 Found 40 hits with Last Name = 'fischer' and Initial = 'ds'
Found 40 hits with Last Name = 'fischer' and Initial = 'ds' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50370656
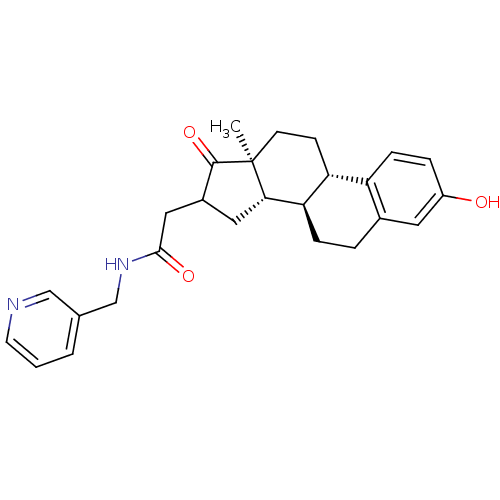
(CHEMBL1627749)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC(CC(=O)NCc1cccnc1)C2=O Show InChI InChI=1S/C26H30N2O3/c1-26-9-8-21-20-7-5-19(29)11-17(20)4-6-22(21)23(26)12-18(25(26)31)13-24(30)28-15-16-3-2-10-27-14-16/h2-3,5,7,10-11,14,18,21-23,29H,4,6,8-9,12-13,15H2,1H3,(H,28,30)/t18?,21-,22-,23+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in T47D cells |
J Med Chem 49: 1325-45 (2006)
Article DOI: 10.1021/jm050830t
BindingDB Entry DOI: 10.7270/Q2C24X7J |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50172493

((13S)-3-hydroxy-16-(hydroxymethylene)-13-methyl-7,...)Show InChI InChI=1S/C19H22O3/c1-19-7-6-15-14-5-3-13(21)8-11(14)2-4-16(15)17(19)9-12(10-20)18(19)22/h3,5,8,10,12,15-17,21H,2,4,6-7,9H2,1H3/t12?,15?,16?,17?,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estrone |
J Med Chem 48: 5749-70 (2005)
Article DOI: 10.1021/jm050348a
BindingDB Entry DOI: 10.7270/Q2QJ7J38 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50172493

((13S)-3-hydroxy-16-(hydroxymethylene)-13-methyl-7,...)Show InChI InChI=1S/C19H22O3/c1-19-7-6-15-14-5-3-13(21)8-11(14)2-4-16(15)17(19)9-12(10-20)18(19)22/h3,5,8,10,12,15-17,21H,2,4,6-7,9H2,1H3/t12?,15?,16?,17?,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in T47D cells |
J Med Chem 49: 1325-45 (2006)
Article DOI: 10.1021/jm050830t
BindingDB Entry DOI: 10.7270/Q2C24X7J |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50172503
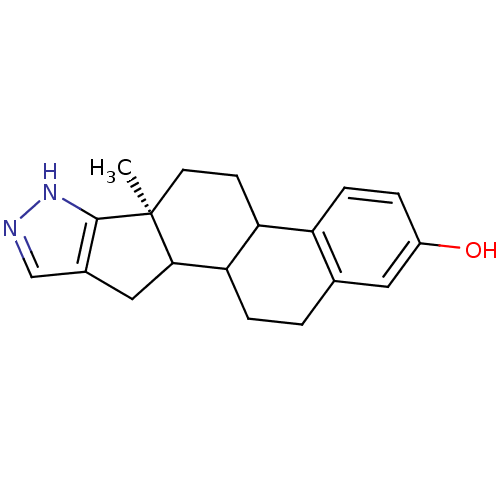
((S)-6a-Methyl-4b,5,6,6a,8,10,10a,10b,11,12-decahyd...)Show InChI InChI=1S/C19H22N2O/c1-19-7-6-15-14-5-3-13(22)8-11(14)2-4-16(15)17(19)9-12-10-20-21-18(12)19/h3,5,8,10,15-17,22H,2,4,6-7,9H2,1H3,(H,20,21)/t15?,16?,17?,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in T47D cells |
J Med Chem 49: 1325-45 (2006)
Article DOI: 10.1021/jm050830t
BindingDB Entry DOI: 10.7270/Q2C24X7J |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50172503

((S)-6a-Methyl-4b,5,6,6a,8,10,10a,10b,11,12-decahyd...)Show InChI InChI=1S/C19H22N2O/c1-19-7-6-15-14-5-3-13(22)8-11(14)2-4-16(15)17(19)9-12-10-20-21-18(12)19/h3,5,8,10,15-17,22H,2,4,6-7,9H2,1H3,(H,20,21)/t15?,16?,17?,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estrone |
J Med Chem 48: 5749-70 (2005)
Article DOI: 10.1021/jm050348a
BindingDB Entry DOI: 10.7270/Q2QJ7J38 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50172504

