(14-19(24(29)30)11-12-21-5-4-13-31-21)26(22)16-17-7-9-18(10-8-17)23(27)28/h4-5,7-10,13-15H,2-3,6,11-12,16H2,1H3,(H,27,28)(H,29,30)/b19-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230792
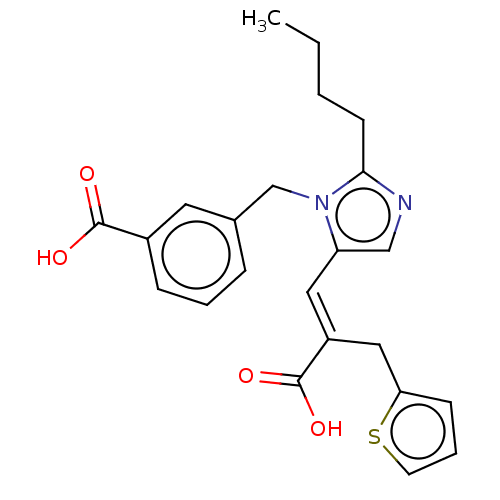
(CHEMBL56497)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1cccc(c1)C(O)=O Show InChI InChI=1S/C23H24N2O4S/c1-2-3-9-21-24-14-19(12-18(23(28)29)13-20-8-5-10-30-20)25(21)15-16-6-4-7-17(11-16)22(26)27/h4-8,10-12,14H,2-3,9,13,15H2,1H3,(H,26,27)(H,28,29)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230774
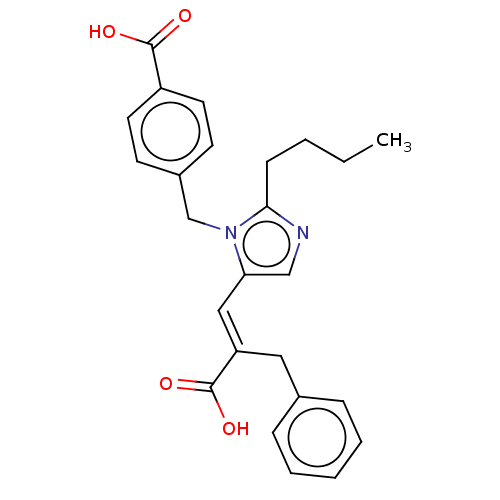
(CHEMBL291955)Show SMILES CCCCc1ncc(\C=C(/Cc2ccccc2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H26N2O4/c1-2-3-9-23-26-16-22(15-21(25(30)31)14-18-7-5-4-6-8-18)27(23)17-19-10-12-20(13-11-19)24(28)29/h4-8,10-13,15-16H,2-3,9,14,17H2,1H3,(H,28,29)(H,30,31)/b21-15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230762

(CHEMBL56160)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H25N3O4S2/c1-2-3-6-21-24-14-18(12-17(22(26)27)13-19-5-4-11-30-19)25(21)15-16-7-9-20(10-8-16)31(23,28)29/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,26,27)(H2,23,28,29)/b17-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230810
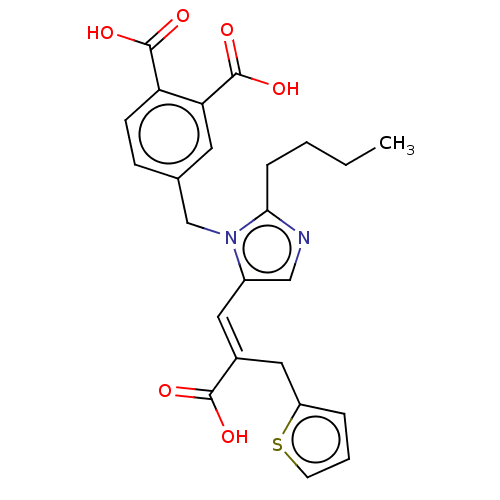
(CHEMBL299064)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C24H24N2O6S/c1-2-3-6-21-25-13-17(11-16(22(27)28)12-18-5-4-9-33-18)26(21)14-15-7-8-19(23(29)30)20(10-15)24(31)32/h4-5,7-11,13H,2-3,6,12,14H2,1H3,(H,27,28)(H,29,30)(H,31,32)/b16-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230764
