

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM221898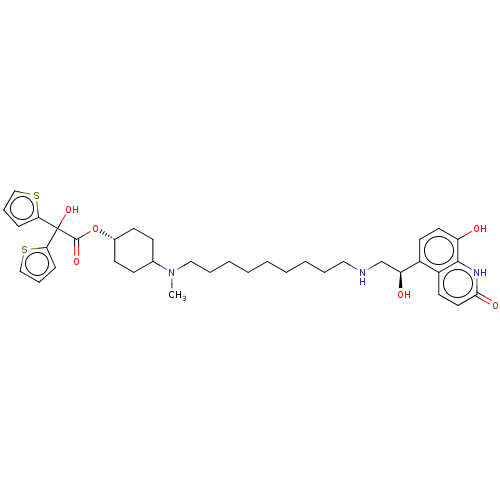 (US9315463, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM221909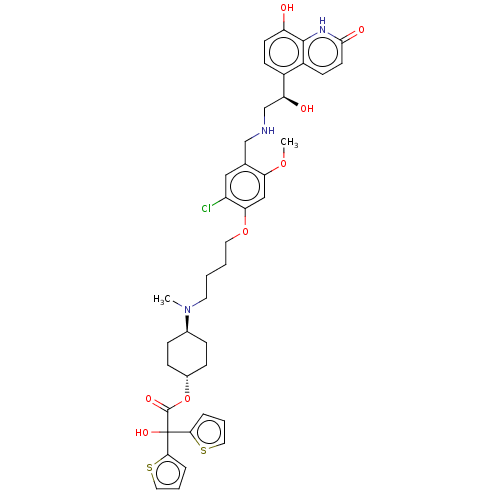 (US9315463, 25) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221910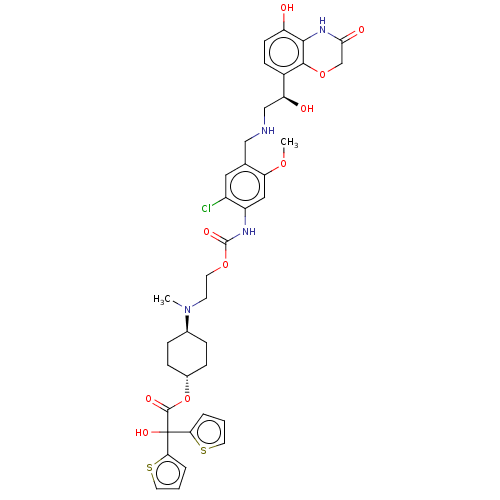 (US9315463, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM221907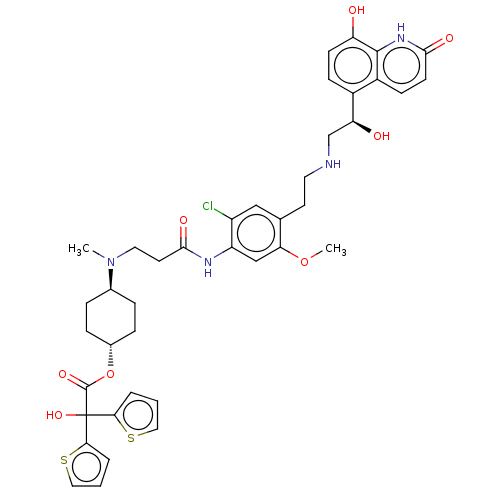 (US9315463, 20) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM221904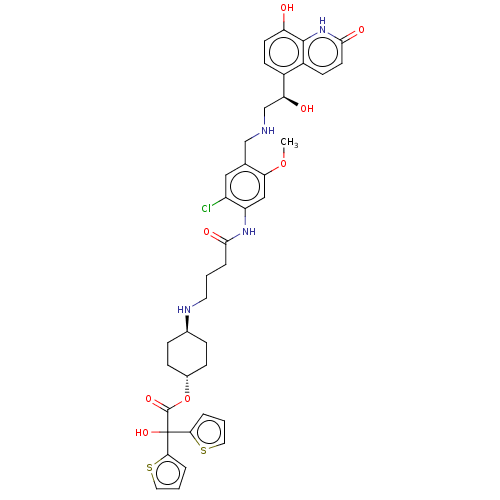 (US9315463, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM221900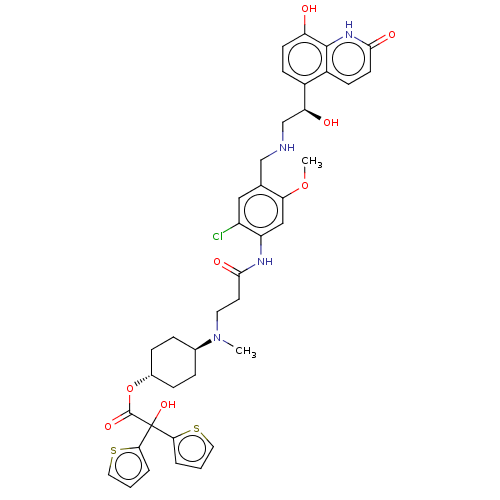 (US9315463, 