((S)-2-Hydroxy-6a-methyl-4b,5,6,6a,8,10,10a,10b,11,...)Show SMILES C[C@]12CCC3C(CCc4cc(O)ccc34)C1Cc1c(n[nH]c21)C(=O)NCCc1cccnc1 Show InChI InChI=1S/C27H30N4O2/c1-27-10-8-20-19-7-5-18(32)13-17(19)4-6-21(20)23(27)14-22-24(30-31-25(22)27)26(33)29-12-9-16-3-2-11-28-15-16/h2-3,5,7,11,13,15,20-21,23,32H,4,6,8-10,12,14H2,1H3,(H,29,33)(H,30,31)/t20?,21?,23?,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estrone |
J Med Chem 48: 5749-70 (2005)
Article DOI: 10.1021/jm050348a
BindingDB Entry DOI: 10.7270/Q2QJ7J38 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50370701
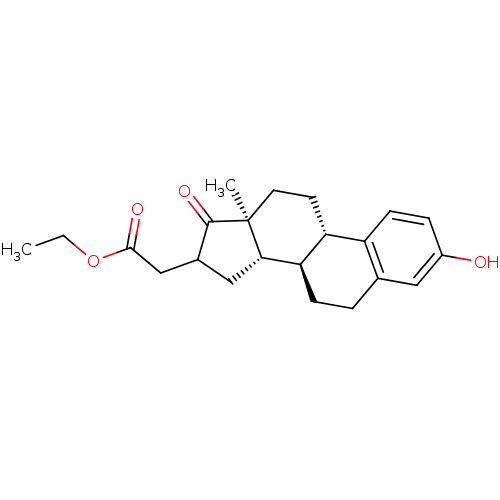
(CHEMBL1627773)Show SMILES CCOC(=O)CC1C[C@H]2[C@@H]3CCc4cc(O)ccc4[C@H]3CC[C@]2(C)C1=O Show InChI InChI=1S/C22H28O4/c1-3-26-20(24)12-14-11-19-18-6-4-13-10-15(23)5-7-16(13)17(18)8-9-22(19,2)21(14)25/h5,7,10,14,17-19,23H,3-4,6,8-9,11-12H2,1-2H3/t14?,17-,18-,19+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in T47D cells |
J Med Chem 49: 1325-45 (2006)
Article DOI: 10.1021/jm050830t
BindingDB Entry DOI: 10.7270/Q2C24X7J |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50370702
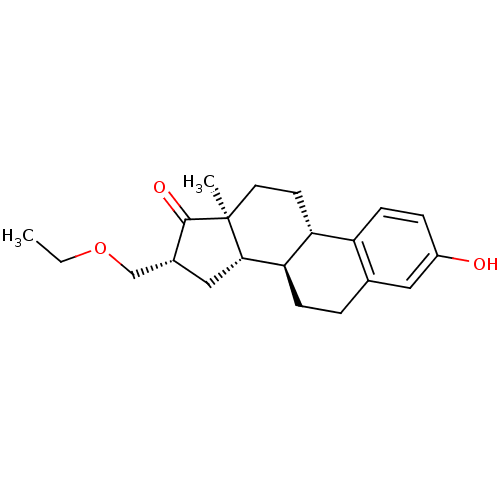
(CHEMBL1627790)Show SMILES CCOC[C@H]1C[C@H]2[C@@H]3CCc4cc(O)ccc4[C@H]3CC[C@]2(C)C1=O Show InChI InChI=1S/C21H28O3/c1-3-24-12-14-11-19-18-6-4-13-10-15(22)5-7-16(13)17(18)8-9-21(19,2)20(14)23/h5,7,10,14,17-19,22H,3-4,6,8-9,11-12H2,1-2H3/t14-,17-,18-,19+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in T47D cells |
J Med Chem 49: 1325-45 (2006)
Article DOI: 10.1021/jm050830t
BindingDB Entry DOI: 10.7270/Q2C24X7J |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM17289

((1S,10R,11S,15S)-5-hydroxy-15-methyltetracyclo[8.7...)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in T47D cells |
J Med Chem 49: 1325-45 (2006)
Article DOI: 10.1021/jm050830t
BindingDB Entry DOI: 10.7270/Q2C24X7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50370698
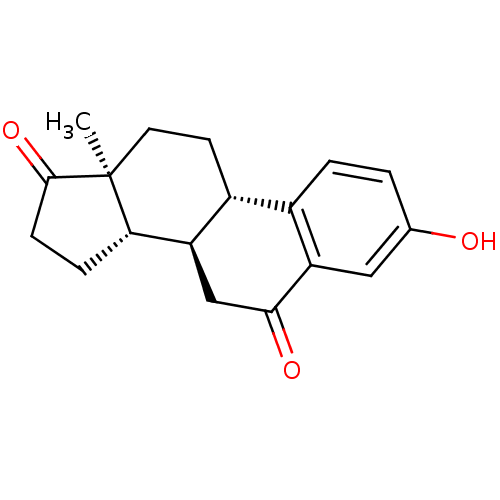
(CHEMBL1628005)Show SMILES C[C@]12CC[C@H]3[C@@H](CC(=O)c4cc(O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H20O3/c1-18-7-6-12-11-3-2-10(19)8-14(11)16(20)9-13(12)15(18)4-5-17(18)21/h2-3,8,12-13,15,19H,4-7,9H2,1H3/t12-,13-,15+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in T47D cells |
J Med Chem 49: 1325-45 (2006)
Article DOI: 10.1021/jm050830t
BindingDB Entry DOI: 10.7270/Q2C24X7J |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50370695

