

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM16589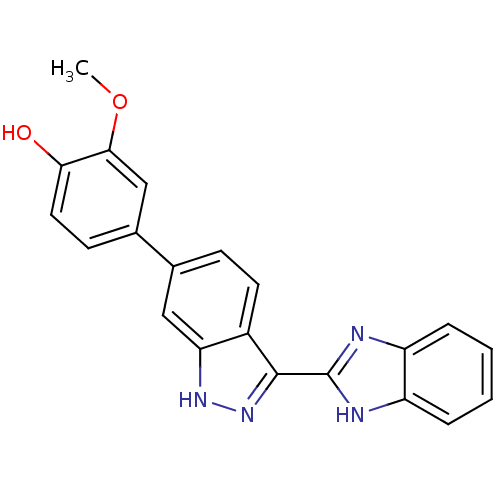 (4-[3-(1H-1,3-benzodiazol-2-yl)-1H-indazol-6-yl]-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 26 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description For Ki determinations a matrix of inhibitor and substrate concentrations were tested. Inhibitor concentrations were tested from four times IC50 with ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM16591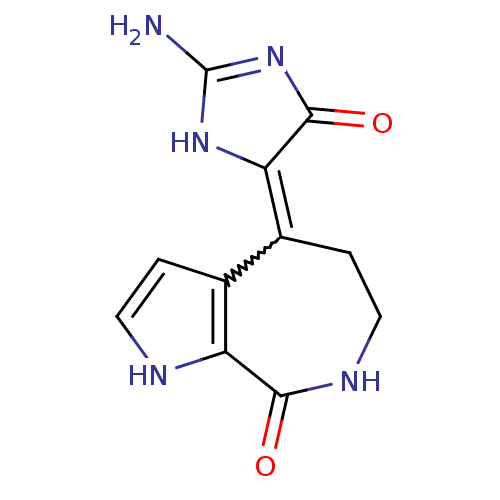 ((4Z)-4-(2-amino-5-oxo-3,5-dihydro-4H-imidazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 659 | -35.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description For Ki determinations a matrix of inhibitor and substrate concentrations were tested. Inhibitor concentrations were tested from four times IC50 with ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM16590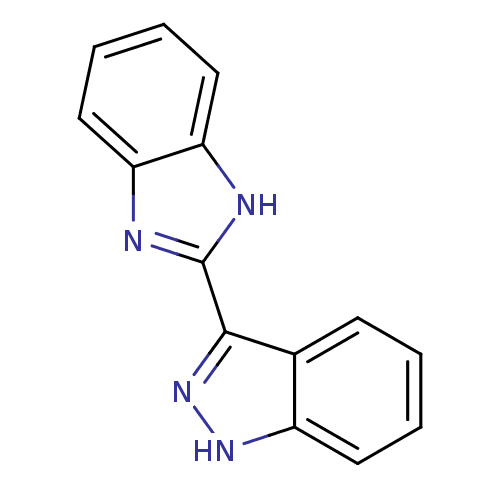 (2-(1H-indazol-3-yl)-1H-1,3-benzodiazole | 3-(1H-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 8.58E+3 | -29.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description For Ki determinations a matrix of inhibitor and substrate concentrations were tested. Inhibitor concentrations were tested from four times IC50 with ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM16589 (4-[3-(1H-1,3-benzodiazol-2-yl)-1H-indazol-6-yl]-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM16589 (4-[3-(1H-1,3-benzodiazol-2-yl)-1H-indazol-6-yl]-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM16589 (4-[3-(1H-1,3-benzodiazol-2-yl)-1H-indazol-6-yl]-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM16591 ((4Z)-4-(2-amino-5-oxo-3,5-dihydro-4H-imidazol-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 168 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Vernalis (R&D) Ltd | Assay Description Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Homo sapiens (Human)) | BDBM16591 ((4Z)-4-(2-amino-5-oxo-3,5-dihydro-4H-imidazol-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50197073 (3-amino-7-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant full-length C-terminal His6-tagged Escherichia coli DNA gyrase AB supercoiling activity using relaxed DNA as substrate prei... | J