 Found 60 hits with Last Name = 'flaumenhaft' and Initial = 'r'
Found 60 hits with Last Name = 'flaumenhaft' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271332
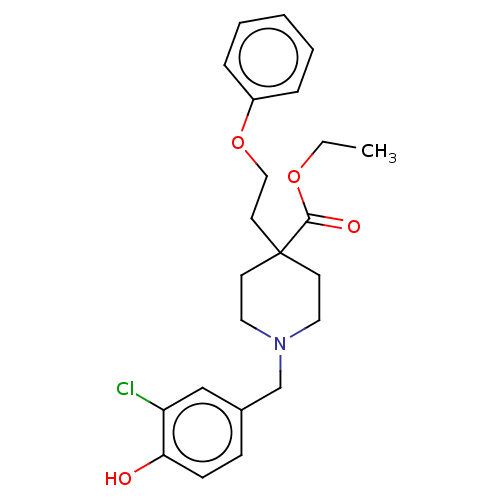
(US10064853, Compound 1)Show SMILES CCOC(=O)C1(CCOc2ccccc2)CCN(Cc2ccc(O)c(Cl)c2)CC1 Show InChI InChI=1S/C23H28ClNO4/c1-2-28-22(27)23(12-15-29-19-6-4-3-5-7-19)10-13-25(14-11-23)17-18-8-9-21(26)20(24)16-18/h3-9,16,26H,2,10-15,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271332

(US10064853, Compound 1)Show SMILES CCOC(=O)C1(CCOc2ccccc2)CCN(Cc2ccc(O)c(Cl)c2)CC1 Show InChI InChI=1S/C23H28ClNO4/c1-2-28-22(27)23(12-15-29-19-6-4-3-5-7-19)10-13-25(14-11-23)17-18-8-9-21(26)20(24)16-18/h3-9,16,26H,2,10-15,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386391
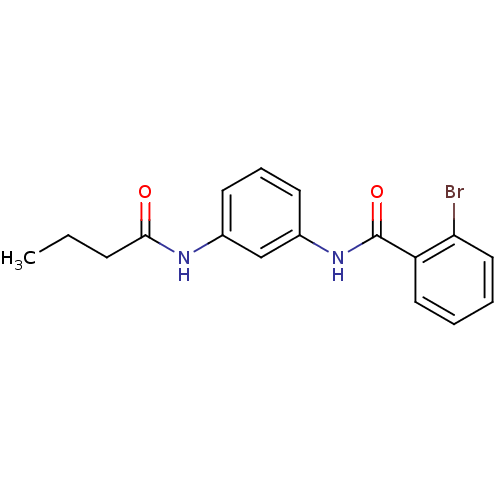
(CHEMBL1411333)Show InChI InChI=1S/C17H17BrN2O2/c1-2-6-16(21)19-12-7-5-8-13(11-12)20-17(22)14-9-3-4-10-15(14)18/h3-5,7-11H,2,6H2,1H3,(H,19,21)(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386409

(CHEMBL2047299)Show InChI InChI=1S/C18H19BrN2O2/c1-12(2)10-17(22)20-13-6-5-7-14(11-13)21-18(23)15-8-3-4-9-16(15)19/h3-9,11-12H,10H2,1-2H3,(H,20,22)(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386408

(CHEMBL2047297)Show InChI InChI=1S/C17H19BrN2O/c1-2-3-11-19-13-7-6-8-14(12-13)20-17(21)15-9-4-5-10-16(15)18/h4-10,12,19H,2-3,11H2,1H3,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386400

(CHEMBL2049115)Show InChI InChI=1S/C17H16BrClN2O2/c1-2-4-16(22)20-12-5-3-6-13(10-12)21-17(23)14-9-11(19)7-8-15(14)18/h3,5-10H,2,4H2,1H3,(H,20,22)(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386398

(CHEMBL2049113)Show InChI InChI=1S/C17H16BrFN2O2/c1-2-4-16(22)20-12-5-3-6-13(10-12)21-17(23)14-8-7-11(19)9-15(14)18/h3,5-10H,2,4H2,1H3,(H,20,22)(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386397
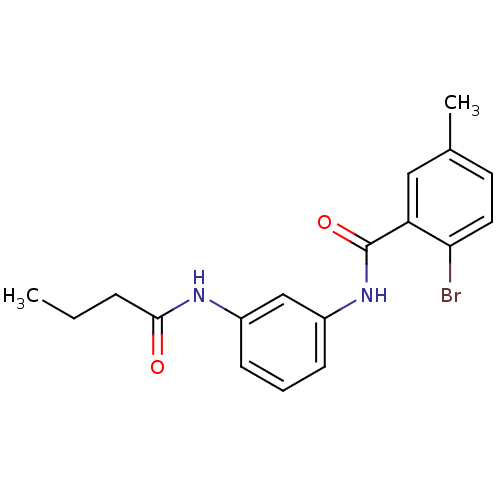
(CHEMBL2049112)Show InChI InChI=1S/C18H19BrN2O2/c1-3-5-17(22)20-13-6-4-7-14(11-13)21-18(23)15-10-12(2)8-9-16(15)19/h4,6-11H,3,5H2,1-2H3,(H,20,22)(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386406
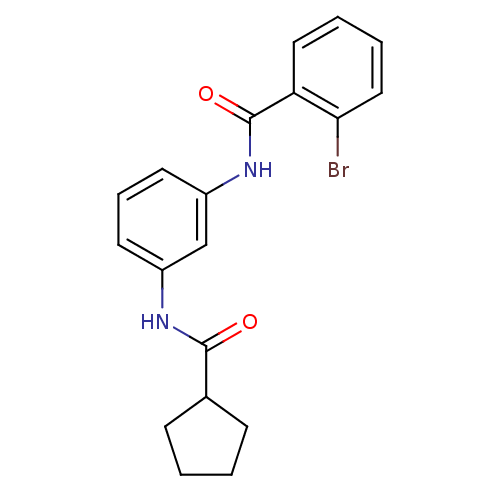
(CHEMBL1718628)Show InChI InChI=1S/C19H19BrN2O2/c20-17-11-4-3-10-16(17)19(24)22-15-9-5-8-14(12-15)21-18(23)13-6-1-2-7-13/h3-5,8-13H,1-2,6-7H2,(H,21,23)(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386394