(CHEMBL1628021)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC(CC(=O)NCC1CCCO1)C2=O Show InChI InChI=1S/C25H33NO4/c1-25-9-8-20-19-7-5-17(27)11-15(19)4-6-21(20)22(25)12-16(24(25)29)13-23(28)26-14-18-3-2-10-30-18/h5,7,11,16,18,20-22,27H,2-4,6,8-10,12-14H2,1H3,(H,26,28)/t16?,18?,20-,21-,22+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in T47D cells |
J Med Chem 49: 1325-45 (2006)
Article DOI: 10.1021/jm050830t
BindingDB Entry DOI: 10.7270/Q2C24X7J |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50366344
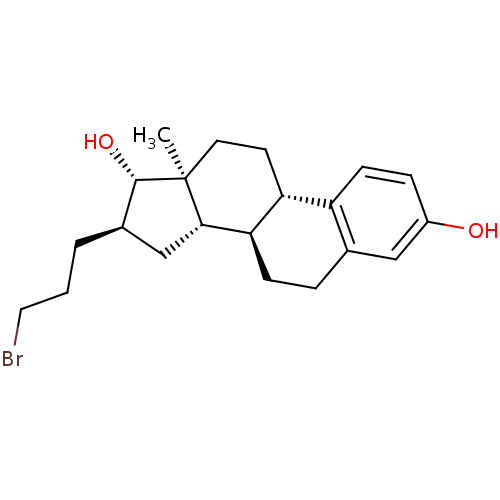
(CHEMBL1627450)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1C[C@@H](CCCBr)[C@@H]2O Show InChI InChI=1S/C21H29BrO2/c1-21-9-8-17-16-7-5-15(23)11-13(16)4-6-18(17)19(21)12-14(20(21)24)3-2-10-22/h5,7,11,14,17-20,23-24H,2-4,6,8-10,12H2,1H3/t14-,17-,18-,19+,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estrone |
J Med Chem 48: 5749-70 (2005)
Article DOI: 10.1021/jm050348a
BindingDB Entry DOI: 10.7270/Q2QJ7J38 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50182496

(2-hydroxy-6a-methyl-5,6,6a,8,10,10a,10b,11-octahyd...)Show SMILES C[C@]12CCC3C(CC(=O)c4cc(O)ccc34)C1Cc1cn[nH]c21 Show InChI InChI=1S/C19H20N2O2/c1-19-5-4-13-12-3-2-11(22)7-15(12)17(23)8-14(13)16(19)6-10-9-20-21-18(10)19/h2-3,7,9,13-14,16,22H,4-6,8H2,1H3,(H,20,21)/t13?,14?,16?,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in T47D cells |
J Med Chem 49: 1325-45 (2006)
Article DOI: 10.1021/jm050830t
BindingDB Entry DOI: 10.7270/Q2C24X7J |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50370696
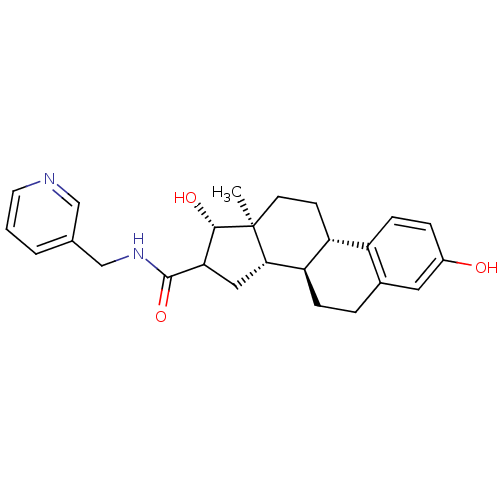
(CHEMBL1627763)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC([C@@H]2O)C(=O)NCc1cccnc1 Show InChI InChI=1S/C25H30N2O3/c1-25-9-8-19-18-7-5-17(28)11-16(18)4-6-20(19)22(25)12-21(23(25)29)24(30)27-14-15-3-2-10-26-13-15/h2-3,5,7,10-11,13,19-23,28-29H,4,6,8-9,12,14H2,1H3,(H,27,30)/t19-,20-,21?,22+,23+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in T47D cells |
J Med Chem 49: 1325-45 (2006)
Article DOI: 10.1021/jm050830t
BindingDB Entry DOI: 10.7270/Q2C24X7J |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50172495
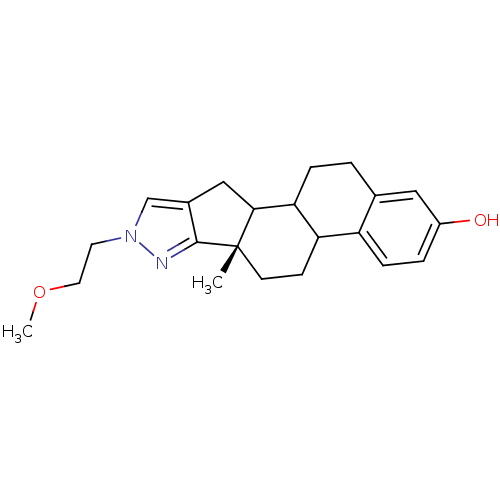
((S)-8-(2-Methoxy-ethyl)-6a-methyl-4b,5,6,6a,8,10,1...)Show SMILES COCCn1cc2CC3C4CCc5cc(O)ccc5C4CC[C@]3(C)c2n1 Show InChI InChI=1S/C22H28N2O2/c1-22-8-7-18-17-6-4-16(25)11-14(17)3-5-19(18)20(22)12-15-13-24(9-10-26-2)23-21(15)22/h4,6,11,13,18-20,25H,3,5,7-10,12H2,1-2H3/t18?,19?,20?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estrone |
J Med Chem 48: 5749-70 (2005)
Article DOI: 10.1021/jm050348a
BindingDB Entry DOI: 10.7270/Q2QJ7J38 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50172499