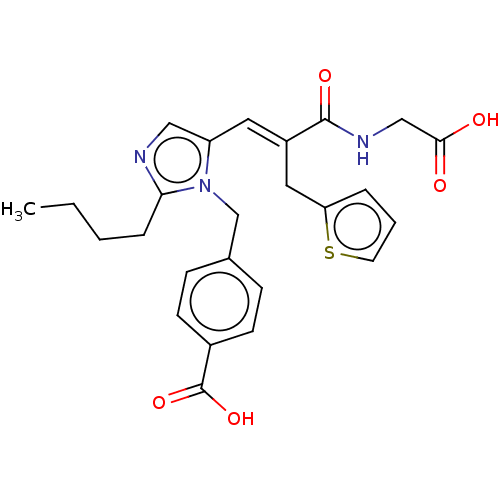
(CHEMBL299954)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(=O)NCC(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H27N3O5S/c1-2-3-6-22-26-14-20(28(22)16-17-7-9-18(10-8-17)25(32)33)12-19(13-21-5-4-11-34-21)24(31)27-15-23(29)30/h4-5,7-12,14H,2-3,6,13,15-16H2,1H3,(H,27,31)(H,29,30)(H,32,33)/b19-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230767
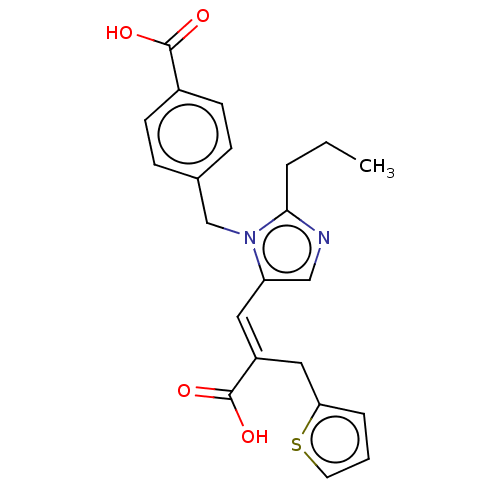
(CHEMBL56522)Show SMILES CCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H22N2O4S/c1-2-4-20-23-13-18(11-17(22(27)28)12-19-5-3-10-29-19)24(20)14-15-6-8-16(9-7-15)21(25)26/h3,5-11,13H,2,4,12,14H2,1H3,(H,25,26)(H,27,28)/b17-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230796

(CHEMBL56690)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C29H28N2O4S/c1-2-3-10-27-30-18-23(16-22(28(32)33)17-24-7-6-15-36-24)31(27)19-20-11-13-21(14-12-20)25-8-4-5-9-26(25)29(34)35/h4-9,11-16,18H,2-3,10,17,19H2,1H3,(H,32,33)(H,34,35)/b22-16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230772

(CHEMBL59043)Show SMILES CCCc1cccc(\C=C(/Cc2cccs2)C(O)=O)c1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H24O4S/c1-2-5-18-6-3-7-20(15-21(25(28)29)16-22-8-4-13-30-22)23(18)14-17-9-11-19(12-10-17)24(26)27/h3-4,6-13,15H,2,5,14,16H2,1H3,(H,26,27)(H,28,29)/b21-15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230805
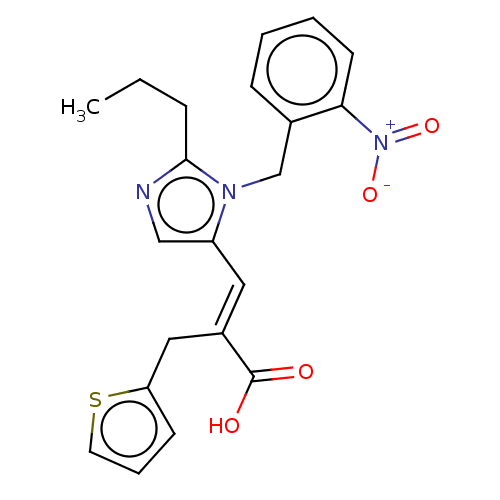