(CHEMBL2048423)Show InChI InChI=1S/C19H22N2O2/c1-3-8-18(22)20-15-10-7-11-16(13-15)21-19(23)17-12-6-5-9-14(17)4-2/h5-7,9-13H,3-4,8H2,1-2H3,(H,20,22)(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386399
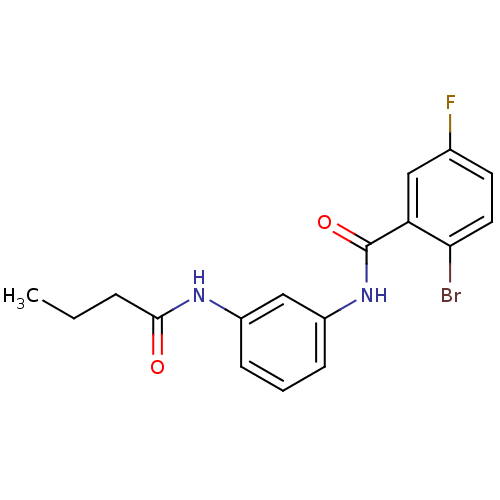
(CHEMBL2049114)Show InChI InChI=1S/C17H16BrFN2O2/c1-2-4-16(22)20-12-5-3-6-13(10-12)21-17(23)14-9-11(19)7-8-15(14)18/h3,5-10H,2,4H2,1H3,(H,20,22)(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271339
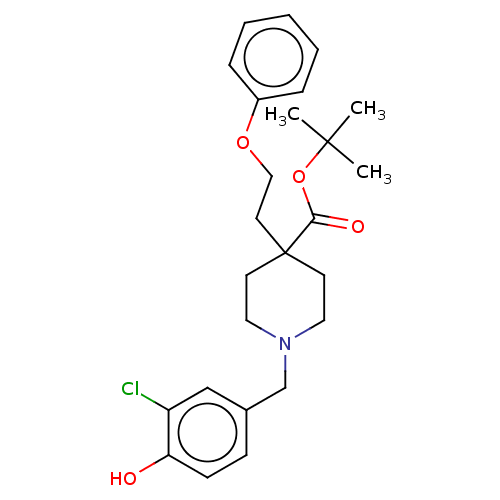
(US10064853, Compound 14)Show SMILES CC(C)(C)OC(=O)C1(CCOc2ccccc2)CCN(Cc2ccc(O)c(Cl)c2)CC1 Show InChI InChI=1S/C25H32ClNO4/c1-24(2,3)31-23(29)25(13-16-30-20-7-5-4-6-8-20)11-14-27(15-12-25)18-19-9-10-22(28)21(26)17-19/h4-10,17,28H,11-16,18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271339

(US10064853, Compound 14)Show SMILES CC(C)(C)OC(=O)C1(CCOc2ccccc2)CCN(Cc2ccc(O)c(Cl)c2)CC1 Show InChI InChI=1S/C25H32ClNO4/c1-24(2,3)31-23(29)25(13-16-30-20-7-5-4-6-8-20)11-14-27(15-12-25)18-19-9-10-22(28)21(26)17-19/h4-10,17,28H,11-16,18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386395
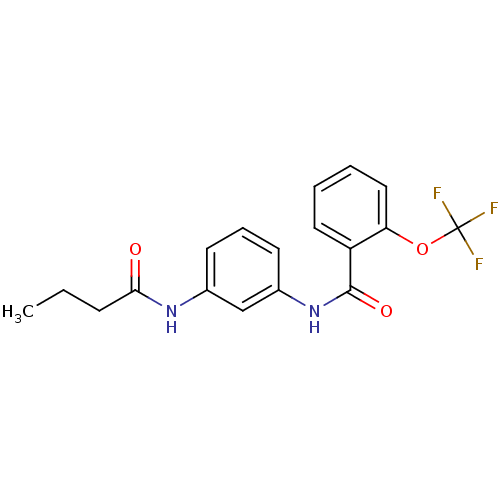
(CHEMBL2048425)Show InChI InChI=1S/C18H17F3N2O3/c1-2-6-16(24)22-12-7-5-8-13(11-12)23-17(25)14-9-3-4-10-15(14)26-18(19,20)21/h3-5,7-11H,2,6H2,1H3,(H,22,24)(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386393
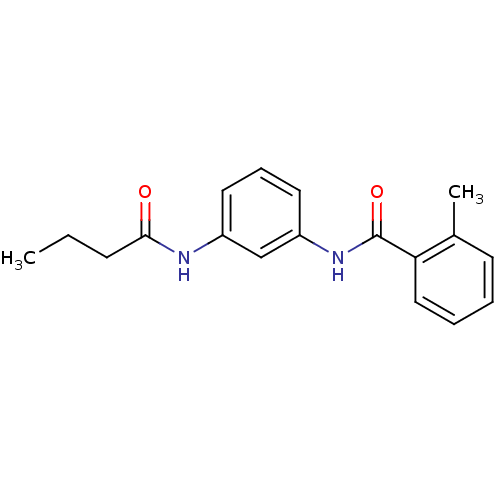
(CHEMBL1721173)Show InChI InChI=1S/C18H20N2O2/c1-3-7-17(21)19-14-9-6-10-15(12-14)20-18(22)16-11-5-4-8-13(16)2/h4-6,8-12H,3,7H2,1-2H3,(H,19,21)(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271338