(3-((S)-2-Hydroxy-6a-methyl-4b,6,6a,10,10a,10b,11,1...)Show SMILES C[C@]12CCC3C(CCc4cc(O)ccc34)C1Cc1cn(CCC#N)nc21 Show InChI InChI=1S/C22H25N3O/c1-22-8-7-18-17-6-4-16(26)11-14(17)3-5-19(18)20(22)12-15-13-25(10-2-9-23)24-21(15)22/h4,6,11,13,18-20,26H,2-3,5,7-8,10,12H2,1H3/t18?,19?,20?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estrone |
J Med Chem 48: 5749-70 (2005)
Article DOI: 10.1021/jm050348a
BindingDB Entry DOI: 10.7270/Q2QJ7J38 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50172496
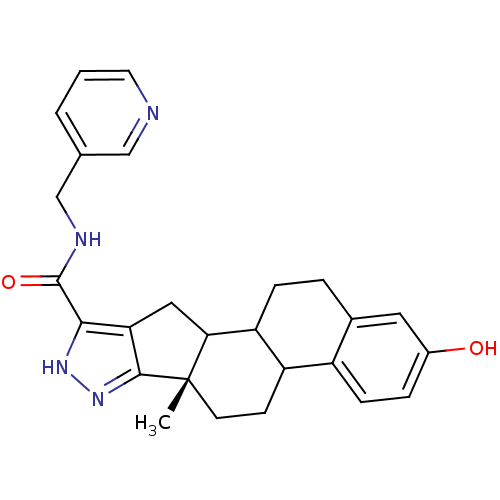
((S)-2-Hydroxy-6a-methyl-4b,5,6,6a,8,10,10a,10b,11,...)Show SMILES C[C@]12CCC3C(CCc4cc(O)ccc34)C1Cc1c([nH]nc21)C(=O)NCc1cccnc1 Show InChI InChI=1S/C26H28N4O2/c1-26-9-8-19-18-7-5-17(31)11-16(18)4-6-20(19)22(26)12-21-23(29-30-24(21)26)25(32)28-14-15-3-2-10-27-13-15/h2-3,5,7,10-11,13,19-20,22,31H,4,6,8-9,12,14H2,1H3,(H,28,32)(H,29,30)/t19?,20?,22?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estrone |
J Med Chem 48: 5749-70 (2005)
Article DOI: 10.1021/jm050348a
BindingDB Entry DOI: 10.7270/Q2QJ7J38 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50370703
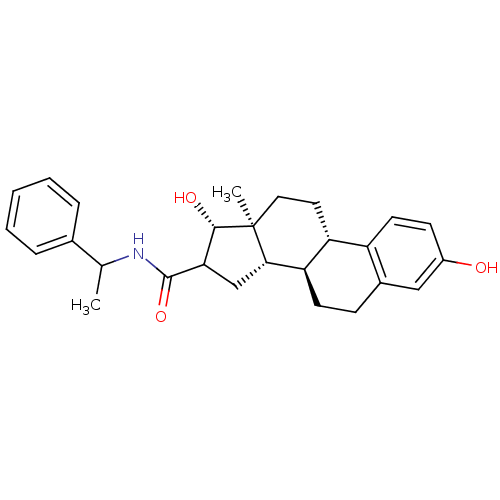
(CHEMBL1627771)Show SMILES CC(NC(=O)C1C[C@H]2[C@@H]3CCc4cc(O)ccc4[C@H]3CC[C@]2(C)[C@H]1O)c1ccccc1 Show InChI InChI=1S/C27H33NO3/c1-16(17-6-4-3-5-7-17)28-26(31)23-15-24-22-10-8-18-14-19(29)9-11-20(18)21(22)12-13-27(24,2)25(23)30/h3-7,9,11,14,16,21-25,29-30H,8,10,12-13,15H2,1-2H3,(H,28,31)/t16?,21-,22-,23?,24+,25+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in T47D cells |
J Med Chem 49: 1325-45 (2006)
Article DOI: 10.1021/jm050830t
BindingDB Entry DOI: 10.7270/Q2C24X7J |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50172500

((S)-2-Hydroxy-6a-methyl-4b,5,6,6a,8,10,10a,10b,11,...)Show SMILES C[C@]12CCC3C(CCc4cc(O)ccc34)C1Cc1c([nH]nc21)C(=O)NCc1ccccn1 Show InChI InChI=1S/C26H28N4O2/c1-26-10-9-19-18-8-6-17(31)12-15(18)5-7-20(19)22(26)13-21-23(29-30-24(21)26)25(32)28-14-16-4-2-3-11-27-16/h2-4,6,8,11-12,19-20,22,31H,5,7,9-10,13-14H2,1H3,(H,28,32)(H,29,30)/t19?,20?,22?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estrone |
J Med Chem 48: 5749-70 (2005)
Article DOI: 10.1021/jm050348a
BindingDB Entry DOI: 10.7270/Q2QJ7J38 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50172494