(CHEMBL54645)Show SMILES CCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccccc1[N+]([O-])=O Show InChI InChI=1S/C21H21N3O4S/c1-2-6-20-22-13-17(11-16(21(25)26)12-18-8-5-10-29-18)23(20)14-15-7-3-4-9-19(15)24(27)28/h3-5,7-11,13H,2,6,12,14H2,1H3,(H,25,26)/b16-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50011978
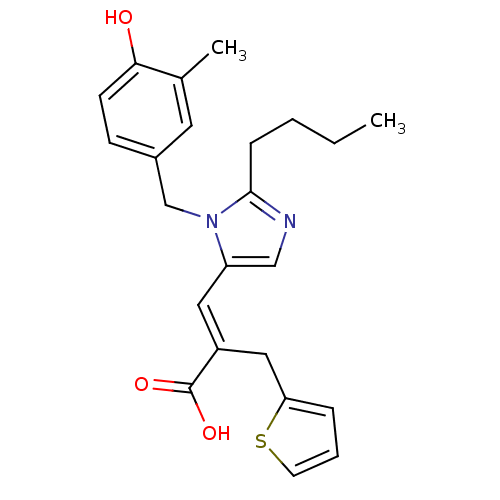
(3-[2-Butyl-3-(4-hydroxy-3-methyl-benzyl)-3H-imidaz...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(O)c(C)c1 Show InChI InChI=1S/C23H26N2O3S/c1-3-4-7-22-24-14-19(12-18(23(27)28)13-20-6-5-10-29-20)25(22)15-17-8-9-21(26)16(2)11-17/h5-6,8-12,14,26H,3-4,7,13,15H2,1-2H3,(H,27,28)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230785
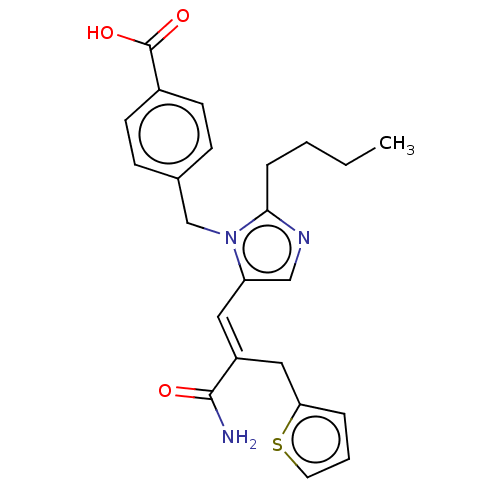
(CHEMBL300566)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(N)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H25N3O3S/c1-2-3-6-21-25-14-19(12-18(22(24)27)13-20-5-4-11-30-20)26(21)15-16-7-9-17(10-8-16)23(28)29/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H2,24,27)(H,28,29)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50011978

(3-[2-Butyl-3-(4-hydroxy-3-methyl-benzyl)-3H-imidaz...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(O)c(C)c1 Show InChI InChI=1S/C23H26N2O3S/c1-3-4-7-22-24-14-19(12-18(23(27)28)13-20-6-5-10-29-20)25(22)15-17-8-9-21(26)16(2)11-17/h5-6,8-12,14,26H,3-4,7,13,15H2,1-2H3,(H,27,28)/b18-12+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of [125I]-AII specific binding towards Angiotensin II receptor in rat mesenteric membranes. |
J Med Chem 34: 1514-7 (1991)
BindingDB Entry DOI: 10.7270/Q2TD9WBV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230783