(US10064853, Compound 13)Show SMILES CC(C)OC(=O)C1(CCOc2ccccc2)CCN(Cc2ccc(O)c(Cl)c2)CC1 Show InChI InChI=1S/C24H30ClNO4/c1-18(2)30-23(28)24(12-15-29-20-6-4-3-5-7-20)10-13-26(14-11-24)17-19-8-9-22(27)21(25)16-19/h3-9,16,18,27H,10-15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271338

(US10064853, Compound 13)Show SMILES CC(C)OC(=O)C1(CCOc2ccccc2)CCN(Cc2ccc(O)c(Cl)c2)CC1 Show InChI InChI=1S/C24H30ClNO4/c1-18(2)30-23(28)24(12-15-29-20-6-4-3-5-7-20)10-13-26(14-11-24)17-19-8-9-22(27)21(25)16-19/h3-9,16,18,27H,10-15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386389

(CHEMBL1729550)Show InChI InChI=1S/C17H17FN2O2/c1-2-6-16(21)19-12-7-5-8-13(11-12)20-17(22)14-9-3-4-10-15(14)18/h3-5,7-11H,2,6H2,1H3,(H,19,21)(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386390

(CHEMBL1734760)Show InChI InChI=1S/C17H17ClN2O2/c1-2-6-16(21)19-12-7-5-8-13(11-12)20-17(22)14-9-3-4-10-15(14)18/h3-5,7-11H,2,6H2,1H3,(H,19,21)(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386410
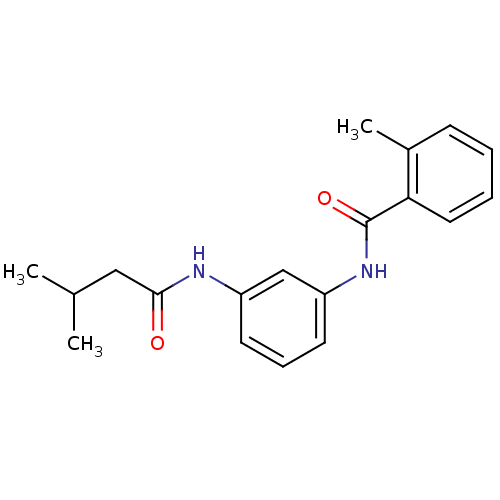
(CHEMBL2047300)Show InChI InChI=1S/C19H22N2O2/c1-13(2)11-18(22)20-15-8-6-9-16(12-15)21-19(23)17-10-5-4-7-14(17)3/h4-10,12-13H,11H2,1-3H3,(H,20,22)(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386392
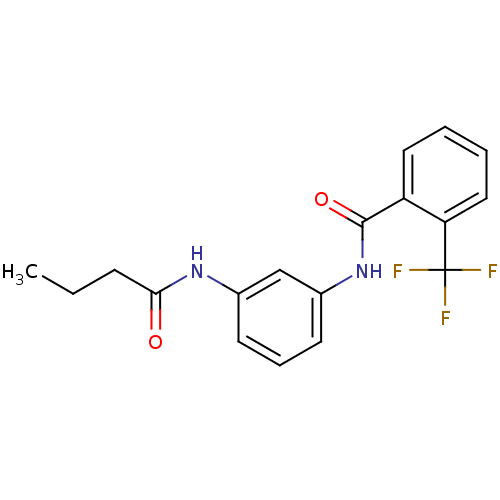
(CHEMBL2048420)Show InChI InChI=1S/C18H17F3N2O2/c1-2-6-16(24)22-12-7-5-8-13(11-12)23-17(25)14-9-3-4-10-15(14)18(19,20)21/h3-5,7-11H,2,6H2,1H3,(H,22,24)(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386405

(CHEMBL2049121)Show InChI InChI=1S/C17H17BrN2O2/c1-11(2)16(21)19-12-6-5-7-13(10-12)20-17(22)14-8-3-4-9-15(14)18/h3-11H,1-2H3,(H,19,21)(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386404

(CHEMBL1332325)Show InChI InChI=1S/C16H15BrN2O2/c1-2-15(20)18-11-6-5-7-12(10-11)19-16(21)13-8-3-4-9-14(13)17/h3-10H,2H2,1H3,(H,18,20)(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386411