(((S)-2-Hydroxy-6a-methyl-4b,6,6a,10,10a,10b,11,12-...)Show SMILES COC(=O)Cn1cc2CC3C4CCc5cc(O)ccc5C4CC[C@]3(C)c2n1 Show InChI InChI=1S/C22H26N2O3/c1-22-8-7-17-16-6-4-15(25)9-13(16)3-5-18(17)19(22)10-14-11-24(23-21(14)22)12-20(26)27-2/h4,6,9,11,17-19,25H,3,5,7-8,10,12H2,1-2H3/t17?,18?,19?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estrone |
J Med Chem 48: 5749-70 (2005)
Article DOI: 10.1021/jm050348a
BindingDB Entry DOI: 10.7270/Q2QJ7J38 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50172502

((S)-9-Hydroxymethyl-6a-methyl-4b,5,6,6a,8,10,10a,1...)Show SMILES C[C@]12CCC3C(CCc4cc(O)ccc34)C1Cc1c(CO)n[nH]c21 Show InChI InChI=1S/C20H24N2O2/c1-20-7-6-14-13-5-3-12(24)8-11(13)2-4-15(14)17(20)9-16-18(10-23)21-22-19(16)20/h3,5,8,14-15,17,23-24H,2,4,6-7,9-10H2,1H3,(H,21,22)/t14?,15?,17?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estrone |
J Med Chem 48: 5749-70 (2005)
Article DOI: 10.1021/jm050348a
BindingDB Entry DOI: 10.7270/Q2QJ7J38 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50370664

(CHEMBL1627756)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC(N=O)C2=O Show InChI InChI=1S/C18H21NO3/c1-18-7-6-13-12-5-3-11(20)8-10(12)2-4-14(13)15(18)9-16(19-22)17(18)21/h3,5,8,13-16,20H,2,4,6-7,9H2,1H3/t13-,14-,15+,16?,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in T47D cells |
J Med Chem 49: 1325-45 (2006)
Article DOI: 10.1021/jm050830t
BindingDB Entry DOI: 10.7270/Q2C24X7J |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50370664

(CHEMBL1627756)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC(N=O)C2=O Show InChI InChI=1S/C18H21NO3/c1-18-7-6-13-12-5-3-11(20)8-10(12)2-4-14(13)15(18)9-16(19-22)17(18)21/h3,5,8,13-16,20H,2,4,6-7,9H2,1H3/t13-,14-,15+,16?,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estrone |
J Med Chem 48: 5749-70 (2005)
Article DOI: 10.1021/jm050348a
BindingDB Entry DOI: 10.7270/Q2QJ7J38 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50370700
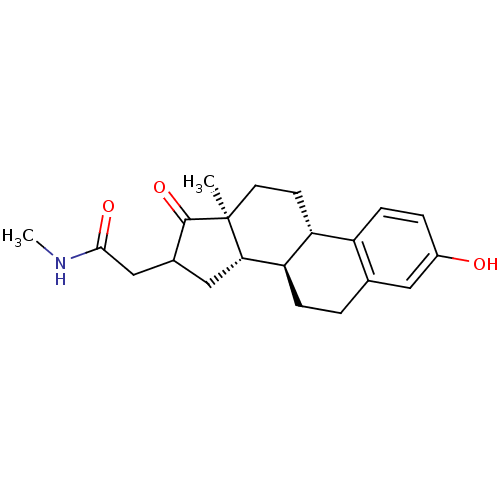
(CHEMBL1628135)Show SMILES CNC(=O)CC1C[C@H]2[C@@H]3CCc4cc(O)ccc4[C@H]3CC[C@]2(C)C1=O Show InChI InChI=1S/C21H27NO3/c1-21-8-7-16-15-6-4-14(23)9-12(15)3-5-17(16)18(21)10-13(20(21)25)11-19(24)22-2/h4,6,9,13,16-18,23H,3,5,7-8,10-11H2,1-2H3,(H,22,24)/t13?,16-,17-,18+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in T47D cells |
J Med Chem 49: 1325-45 (2006)
Article DOI: 10.1021/jm050830t
BindingDB Entry DOI: 10.7270/Q2C24X7J |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50172491