Med Chem 63: 7773-7816 (2020) Article DOI: 10.1021/acs.jmedchem.0c00347 BindingDB Entry DOI: 10.7270/Q2MW2MQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM16591 ((4Z)-4-(2-amino-5-oxo-3,5-dihydro-4H-imidazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 266 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM16589 (4-[3-(1H-1,3-benzodiazol-2-yl)-1H-indazol-6-yl]-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 314 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Vernalis (R&D) Ltd | Assay Description Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM16591 ((4Z)-4-(2-amino-5-oxo-3,5-dihydro-4H-imidazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 353 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50542206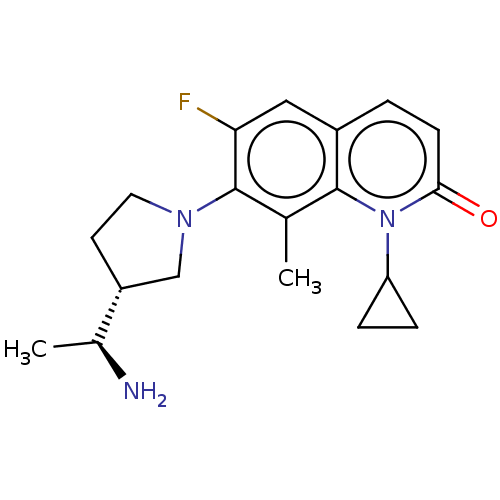 (CHEMBL4640061) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant full-length C-terminal His6-tagged Escherichia coli DNA gyrase AB supercoiling activity using relaxed DNA as substrate prei... | J Med Chem 63: 7773-7816 (2020) Article DOI: 10.1021/acs.jmedchem.0c00347 BindingDB Entry DOI: 10.7270/Q2MW2MQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM16589 (4-[3-(1H-1,3-benzodiazol-2-yl)-1H-indazol-6-yl]-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 443 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM21690 (1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant full-length C-terminal His6-tagged Escherichia coli DNA gyrase AB supercoiling activity using relaxed DNA as substrate prei... | J Med Chem 63: 7773-7816 (2020) Article DOI: 10.1021/acs.jmedchem.0c00347 BindingDB Entry DOI: 10.7270/Q2MW2MQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM16590 (2-(1H-indazol-3-yl)-1H-1,3-benzodiazole | 3-(1H-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 744 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Vernalis (R&D) Ltd | Assay Description Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM16590 (2-(1H-indazol-3-yl)-1H-1,3-benzodiazole | 3-(1H-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 845 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM16591 ((4Z)-4-(2-amino-5-oxo-3,5-dihydro-4H-imidazol-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 856 