(CHEMBL2047284)Show InChI InChI=1S/C17H17ClN2O2/c1-2-10-19-16(21)12-6-5-7-13(11-12)20-17(22)14-8-3-4-9-15(14)18/h3-9,11H,2,10H2,1H3,(H,19,21)(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386396
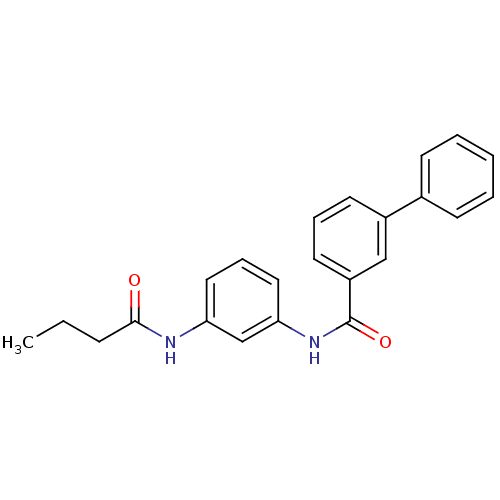
(CHEMBL2048430)Show SMILES CCCC(=O)Nc1cccc(NC(=O)c2cccc(c2)-c2ccccc2)c1 Show InChI InChI=1S/C23H22N2O2/c1-2-8-22(26)24-20-13-7-14-21(16-20)25-23(27)19-12-6-11-18(15-19)17-9-4-3-5-10-17/h3-7,9-16H,2,8H2,1H3,(H,24,26)(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271337
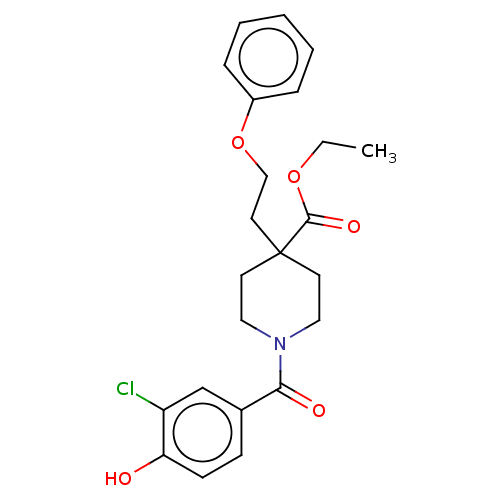
(US10064853, Compound 9)Show SMILES CCOC(=O)C1(CCOc2ccccc2)CCN(CC1)C(=O)c1ccc(O)c(Cl)c1 Show InChI InChI=1S/C23H26ClNO5/c1-2-29-22(28)23(12-15-30-18-6-4-3-5-7-18)10-13-25(14-11-23)21(27)17-8-9-20(26)19(24)16-17/h3-9,16,26H,2,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271337

(US10064853, Compound 9)Show SMILES CCOC(=O)C1(CCOc2ccccc2)CCN(CC1)C(=O)c1ccc(O)c(Cl)c1 Show InChI InChI=1S/C23H26ClNO5/c1-2-29-22(28)23(12-15-30-18-6-4-3-5-7-18)10-13-25(14-11-23)21(27)17-8-9-20(26)19(24)16-17/h3-9,16,26H,2,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386401
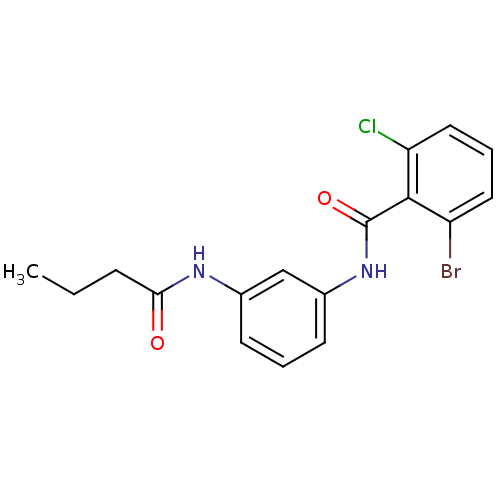
(CHEMBL2049116)Show InChI InChI=1S/C17H16BrClN2O2/c1-2-5-15(22)20-11-6-3-7-12(10-11)21-17(23)16-13(18)8-4-9-14(16)19/h3-4,6-10H,2,5H2,1H3,(H,20,22)(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386407
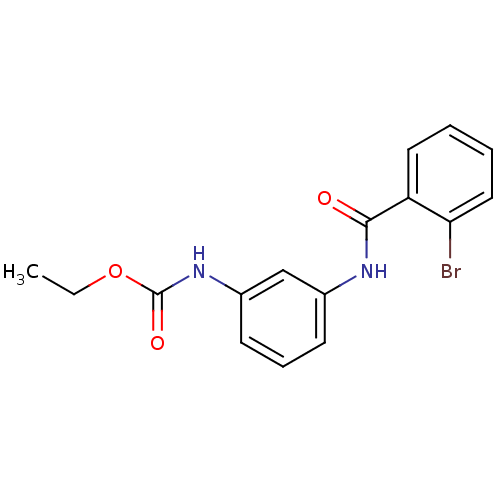
(CHEMBL2047286)Show InChI InChI=1S/C16H15BrN2O3/c1-2-22-16(21)19-12-7-5-6-11(10-12)18-15(20)13-8-3-4-9-14(13)17/h3-10H,2H2,1H3,(H,18,20)(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM645352
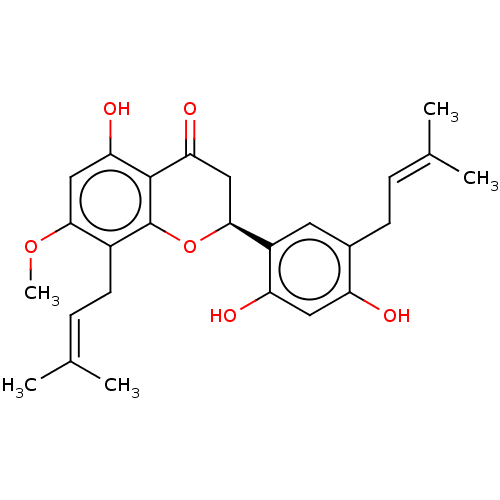
(Maackiaflavanone | US20240016777, Table1a.2)Show SMILES [#6]-[#8]-c1cc(-[#8])c2-[#6](=O)-[#6]-[#6@H](-[#8]-c2c1-[#6]\[#6]=[#6](/[#6])-[#6])-c1cc(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])cc1-[#8] |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386403

