

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50386376 (CHEMBL2046865) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting | ACS Med Chem Lett 3: 222-226 (2012) Article DOI: 10.1021/ml200265m BindingDB Entry DOI: 10.7270/Q2ZP475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50386365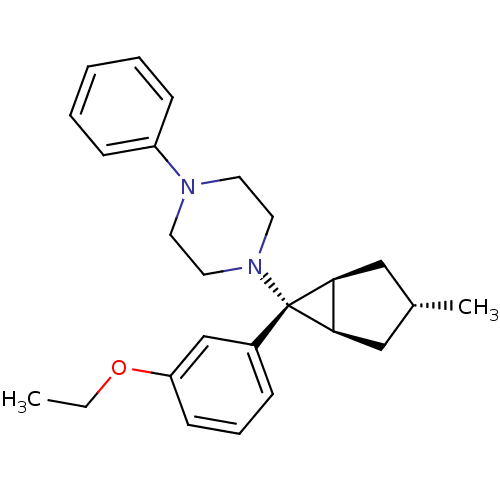 (CHEMBL2046867) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting | ACS Med Chem Lett 3: 222-226 (2012) Article DOI: 10.1021/ml200265m BindingDB Entry DOI: 10.7270/Q2ZP475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50386375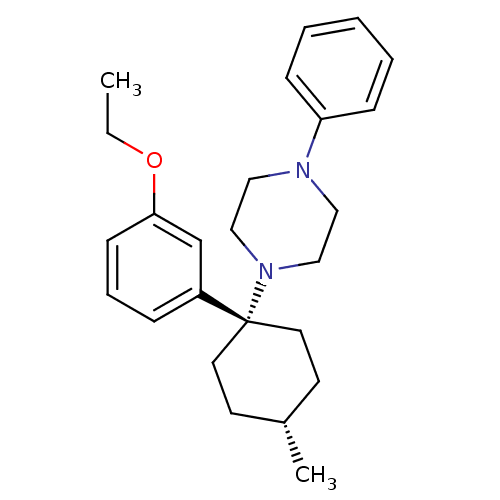 (CHEMBL2046864) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting | ACS Med Chem Lett 3: 222-226 (2012) Article DOI: 10.1021/ml200265m BindingDB Entry DOI: 10.7270/Q2ZP475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50386372 (CHEMBL2046861) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting | ACS Med Chem Lett 3: 222-226 (2012) Article DOI: 10.1021/ml200265m BindingDB Entry DOI: 10.7270/Q2ZP475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50386374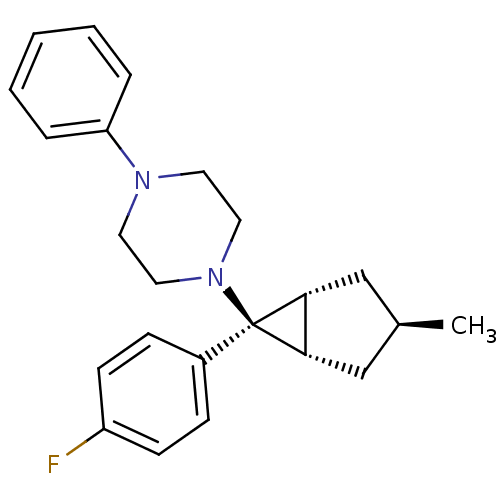 (CHEMBL2046863) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting | ACS Med Chem Lett 3: 222-226 (2012) Article DOI: 10.1021/ml200265m BindingDB Entry DOI: 10.7270/Q2ZP475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50386369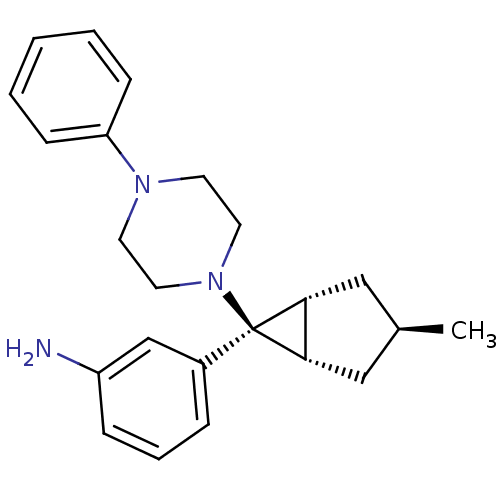 (CHEMBL2046858) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting | ACS Med Chem Lett 3: 222-226 (2012) Article DOI: 10.1021/ml200265m BindingDB Entry DOI: 10.7270/Q2ZP475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50386368 (CHEMBL2046870) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting | ACS Med Chem Lett 3: 222-226 (2012) Article DOI: 10.1021/ml200265m BindingDB Entry DOI: 10.7270/Q2ZP475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50386371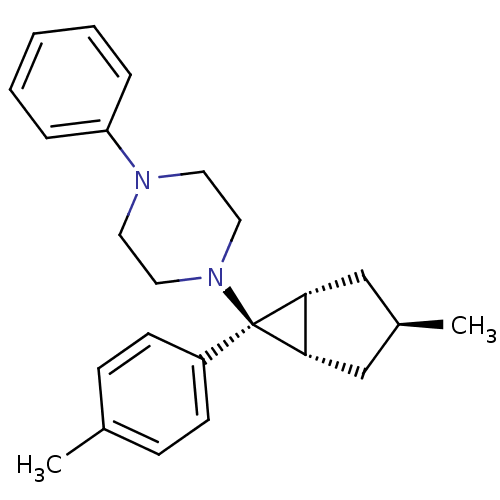 (CHEMBL2046860) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting | ACS Med Chem Lett 3: 222-226 (2012) Article DOI: 10.1021/ml200265m BindingDB Entry DOI: 10.7270/Q2ZP475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50386373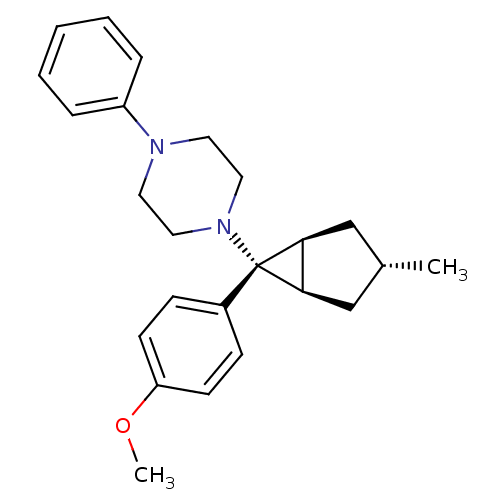 (CHEMBL2046862) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting | ACS Med Chem Lett 3: 222-226 (2012) Article DOI: 10.1021/ml200265m BindingDB Entry DOI: 10.7270/Q2ZP475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50386367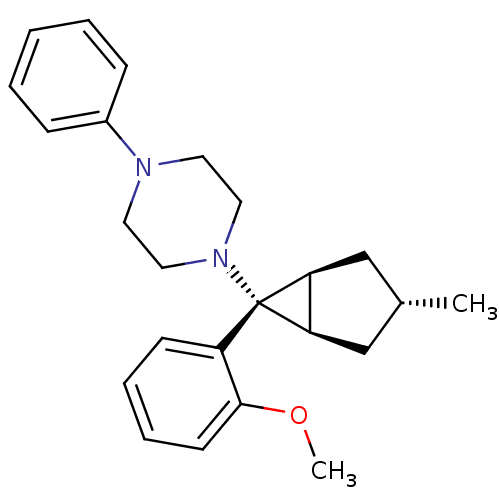 (CHEMBL2046869) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting | ACS Med Chem Lett 3: 222-226 (2012) Article DOI: 10.1021/ml200265m BindingDB Entry DOI: 10.7270/Q2ZP475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50386366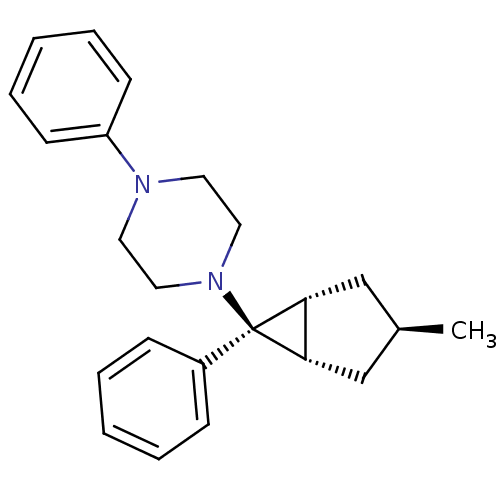 (CHEMBL2046868) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting | ACS Med Chem Lett 3: 222-226 (2012) Article DOI: 10.1021/ml200265m BindingDB Entry DOI: 10.7270/Q2ZP475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50386370 (CHEMBL2046859) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]peptideYY from NPY1 receptor in human SK-N-MC cells after 1 hr by scintillation counting | ACS Med Chem Lett 3: 222-226 (2012) Article DOI: 10.1021/ml200265m BindingDB Entry DOI: 10.7270/Q2ZP475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM20875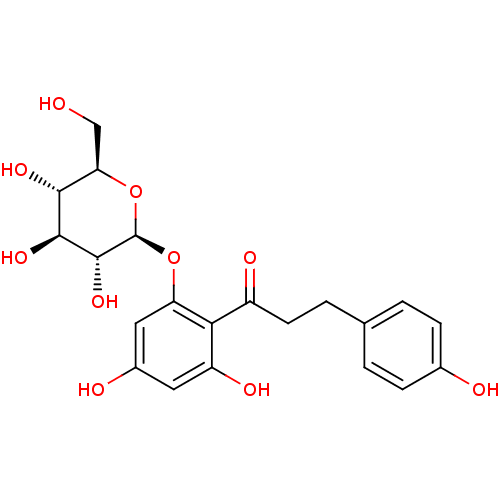 (1-(2,4-dihydroxy-6-{[(2S,3R,4S,5S,6R)-3,4,5-trihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 35.6 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM20877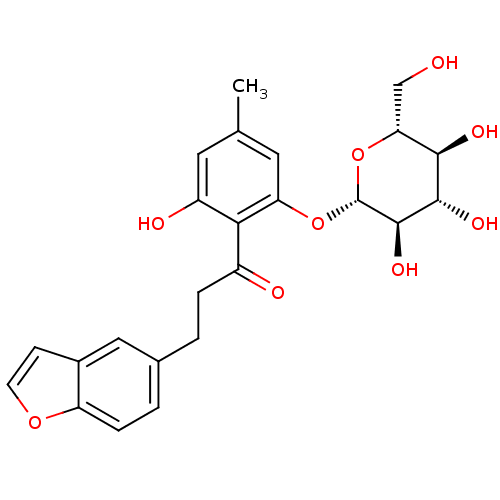 (3-(1-benzofuran-5-yl)-1-(2-hydroxy-4-methyl-6-{[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.60 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM20880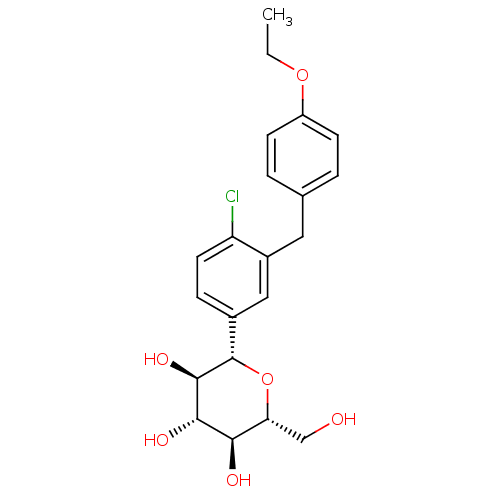 ((2S,3R,4R,5S,6R)-2-{4-chloro-3-[(4-ethoxyphenyl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.39E+3 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM20879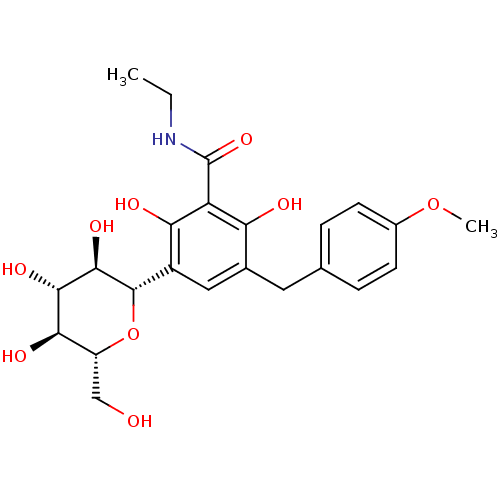 (C-aryl glucoside, 5 | CHEMBL429911 | N-ethyl-2,6-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >8.00E+3 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM20878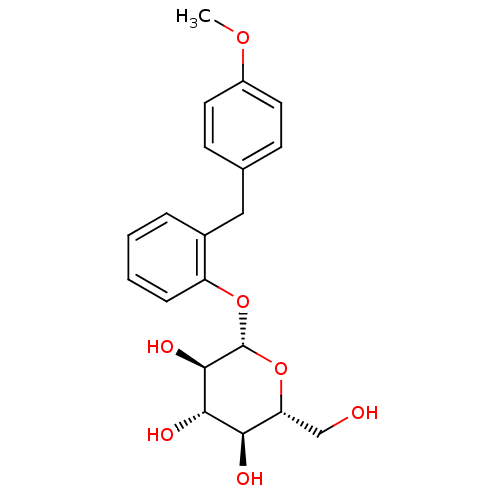 ((2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-{2-[(4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >8.00E+3 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM20877 (3-(1-benzofuran-5-yl)-1-(2-hydroxy-4-methyl-6-{[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 211 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM20875 (1-(2,4-dihydroxy-6-{[(2S,3R,4S,5S,6R)-3,4,5-trihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 330 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM20878 ((2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-{2-[(4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.20 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM20879 (C-aryl glucoside, 5 | CHEMBL429911 | N-ethyl-2,6-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM20880 ((2S,3R,4R,5S,6R)-2-{4-chloro-3-[(4-ethoxyphenyl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||