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50542207 (CHEMBL4634552) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant full-length C-terminal His6-tagged Escherichia coli DNA gyrase AB supercoiling activity using relaxed DNA as substrate prei... | J Med Chem 63: 7773-7816 (2020) Article DOI: 10.1021/acs.jmedchem.0c00347 BindingDB Entry DOI: 10.7270/Q2MW2MQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM14805 (3-(5,6-Diphenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Chk1 kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor in the presence of 100uM ATP/[gamma-33P] ATP.... | J Med Chem 48: 4332-45 (2005) Article DOI: 10.1021/jm049022c BindingDB Entry DOI: 10.7270/Q2WW7FXS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM14805 (3-(5,6-Diphenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Human CDK1 kinase activity was assayed in reaction buffer containing substrate Histone H1, enzyme, and inhibitor in the presence of 100uM ATP/[gamma-... | J Med Chem 48: 4332-45 (2005) Article DOI: 10.1021/jm049022c BindingDB Entry DOI: 10.7270/Q2WW7FXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM14803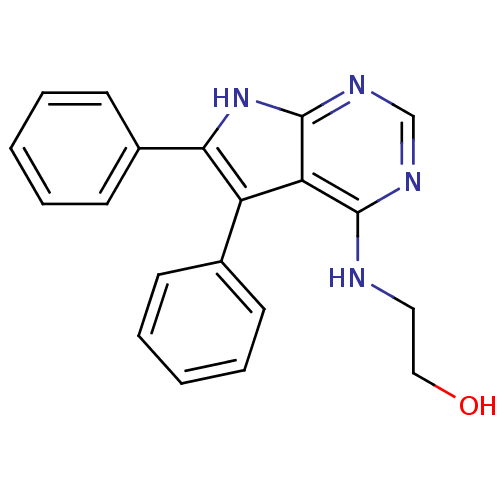 (2-(5,6-Diphenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Chk1 kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor in the presence of 100uM ATP/[gamma-33P] ATP.... | J Med Chem 48: 4332-45 (2005) Article DOI: 10.1021/jm049022c BindingDB Entry DOI: 10.7270/Q2WW7FXS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566539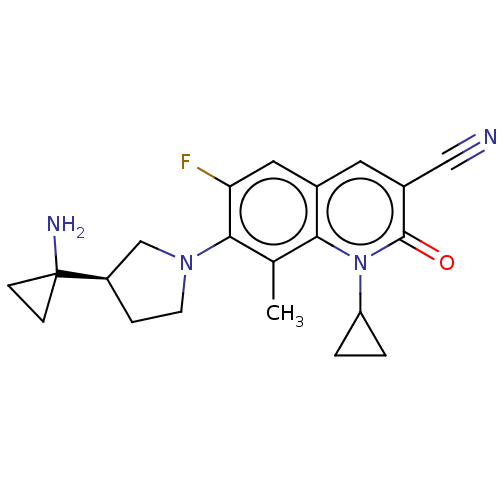 (CHEMBL4860033) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM16590 (2-(1H-indazol-3-yl)-1H-1,3-benzodiazole | 3-(1H-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.71E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50542205 (CHEMBL4643875) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant full-length C-terminal His6-tagged Escherichia coli DNA gyrase AB supercoiling activity using relaxed DNA as substrate prei... | J Med Chem 63: 7773-7816 (2020) Article DOI: 10.1021/acs.jmedchem.0c00347 BindingDB Entry DOI: 10.7270/Q2MW2MQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM14804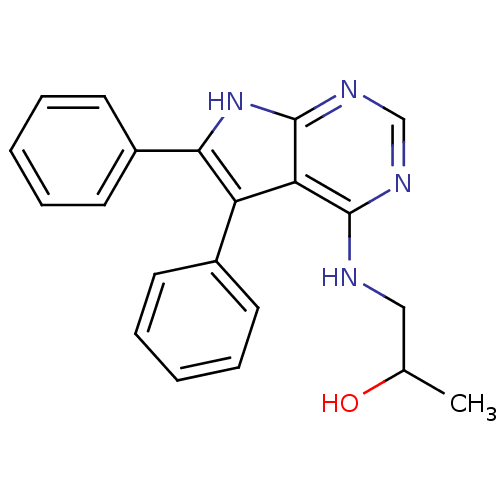 (1-(5,6-Diphenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Human