(CHEMBL2049118)Show InChI InChI=1S/C19H22N2O2/c1-4-7-17(22)20-15-10-6-11-16(12-15)21-19(23)18-13(2)8-5-9-14(18)3/h5-6,8-12H,4,7H2,1-3H3,(H,20,22)(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271341

(US10064853, Compound 16)Show SMILES CCN(C)C(=O)C1(CCOc2ccccc2)CCN(Cc2ccc(O)c(Cl)c2)CC1 Show InChI InChI=1S/C24H31ClN2O3/c1-3-26(2)23(29)24(13-16-30-20-7-5-4-6-8-20)11-14-27(15-12-24)18-19-9-10-22(28)21(25)17-19/h4-10,17,28H,3,11-16,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271341

(US10064853, Compound 16)Show SMILES CCN(C)C(=O)C1(CCOc2ccccc2)CCN(Cc2ccc(O)c(Cl)c2)CC1 Show InChI InChI=1S/C24H31ClN2O3/c1-3-26(2)23(29)24(13-16-30-20-7-5-4-6-8-20)11-14-27(15-12-24)18-19-9-10-22(28)21(25)17-19/h4-10,17,28H,3,11-16,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50386402

(CHEMBL2049117)Show InChI InChI=1S/C17H16Cl2N2O2/c1-2-5-15(22)20-11-6-3-7-12(10-11)21-17(23)13-8-4-9-14(18)16(13)19/h3-4,6-10H,2,5H2,1H3,(H,20,22)(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SFLLRN-NH2-stimulated PAR1-mediated platelet activation in platelet-rich human plasma assessed as surface expression of P-selectin afte... |
ACS Med Chem Lett 3: 232-237 (2012)
Article DOI: 10.1021/ml2002696
BindingDB Entry DOI: 10.7270/Q2Q52QP8 |
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271342
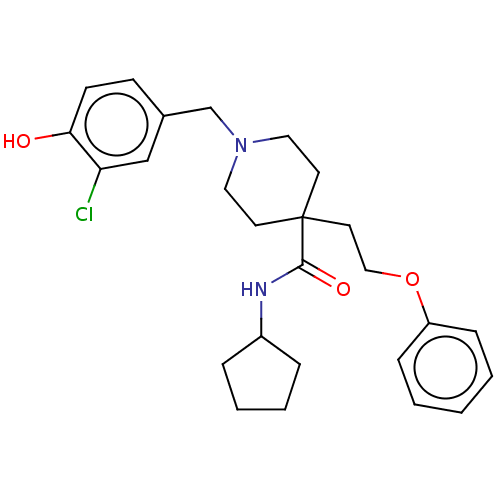
(US10064853, Compound 17)Show SMILES Oc1ccc(CN2CCC(CCOc3ccccc3)(CC2)C(=O)NC2CCCC2)cc1Cl Show InChI InChI=1S/C26H33ClN2O3/c27-23-18-20(10-11-24(23)30)19-29-15-12-26(13-16-29,25(31)28-21-6-4-5-7-21)14-17-32-22-8-2-1-3-9-22/h1-3,8-11,18,21,30H,4-7,12-17,19H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271342