((S)-2-Hydroxy-6a-methyl-4b,5,6,6a,8,10,10a,10b,11,...)Show SMILES CCOC(=O)c1n[nH]c2c1CC1C3CCc4cc(O)ccc4C3CC[C@]21C Show InChI InChI=1S/C22H26N2O3/c1-3-27-21(26)19-17-11-18-16-6-4-12-10-13(25)5-7-14(12)15(16)8-9-22(18,2)20(17)24-23-19/h5,7,10,15-16,18,25H,3-4,6,8-9,11H2,1-2H3,(H,23,24)/t15?,16?,18?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estrone |
J Med Chem 48: 5749-70 (2005)
Article DOI: 10.1021/jm050348a
BindingDB Entry DOI: 10.7270/Q2QJ7J38 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50370699
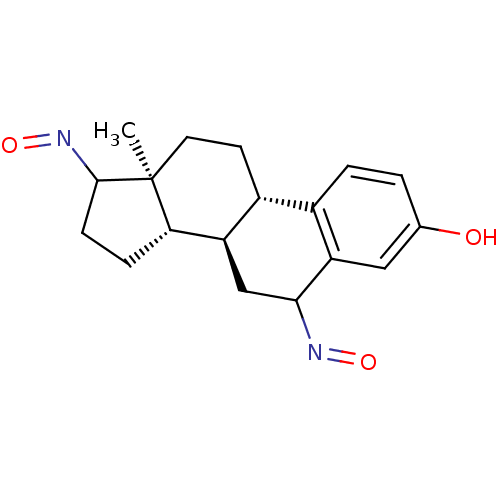
(CHEMBL1627779)Show SMILES C[C@]12CC[C@H]3[C@@H](CC(N=O)c4cc(O)ccc34)[C@@H]1CCC2N=O Show InChI InChI=1S/C18H22N2O3/c1-18-7-6-12-11-3-2-10(21)8-14(11)16(19-22)9-13(12)15(18)4-5-17(18)20-23/h2-3,8,12-13,15-17,21H,4-7,9H2,1H3/t12-,13-,15+,16?,17?,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in T47D cells |
J Med Chem 49: 1325-45 (2006)
Article DOI: 10.1021/jm050830t
BindingDB Entry DOI: 10.7270/Q2C24X7J |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50172497
((S)-2-Hydroxy-6a-methyl-4b,5,6,6a,8,10,10a,10b,11,...)Show SMILES Cn1cccc1CNC(=O)c1[nH]nc2c1CC1C3CCc4cc(O)ccc4C3CC[C@]21C Show InChI InChI=1S/C26H30N4O2/c1-26-10-9-19-18-8-6-17(31)12-15(18)5-7-20(19)22(26)13-21-23(28-29-24(21)26)25(32)27-14-16-4-3-11-30(16)2/h3-4,6,8,11-12,19-20,22,31H,5,7,9-10,13-14H2,1-2H3,(H,27,32)(H,28,29)/t19?,20?,22?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estrone |
J Med Chem 48: 5749-70 (2005)
Article DOI: 10.1021/jm050348a
BindingDB Entry DOI: 10.7270/Q2QJ7J38 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50172501

((S)-6a,8-Dimethyl-4b,5,6,6a,8,10,10a,10b,11,12-dec...)Show InChI InChI=1S/C20H24N2O/c1-20-8-7-16-15-6-4-14(23)9-12(15)3-5-17(16)18(20)10-13-11-22(2)21-19(13)20/h4,6,9,11,16-18,23H,3,5,7-8,10H2,1-2H3/t16?,17?,18?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estrone |
J Med Chem 48: 5749-70 (2005)
Article DOI: 10.1021/jm050348a
BindingDB Entry DOI: 10.7270/Q2QJ7J38 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50370697
(CHEMBL1628134)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1C\C(=C/c1ccncc1)[C@@H]2O Show InChI InChI=1S/C24H27NO2/c1-24-9-6-20-19-5-3-18(26)13-16(19)2-4-21(20)22(24)14-17(23(24)27)12-15-7-10-25-11-8-15/h3,5,7-8,10-13,20-23,26-27H,2,4,6,9,14H2,1H3/b17-12+/t20-,21-,22+,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in T47D cells |