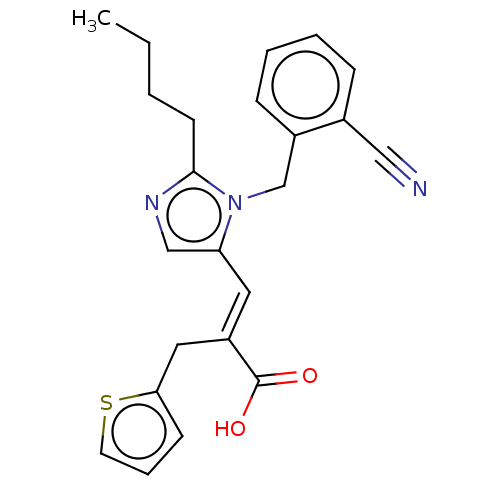
(CHEMBL54711)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccccc1C#N Show InChI InChI=1S/C23H23N3O2S/c1-2-3-10-22-25-15-20(12-19(23(27)28)13-21-9-6-11-29-21)26(22)16-18-8-5-4-7-17(18)14-24/h4-9,11-12,15H,2-3,10,13,16H2,1H3,(H,27,28)/b19-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230809

(CHEMBL57620)Show SMILES CCCC\C(=C/c1cnc(CCCC)n1Cc1ccc(cc1)C(O)=O)C(O)=O Show InChI InChI=1S/C22H28N2O4/c1-3-5-7-18(22(27)28)13-19-14-23-20(8-6-4-2)24(19)15-16-9-11-17(12-10-16)21(25)26/h9-14H,3-8,15H2,1-2H3,(H,25,26)(H,27,28)/b18-13+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230791
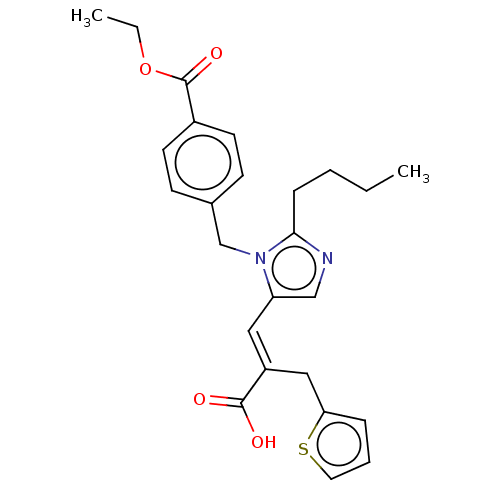
(CHEMBL55950)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(=O)OCC Show InChI InChI=1S/C25H28N2O4S/c1-3-5-8-23-26-16-21(14-20(24(28)29)15-22-7-6-13-32-22)27(23)17-18-9-11-19(12-10-18)25(30)31-4-2/h6-7,9-14,16H,3-5,8,15,17H2,1-2H3,(H,28,29)/b20-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50011974
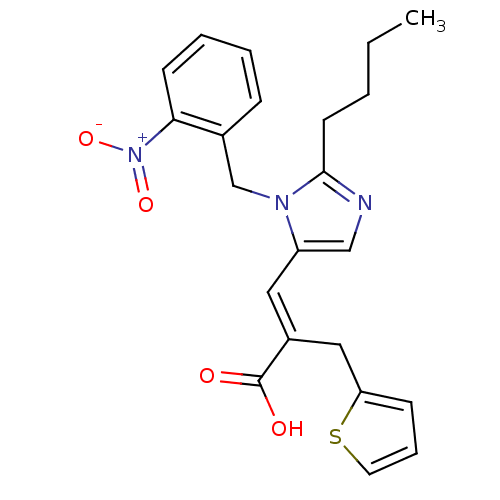
(3-[2-Butyl-3-(2-nitro-benzyl)-3H-imidazol-4-yl]-2-...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccccc1[N+]([O-])=O Show InChI InChI=1S/C22H23N3O4S/c1-2-3-10-21-23-14-18(12-17(22(26)27)13-19-8-6-11-30-19)24(21)15-16-7-4-5-9-20(16)25(28)29/h4-9,11-12,14H,2-3,10,13,15H2,1H3,(H,26,27)/b17-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50011974