(US10064853, Compound 17)Show SMILES Oc1ccc(CN2CCC(CCOc3ccccc3)(CC2)C(=O)NC2CCCC2)cc1Cl Show InChI InChI=1S/C26H33ClN2O3/c27-23-18-20(10-11-24(23)30)19-29-15-12-26(13-16-29,25(31)28-21-6-4-5-7-21)14-17-32-22-8-2-1-3-9-22/h1-3,8-11,18,21,30H,4-7,12-17,19H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM645355
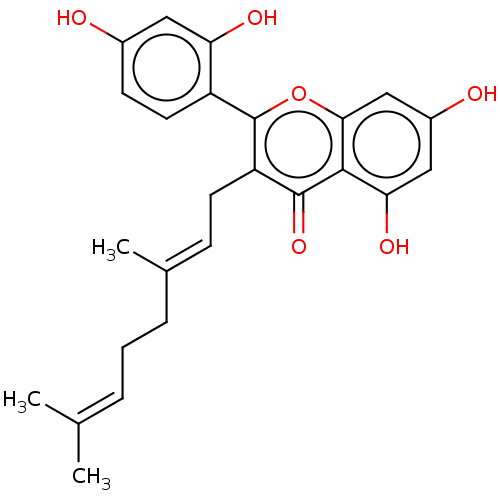
(5,7,2',4'- Tetrahydroxy-3- geranylflavone | US2024...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(oc2cc(-[#8])cc(-[#8])c2c1=O)-c1ccc(-[#8])cc1-[#8] | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM645353
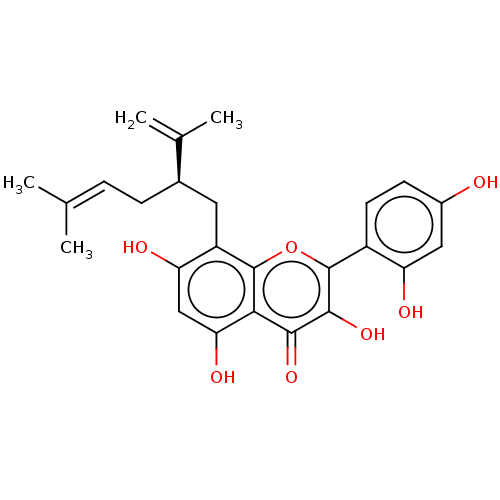
(Kushenol C | US20240016777, Table1a.3)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6@H](-[#6]-c1c(-[#8])cc(-[#8])c2c1oc(c(-[#8])c2=O)-c1ccc(-[#8])cc1-[#8])-[#6](-[#6])=[#6] |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM645364
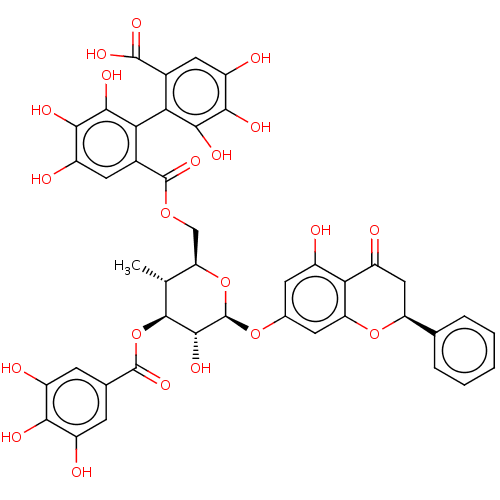
(US20240016777, Table3.6 | pinocembrin 7-O-(3$#8243...)Show SMILES C[C@@H]1[C@@H](COC(=O)c2cc(O)c(O)c(O)c2-c2c(O)c(O)c(O)cc2C(O)=O)O[C@@H](Oc2cc(O)c3C(=O)C[C@H](Oc3c2)c2ccccc2)[C@H](O)[C@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r,wU:29.31,51.57,2.2,wD:39.46,49.54,1.0,(-4,1.15,;-2.67,1.93,;-2.67,3.47,;-4,4.23,;-5.33,3.47,;-6.67,2.69,;-6.67,4.23,;-8,3.47,;-8,5,;-9.34,5.78,;-9.34,7.31,;-10.67,5,;-12,5.78,;-10.67,3.47,;-12,2.69,;-9.34,2.69,;-9.34,1.15,;-10.67,.38,;-12,1.15,;-10.67,-1.15,;-12,-1.93,;-9.34,-1.93,;-9.34,-3.47,;-8,-1.15,;-8,.38,;-6.67,1.15,;-4.34,1.15,;-6.67,-.38,;-1.33,4.23,;,3.47,;1.33,4.23,;2.67,3.47,;2.67,1.93,;4,1.15,;4,-.38,;5.33,1.93,;6.67,1.15,;6.67,-.38,;8,1.93,;8,3.47,;6.67,4.23,;5.33,3.47,;4,4.23,;9.34,4.23,;10.67,3.47,;12,4.23,;12,5.78,;10.67,6.54,;9.34,5.78,;,1.93,;1.33,1.15,;-1.33,1.15,;-1.33,-.38,;,-1.15,;1.33,-.38,;,-2.69,;-1.33,-3.47,;-1.33,-5,;-2.67,-5.78,;,-5.78,;,-7.31,;1.33,-5,;2.67,-5.78,;1.33,-3.47,)| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM645361
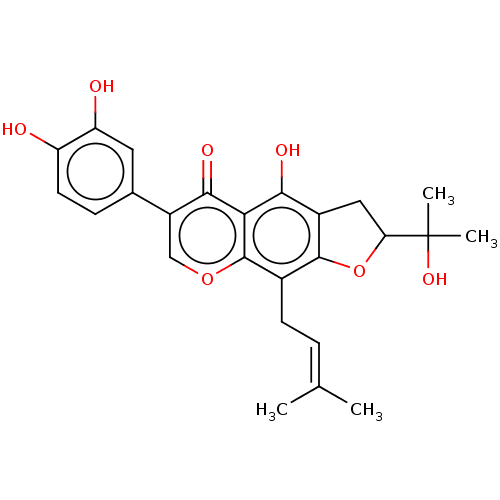
(US20240016777, Table2a.1 | furowanin A)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1c2-[#8]-[#6](-[#6]-c2c(-[#8])c2c1occ(-c1ccc(-[#8])c(-[#8])c1)c2=O)C([#6])([#6])[#8] | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM429466