J Med Chem 49: 1325-45 (2006)
Article DOI: 10.1021/jm050830t
BindingDB Entry DOI: 10.7270/Q2C24X7J |
More data for this
Ligand-Target Pair | |
Penicillin-binding protein 2x
(Streptococcus pneumoniae) | BDBM50324696
(CHEMBL1221990 | Phenoxyacetyl lactivicin)Show SMILES OC(=O)[C@@]1(CCC(=O)O1)N1OC[C@H](NC(=O)COc2ccccc2)C1=O |r| Show InChI InChI=1S/C16H16N2O8/c19-12(9-24-10-4-2-1-3-5-10)17-11-8-25-18(14(11)21)16(15(22)23)7-6-13(20)26-16/h1-5,11H,6-9H2,(H,17,19)(H,22,23)/t11-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Joseph Fourier
Curated by ChEMBL
| Assay Description
Inhibition of penicillin-resistant Streptococcus pneumoniae 5204 PBP2x after 60 mins by SDS-PAGE |
Nat Chem Biol 3: 565-9 (2007)
Article DOI: 10.1038/nchembio.2007.21
BindingDB Entry DOI: 10.7270/Q2JM2BK9 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50370663
(CHEMBL1627759)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC(C#N)C2=O Show InChI InChI=1S/C19H21NO2/c1-19-7-6-15-14-5-3-13(21)8-11(14)2-4-16(15)17(19)9-12(10-20)18(19)22/h3,5,8,12,15-17,21H,2,4,6-7,9H2,1H3/t12?,15-,16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estrone |
J Med Chem 48: 5749-70 (2005)
Article DOI: 10.1021/jm050348a
BindingDB Entry DOI: 10.7270/Q2QJ7J38 |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50300661
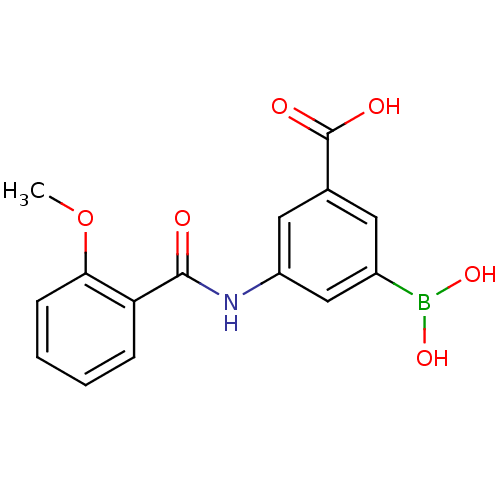
(3-borono-5-(2-methoxybenzamido)benzoic acid | CHEM...)Show InChI InChI=1S/C15H14BNO6/c1-23-13-5-3-2-4-12(13)14(18)17-11-7-9(15(19)20)6-10(8-11)16(21)22/h2-8,21-22H,1H3,(H,17,18)(H,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p... |
J Med Chem 52: 6097-106 (2009)
Article DOI: 10.1021/jm9009718
BindingDB Entry DOI: 10.7270/Q2G73DSS |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50300664
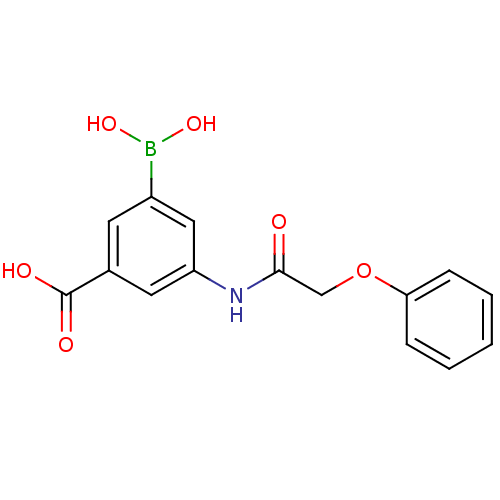
(3-borono-5-(2-phenoxyacetamido)benzoic acid | CHEM...)Show InChI InChI=1S/C15H14BNO6/c18-14(9-23-13-4-2-1-3-5-13)17-12-7-10(15(19)20)6-11(8-12)16(21)22/h1-8,21-22H,9H2,(H,17,18)(H,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p... |
J Med Chem 52: 6097-106 (2009)
Article DOI: 10.1021/jm9009718
BindingDB Entry DOI: 10.7270/Q2G73DSS |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50300662