CDK1 kinase activity was assayed in reaction buffer containing substrate Histone H1, enzyme, and inhibitor in the presence of 100uM ATP/[gamma-... | J Med Chem 48: 4332-45 (2005) Article DOI: 10.1021/jm049022c BindingDB Entry DOI: 10.7270/Q2WW7FXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM14803 (2-(5,6-Diphenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Human CDK1 kinase activity was assayed in reaction buffer containing substrate Histone H1, enzyme, and inhibitor in the presence of 100uM ATP/[gamma-... | J Med Chem 48: 4332-45 (2005) Article DOI: 10.1021/jm049022c BindingDB Entry DOI: 10.7270/Q2WW7FXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM14804 (1-(5,6-Diphenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Chk1 kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor in the presence of 100uM ATP/[gamma-33P] ATP.... | J Med Chem 48: 4332-45 (2005) Article DOI: 10.1021/jm049022c BindingDB Entry DOI: 10.7270/Q2WW7FXS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM14801 (2-({5,6-diphenylfuro[2,3-d]pyrimidin-4-yl}(methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Chk1 kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor in the presence of 100uM ATP/[gamma-33P] ATP.... | J Med Chem 48: 4332-45 (2005) Article DOI: 10.1021/jm049022c BindingDB Entry DOI: 10.7270/Q2WW7FXS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM16590 (2-(1H-indazol-3-yl)-1H-1,3-benzodiazole | 3-(1H-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566543 (CHEMBL4864037) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM16591 ((4Z)-4-(2-amino-5-oxo-3,5-dihydro-4H-imidazol-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.83E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566538 (CHEMBL4875315) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566541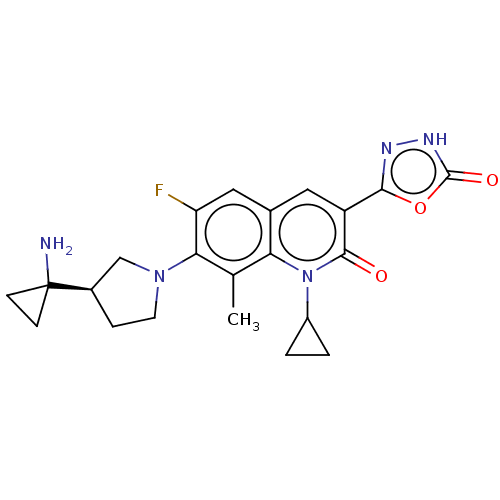 (CHEMBL4867915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566542 (CHEMBL4878164) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566546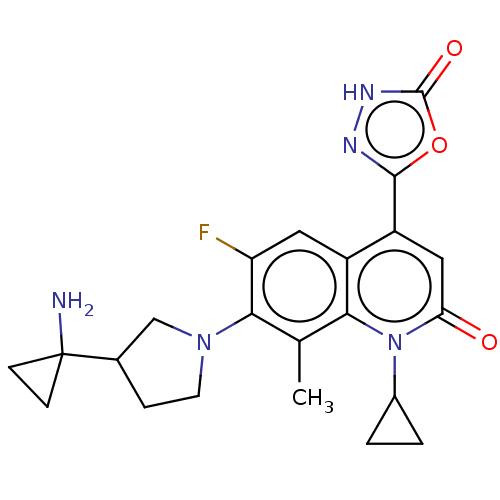 (CHEMBL4875871) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM14818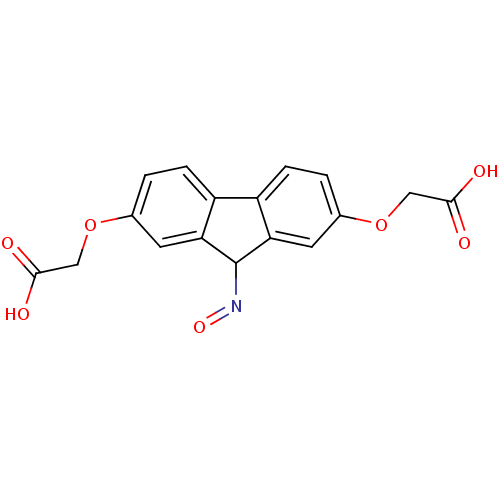 (2-{[7-(carboxymethoxy)-9-(hydroxyimino)-9H-fluoren...