(3-[2-Butyl-3-(2-nitro-benzyl)-3H-imidazol-4-yl]-2-...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccccc1[N+]([O-])=O Show InChI InChI=1S/C22H23N3O4S/c1-2-3-10-21-23-14-18(12-17(22(26)27)13-19-8-6-11-30-19)24(21)15-16-7-4-5-9-20(16)25(28)29/h4-9,11-12,14H,2-3,10,13,15H2,1H3,(H,26,27)/b17-12+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of [125I]-AII specific binding towards Angiotensin II receptor in rat mesenteric membranes. |
J Med Chem 34: 1514-7 (1991)
BindingDB Entry DOI: 10.7270/Q2TD9WBV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230795
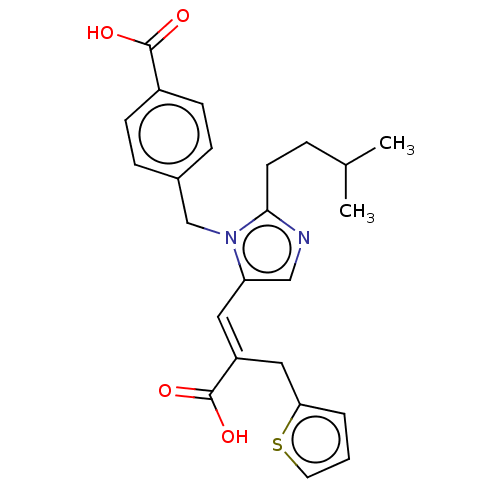
(CHEMBL59580)Show SMILES CC(C)CCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H26N2O4S/c1-16(2)5-10-22-25-14-20(12-19(24(29)30)13-21-4-3-11-31-21)26(22)15-17-6-8-18(9-7-17)23(27)28/h3-4,6-9,11-12,14,16H,5,10,13,15H2,1-2H3,(H,27,28)(H,29,30)/b19-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230781
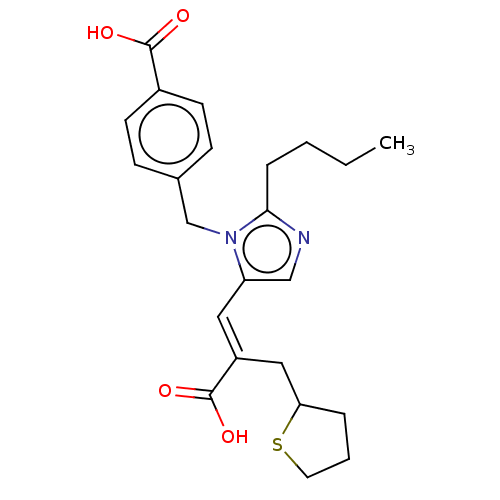
(CHEMBL291688)Show SMILES CCCCc1ncc(\C=C(/CC2CCCS2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H28N2O4S/c1-2-3-6-21-24-14-19(12-18(23(28)29)13-20-5-4-11-30-20)25(21)15-16-7-9-17(10-8-16)22(26)27/h7-10,12,14,20H,2-6,11,13,15H2,1H3,(H,26,27)(H,28,29)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50011971
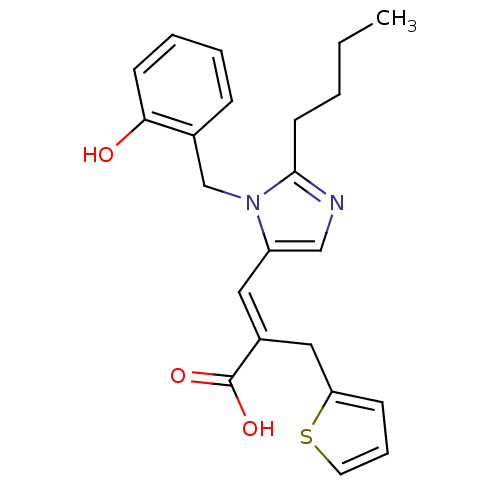
(3-[2-Butyl-3-(2-hydroxy-benzyl)-3H-imidazol-4-yl]-...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccccc1O Show InChI InChI=1S/C22H24N2O3S/c1-2-3-10-21-23-14-18(24(21)15-16-7-4-5-9-20(16)25)12-17(22(26)27)13-19-8-6-11-28-19/h4-9,11-12,14,25H,2-3,10,13,15H2,1H3,(H,26,27)/b17-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Bos taurus) | BDBM50014970