(3-borono-5-(thiophene-2-carboxamido)benzoic acid |...)Show InChI InChI=1S/C12H10BNO5S/c15-11(10-2-1-3-20-10)14-9-5-7(12(16)17)4-8(6-9)13(18)19/h1-6,18-19H,(H,14,15)(H,16,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p... |
J Med Chem 52: 6097-106 (2009)
Article DOI: 10.1021/jm9009718
BindingDB Entry DOI: 10.7270/Q2G73DSS |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50300660
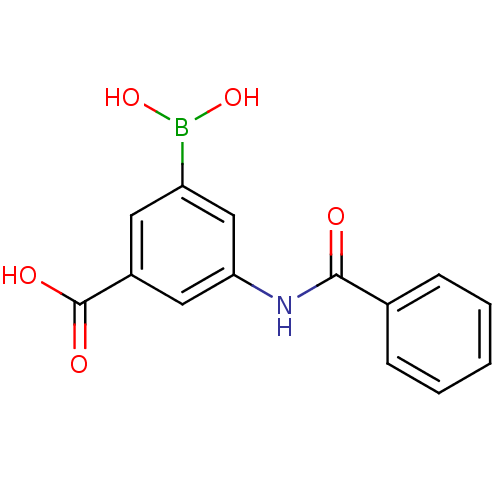
(3-benzamido-5-boronobenzoic acid | CHEMBL575719)Show InChI InChI=1S/C14H12BNO5/c17-13(9-4-2-1-3-5-9)16-12-7-10(14(18)19)6-11(8-12)15(20)21/h1-8,20-21H,(H,16,17)(H,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p... |
J Med Chem 52: 6097-106 (2009)
Article DOI: 10.1021/jm9009718
BindingDB Entry DOI: 10.7270/Q2G73DSS |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50300663
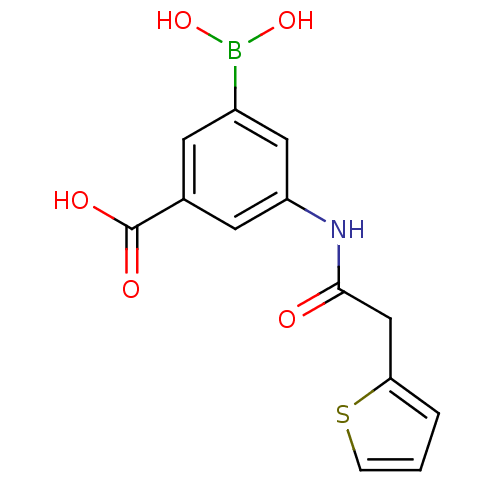
(3-borono-5-(2-(thiophen-2-yl)acetamido)benzoic aci...)Show InChI InChI=1S/C13H12BNO5S/c16-12(7-11-2-1-3-21-11)15-10-5-8(13(17)18)4-9(6-10)14(19)20/h1-6,19-20H,7H2,(H,15,16)(H,17,18) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p... |
J Med Chem 52: 6097-106 (2009)
Article DOI: 10.1021/jm9009718
BindingDB Entry DOI: 10.7270/Q2G73DSS |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50300659
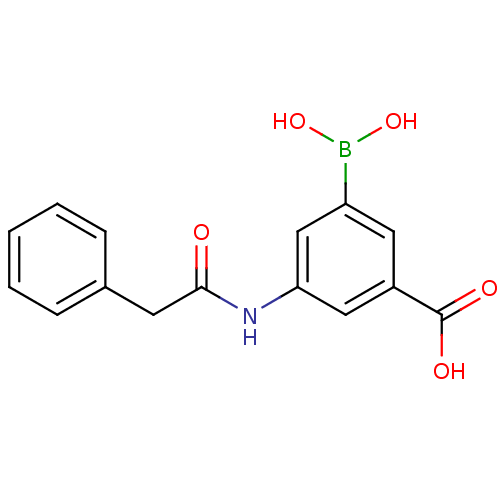
(3-borono-5-(2-phenylacetamido)benzoic acid | CHEMB...)Show InChI InChI=1S/C15H14BNO5/c18-14(6-10-4-2-1-3-5-10)17-13-8-11(15(19)20)7-12(9-13)16(21)22/h1-5,7-9,21-22H,6H2,(H,17,18)(H,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p... |
J Med Chem 52: 6097-106 (2009)
Article DOI: 10.1021/jm9009718
BindingDB Entry DOI: 10.7270/Q2G73DSS |
More data for this
Ligand-Target Pair | |
Penicillin-binding protein 2x
(Streptococcus pneumoniae) | BDBM50324697
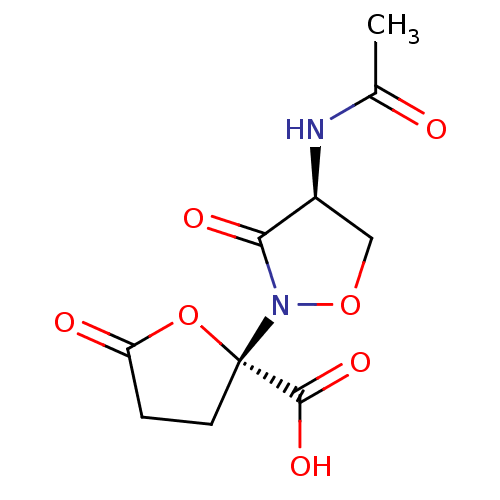
(CHEMBL1221989 | Lactivicin)Show SMILES CC(=O)N[C@H]1CON(C1=O)[C@]1(CCC(=O)O1)C(O)=O |r| Show InChI InChI=1S/C10H12N2O7/c1-5(13)11-6-4-18-12(8(6)15)10(9(16)17)3-2-7(14)19-10/h6H,2-4H2,1H3,(H,11,13)(H,16,17)/t6-,10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Joseph Fourier
Curated by ChEMBL
| Assay Description
Inhibition of penicillin-resistant Streptococcus pneumoniae 5204 PBP2x after 120 mins by SDS-PAGE |
Nat Chem Biol 3: 565-9 (2007)
Article DOI: 10.1038/nchembio.2007.21
BindingDB Entry DOI: 10.7270/Q2JM2BK9 |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50067893
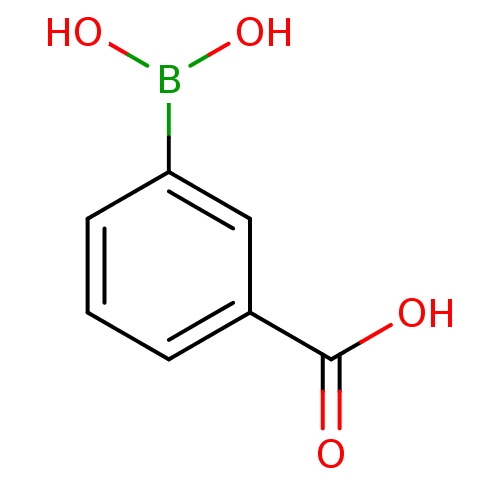
(3-Carboxyphenylboronicacid | 3-boronobenzoic acid ...)Show InChI InChI=1S/C7H7BO4/c9-7(10)5-2-1-3-6(4-5)8(11)12/h1-4,11-12H,(H,9,10) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p... |
J Med Chem 52: 6097-106 (2009)
Article DOI: 10.1021/jm9009718
BindingDB Entry DOI: 10.7270/Q2G73DSS |
More data for this
Ligand-Target Pair | |
Penicillin-binding protein 2x
(Streptococcus pneumoniae) | BDBM50300665

(5-boronothiophene-2-carboxylic acid | CHEMBL573906)Show InChI InChI=1S/C5H5BO4S/c7-5(8)3-1-2-4(11-3)6(9)10/h1-2,9-10H,(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of penicillin-resistant Streptococcus pneumoniae 5204 PBP2X preincubated for 4 hrs before addition of substrate mixture of (R)-[2-(benzoyl... |
J Med Chem 52: 6097-106 (2009)
Article DOI: 10.1021/jm9009718
BindingDB Entry DOI: 10.7270/Q2G73DSS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data