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Chk1 kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor in the presence of 100uM ATP/[gamma-33P] ATP.... | Bioorg Med Chem 14: 4792-802 (2006) Article DOI: 10.1016/j.bmc.2006.03.021 BindingDB Entry DOI: 10.7270/Q2S46Q6S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM16590 (2-(1H-indazol-3-yl)-1H-1,3-benzodiazole | 3-(1H-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM14799 (2-[5,6-Bis-(4-methoxy-phenyl)-furo[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Chk1 kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor in the presence of 100uM ATP/[gamma-33P] ATP.... | J Med Chem 48: 4332-45 (2005) Article DOI: 10.1021/jm049022c BindingDB Entry DOI: 10.7270/Q2WW7FXS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM14823 (2-[(6-amino-9H-purin-8-yl)sulfanyl]acetamide | Asi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Chk1 kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor in the presence of 100uM ATP/[gamma-33P] ATP.... | Bioorg Med Chem 14: 4792-802 (2006) Article DOI: 10.1016/j.bmc.2006.03.021 BindingDB Entry DOI: 10.7270/Q2S46Q6S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM14815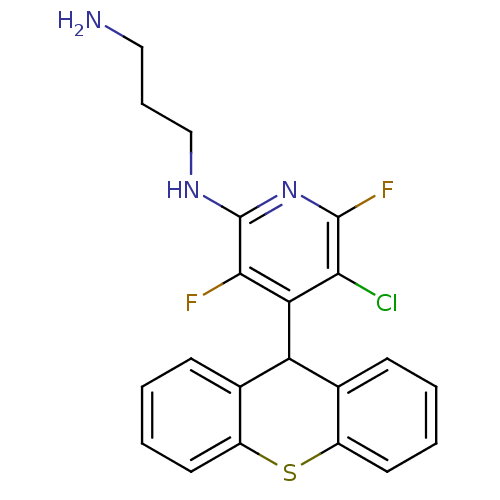 (InterBioScreen compound 2 | N-(3-aminopropyl)-5-ch...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Chk1 kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor in the presence of 100uM ATP/[gamma-33P] ATP.... | Bioorg Med Chem 14: 4792-802 (2006) Article DOI: 10.1016/j.bmc.2006.03.021 BindingDB Entry DOI: 10.7270/Q2S46Q6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566544 (CHEMBL4852170) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM14822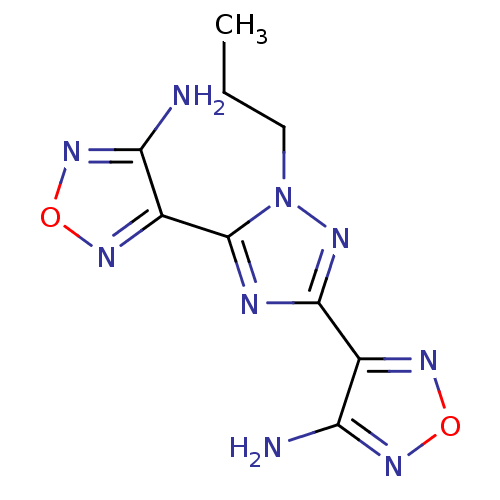 (4-[3-(4-amino-1,2,5-oxadiazol-3-yl)-1-propyl-1H-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Chk1 kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor in the presence of 100uM ATP/[gamma-33P] ATP.... | Bioorg Med Chem 14: 4792-802 (2006) Article DOI: 10.1016/j.bmc.2006.03.021 BindingDB Entry DOI: 10.7270/Q2S46Q6S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566545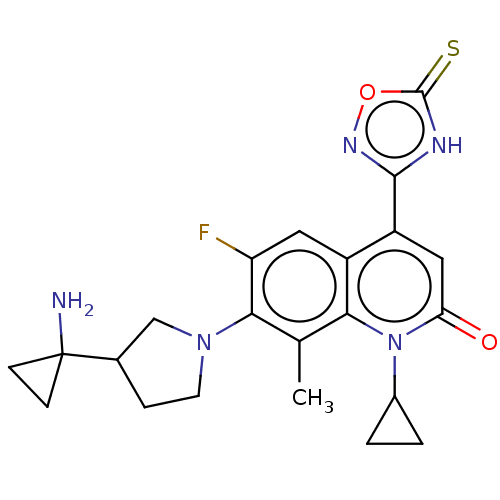 (CHEMBL4861495) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM14819 ((1E)-1-[(6-chloro-2H-1,3-benzodioxol-5-yl)methylid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Chk1 kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor in the presence of 100uM ATP/[gamma-33P] ATP.... | Bioorg Med Chem 14: 4792-802 (2006) Article DOI: 10.1016/j.bmc.2006.03.021 BindingDB Entry DOI: 10.7270/Q2S46Q6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM14816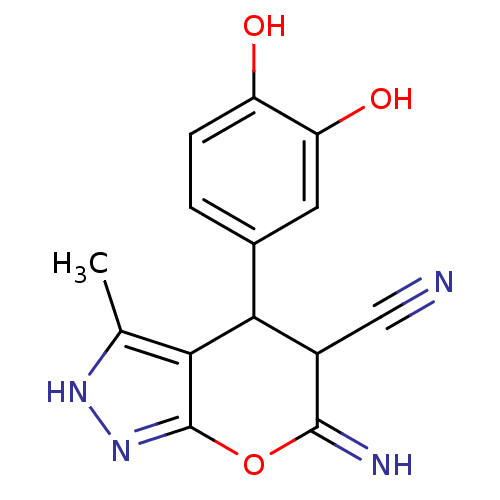 (6-amino-4-(3,4-dihydroxyphenyl)-3-methyl-1H,4H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Chk1 kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor in the presence of 100uM ATP/[gamma-33P] ATP.... | Bioorg Med Chem 14: 4792-802 (2006) Article DOI: 10.1016/j.bmc.2006.03.021 BindingDB Entry DOI: 10.7270/Q2S46Q6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM14800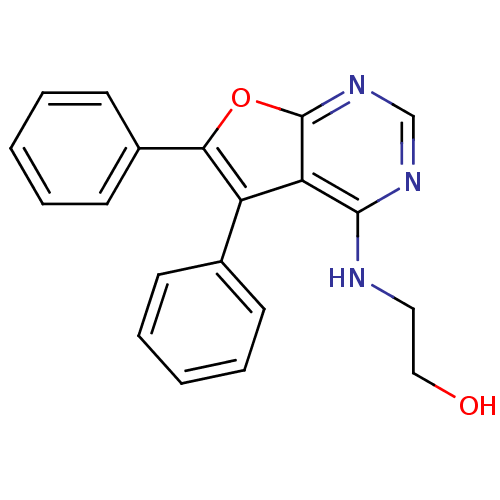 (2-(5,6-Diphenyl-furo[2,3-d]pyrimidin-4-ylamino)-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.09E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Chk1 kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor in the presence of 100uM ATP/[gamma-33P] ATP.... | J Med Chem 48: 4332-45 (2005) Article DOI: 10.1021/jm049022c BindingDB Entry DOI: 10.7270/Q2WW7FXS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM14802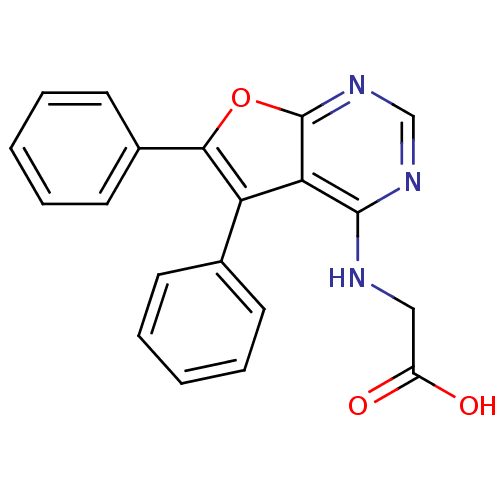 ((5,6-Diphenyl-furo[2,3-d]pyrimidin-4-ylamino)-acet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Chk1 kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor in the presence of 100uM ATP/[gamma-33P] ATP.... | J Med Chem 48: 4332-45 (2005) Article DOI: 10.1021/jm049022c BindingDB Entry DOI: 10.7270/Q2WW7FXS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM16589 (4-[3-(1H-1,3-benzodiazol-2-yl)-1H-indazol-6-yl]-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vernalis (R&D) Ltd | Assay Description Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P ... | Bioorg Med Chem 14: 1792-804 (2006) Article DOI: 10.1016/j.bmc.2005.10.022 BindingDB Entry DOI: 10.7270/Q2154F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566510 (CHEMBL4860105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG expressed in CHO-K1 cells by Qpatch clamp method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 124 total ) | Next | Last >> |