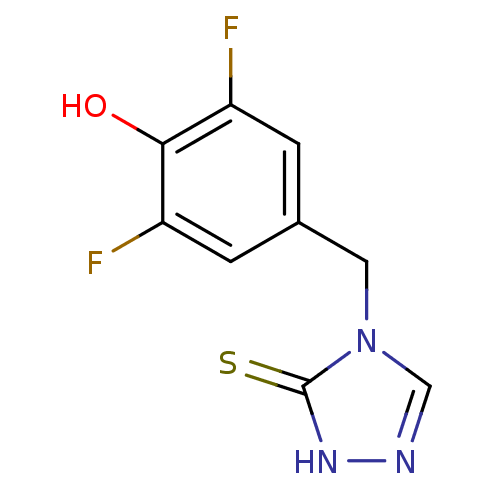
(4-(3,5-Difluoro-4-hydroxy-benzyl)-2,4-dihydro-[1,2...)Show InChI InChI=1S/C9H7F2N3OS/c10-6-1-5(2-7(11)8(6)15)3-14-4-12-13-9(14)16/h1-2,4,15H,3H2,(H,13,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against bovine dopamine beta-hydroxylase(DBH) |
J Med Chem 33: 781-9 (1990)
BindingDB Entry DOI: 10.7270/Q2DZ078G |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50048076
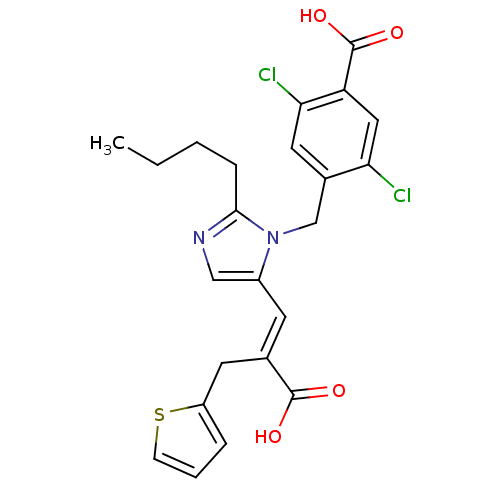
(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1cc(Cl)c(cc1Cl)C(O)=O Show InChI InChI=1S/C23H22Cl2N2O4S/c1-2-3-6-21-26-12-16(8-14(22(28)29)9-17-5-4-7-32-17)27(21)13-15-10-20(25)18(23(30)31)11-19(15)24/h4-5,7-8,10-12H,2-3,6,9,13H2,1H3,(H,28,29)(H,30,31)/b14-8+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230804
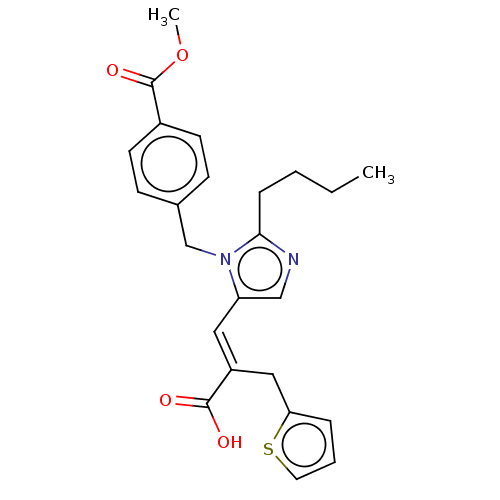
(CHEMBL293706)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(=O)OC Show InChI InChI=1S/C24H26N2O4S/c1-3-4-7-22-25-15-20(13-19(23(27)28)14-21-6-5-12-31-21)26(22)16-17-8-10-18(11-9-17)24(29)30-2/h5-6,8-13,15H,3-4,7,14,16H2,1-2H3,(H,27,28)/b19-13+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230770
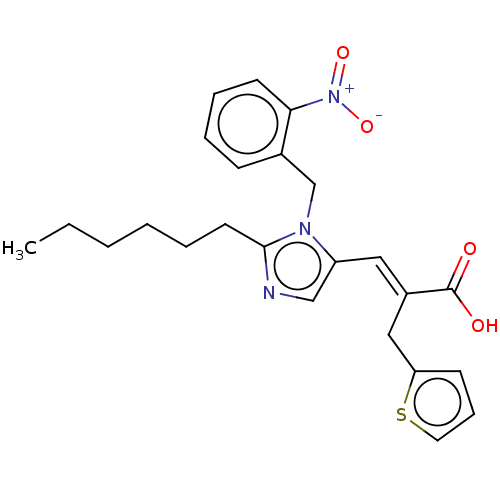
(CHEMBL55216)Show SMILES CCCCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccccc1[N+]([O-])=O Show InChI InChI=1S/C24H27N3O4S/c1-2-3-4-5-12-23-25-16-20(14-19(24(28)29)15-21-10-8-13-32-21)26(23)17-18-9-6-7-11-22(18)27(30)31/h6-11,13-14,16H,2-5,12,15,17H2,1H3,(H,28,29)/b19-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