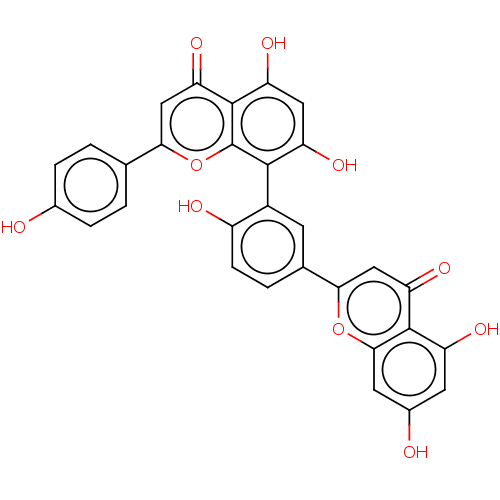
(US20240016777, Table3.7 | acs.jmedchem.1c00409_ST....)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(=O)c4c(O)cc(O)cc4o3)c2o1 |(-10.67,-4.62,;-9.34,-5.39,;-9.34,-6.93,;-8,-7.7,;-6.67,-6.93,;-6.67,-5.39,;-8,-4.62,;-5.33,-7.7,;-5.33,-9.24,;-4,-10.01,;-4,-11.55,;-2.67,-9.24,;-1.33,-10.01,;-1.33,-11.55,;,-9.24,;,-7.7,;1.33,-6.93,;-1.33,-6.93,;-1.33,-5.39,;,-4.62,;,-3.08,;-1.33,-2.31,;-2.67,-3.08,;-2.67,-4.62,;-4,-5.39,;1.33,-2.31,;1.33,-.77,;2.67,,;2.67,1.54,;4,-.77,;5.33,,;5.33,1.54,;6.67,-.77,;6.67,-2.31,;8,-3.08,;5.33,-3.08,;4,-2.31,;2.67,-3.08,;-2.67,-7.7,;-4,-6.93,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-36H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM645366
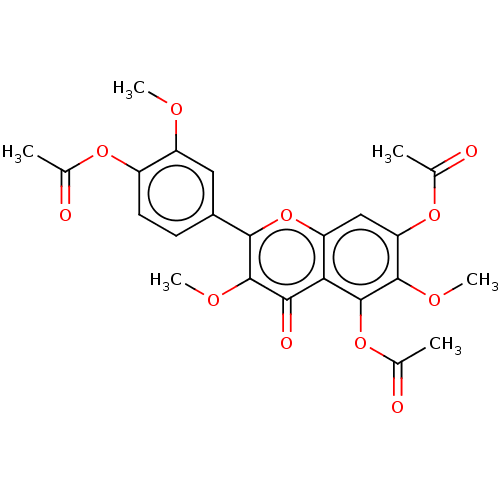
(Jaceidin triacetate | US20240016777, Table3.8)Show SMILES COc1cc(ccc1OC(C)=O)-c1oc2cc(OC(C)=O)c(OC)c(OC(C)=O)c2c(=O)c1OC | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM645360
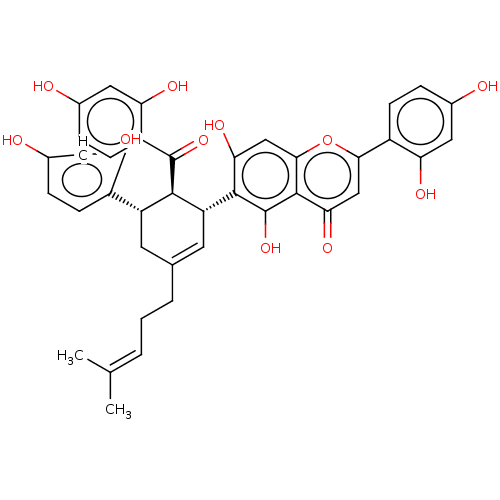
(Multicaulisin | US20240016777, Table1d.1)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]-[#6]-1=[#6]-[#6@H](-[#6@@H](-[#6@H](-[#6]-1)-c1ccc(-[#8])cc1-[#8])-[#6](=O)-c1ccc(-[#8])cc1-[#8])-c1c(-[#8])cc2oc(cc(=O)c2c1-[#8])-c1ccc(-[#8])cc1-[#8] |r,t:6| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM645354
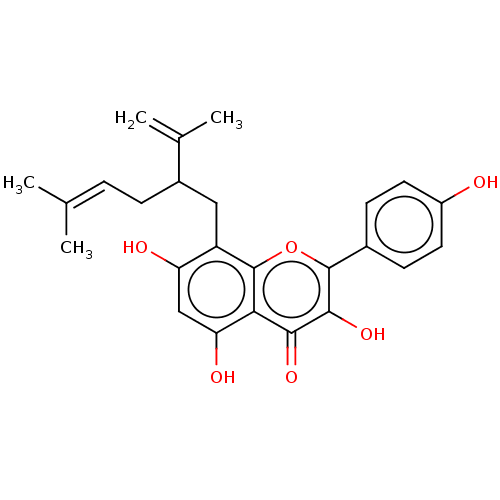
(8- Lavandulylkaempferol | US20240016777, Table1a.5)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6](-[#6]-c1c(-[#8])cc(-[#8])c2c1oc(-c1ccc(-[#8])cc1)c(-[#8])c2=O)-[#6](-[#6])=[#6] | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271344
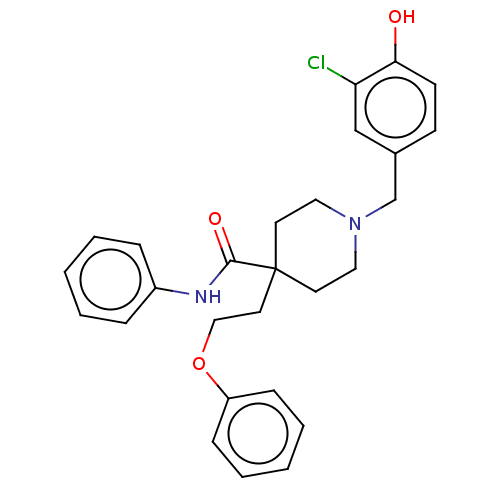
(US10064853, Compound 19)Show SMILES Oc1ccc(CN2CCC(CCOc3ccccc3)(CC2)C(=O)Nc2ccccc2)cc1Cl Show InChI InChI=1S/C27H29ClN2O3/c28-24-19-21(11-12-25(24)31)20-30-16-13-27(14-17-30,15-18-33-23-9-5-2-6-10-23)26(32)29-22-7-3-1-4-8-22/h1-12,19,31H,13-18,20H2,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271344