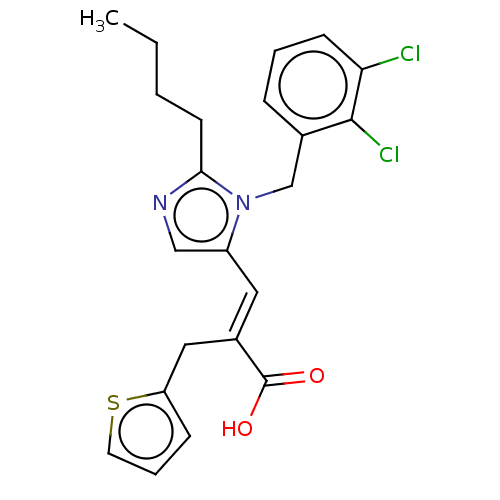
(RAT) | BDBM50230768
(CHEMBL417752)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1cccc(Cl)c1Cl Show InChI InChI=1S/C22H22Cl2N2O2S/c1-2-3-9-20-25-13-17(11-16(22(27)28)12-18-7-5-10-29-18)26(20)14-15-6-4-8-19(23)21(15)24/h4-8,10-11,13H,2-3,9,12,14H2,1H3,(H,27,28)/b16-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
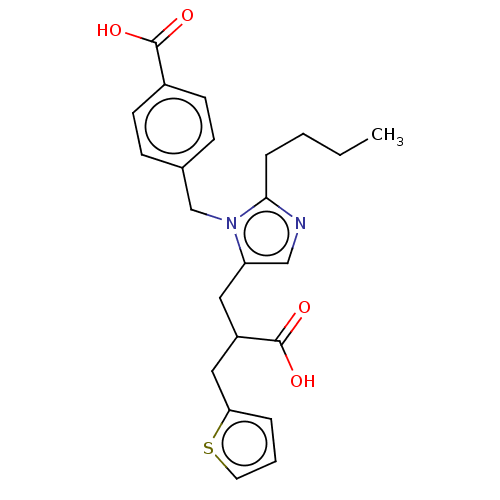
(RAT) | BDBM50230801
(CHEMBL301632)Show SMILES CCCCc1ncc(CC(Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H26N2O4S/c1-2-3-6-21-24-14-19(12-18(23(28)29)13-20-5-4-11-30-20)25(21)15-16-7-9-17(10-8-16)22(26)27/h4-5,7-11,14,18H,2-3,6,12-13,15H2,1H3,(H,26,27)(H,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230088

(CHEMBL125786)Show SMILES CCCCc1ncc(\C=C(/Cc2ccc(O)c(O)c2)C(O)=O)n1Cc1ccccc1Cl Show InChI InChI=1S/C24H25ClN2O4/c1-2-3-8-23-26-14-19(27(23)15-17-6-4-5-7-20(17)25)13-18(24(30)31)11-16-9-10-21(28)22(29)12-16/h4-7,9-10,12-14,28-29H,2-3,8,11,15H2,1H3,(H,30,31)/b18-13+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II |
J Med Chem 35: 3858-72 (1992)
BindingDB Entry DOI: 10.7270/Q23T9G4T |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230769
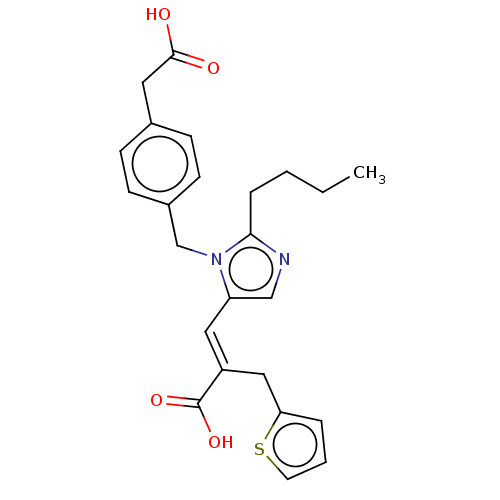
(CHEMBL56085)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C24H26N2O4S/c1-2-3-6-22-25-15-20(13-19(24(29)30)14-21-5-4-11-31-21)26(22)16-18-9-7-17(8-10-18)12-23(27)28/h4-5,7-11,13,15H,2-3,6,12,14,16H2,1H3,(H,27,28)(H,29,30)/b19-13+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data