(US10064853, Compound 19)Show SMILES Oc1ccc(CN2CCC(CCOc3ccccc3)(CC2)C(=O)Nc2ccccc2)cc1Cl Show InChI InChI=1S/C27H29ClN2O3/c28-24-19-21(11-12-25(24)31)20-30-16-13-27(14-17-30,15-18-33-23-9-5-2-6-10-23)26(32)29-22-7-3-1-4-8-22/h1-12,19,31H,13-18,20H2,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271343

(US10064853, Compound 18)Show SMILES Oc1ccc(CN2CCC(CCOc3ccccc3)(CC2)C(=O)N2CCCC2)cc1Cl Show InChI InChI=1S/C25H31ClN2O3/c26-22-18-20(8-9-23(22)29)19-27-15-10-25(11-16-27,24(30)28-13-4-5-14-28)12-17-31-21-6-2-1-3-7-21/h1-3,6-9,18,29H,4-5,10-17,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271340

(US10064853, Compound 15)Show SMILES CCNC(=O)C1(CCOc2ccccc2)CCN(Cc2ccc(O)c(Cl)c2)CC1 Show InChI InChI=1S/C23H29ClN2O3/c1-2-25-22(28)23(12-15-29-19-6-4-3-5-7-19)10-13-26(14-11-23)17-18-8-9-21(27)20(24)16-18/h3-9,16,27H,2,10-15,17H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271336
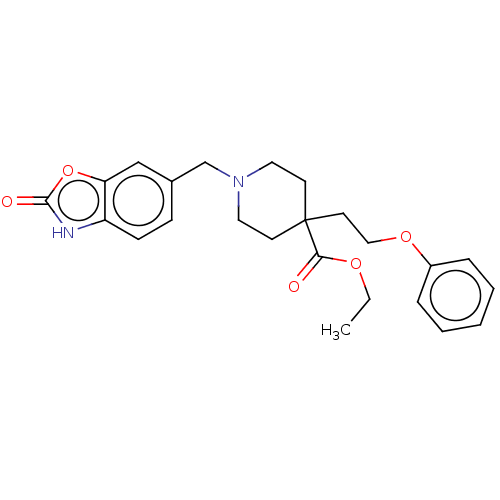
(US10064853, Compound 8)Show SMILES CCOC(=O)C1(CCOc2ccccc2)CCN(Cc2ccc3[nH]c(=O)oc3c2)CC1 Show InChI InChI=1S/C24H28N2O5/c1-2-29-22(27)24(12-15-30-19-6-4-3-5-7-19)10-13-26(14-11-24)17-18-8-9-20-21(16-18)31-23(28)25-20/h3-9,16H,2,10-15,17H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |
Protein disulfide-isomerase
(Homo sapiens (Human)) | BDBM271343

(US10064853, Compound 18)Show SMILES Oc1ccc(CN2CCC(CCOc3ccccc3)(CC2)C(=O)N2CCCC2)cc1Cl Show InChI InChI=1S/C25H31ClN2O3/c26-22-18-20(8-9-23(22)29)19-27-15-10-25(11-16-27,24(30)28-13-4-5-14-28)12-17-31-21-6-2-1-3-7-21/h1-3,6-9,18,29H,4-5,10-17,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beth Israel Deaconess Medical Center, Inc.; The Broad Institute, Inc.
US Patent
| Assay Description
Insulin reductase assay. |
US Patent US10064853 (2018)
BindingDB Entry DOI: 10.7270/Q2SN0C0V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data