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221912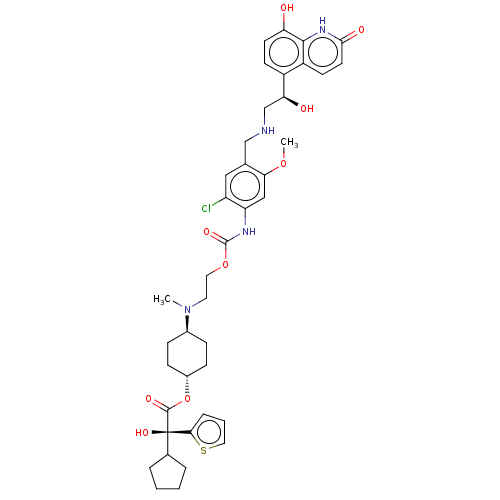 (US9315463, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM221899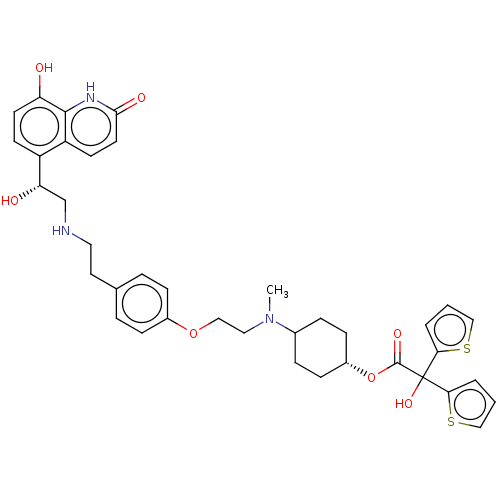 (US9315463, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221911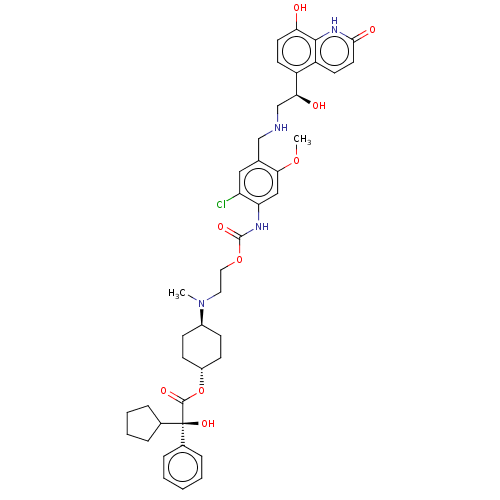 (US9315463, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM221908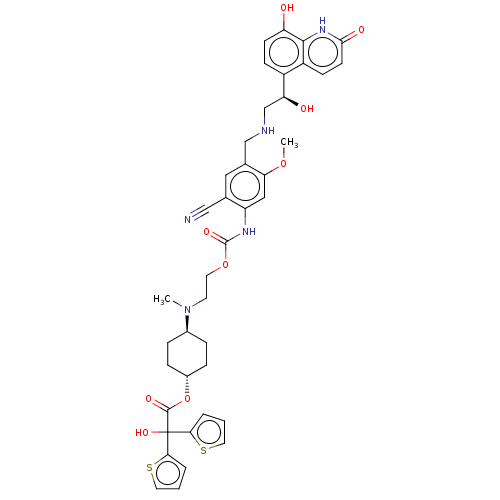 (US9315463, 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM221902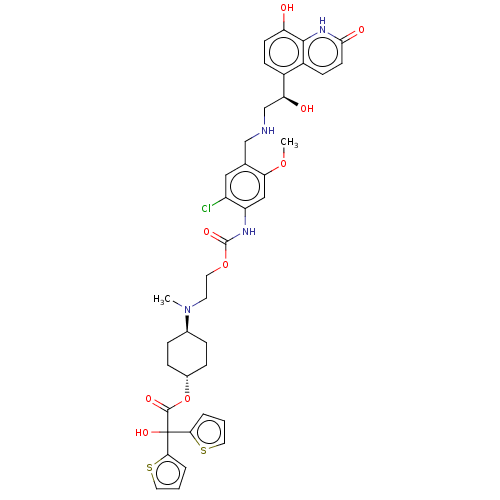 (US9315463, 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221902 (US9315463, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM221906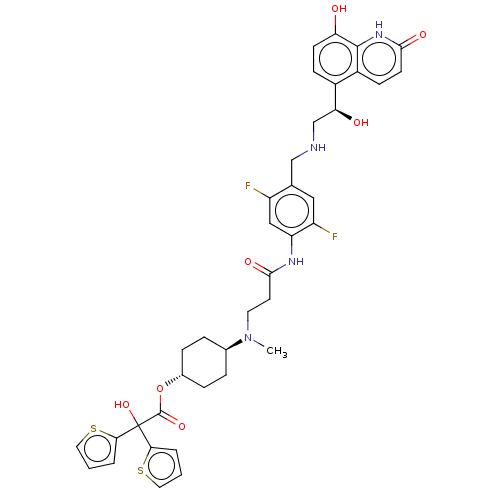 (US9315463, 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM221910 (US9315463, 26) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM221905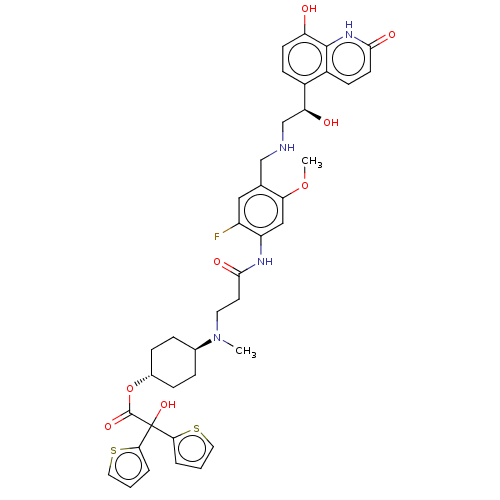 (US9315463, 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102482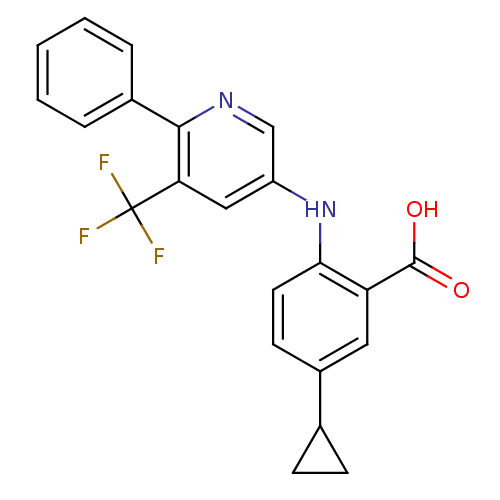 (US8536165, 81) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221904 (US9315463, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM221903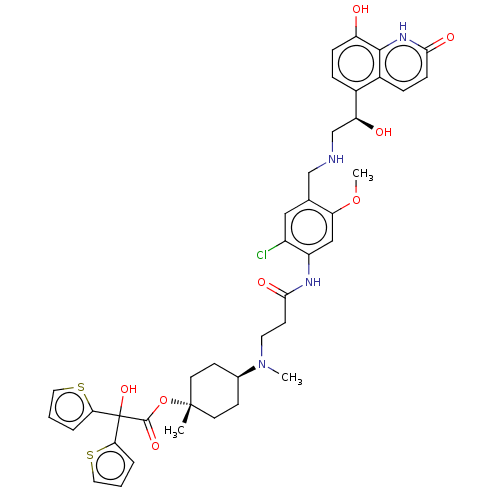 (US9315463, 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221903 (US9315463, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM221901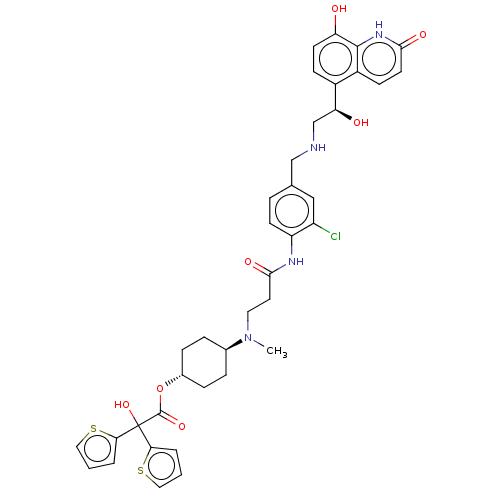 (US9315463, 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102488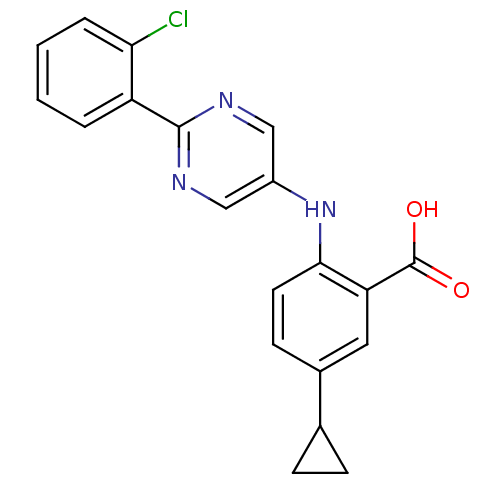 (US8536165, 92) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM221912 (US9315463, 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102485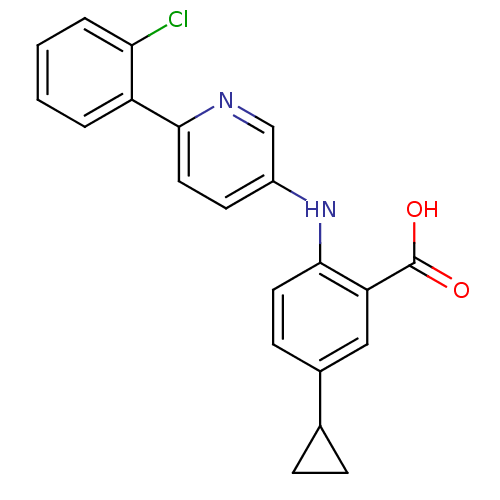 (US8536165, 86) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221908 (US9315463, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221905 (US9315463, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102476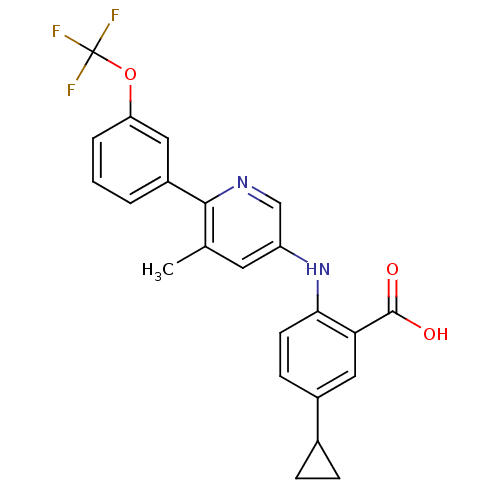 (US8536165, 73) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102480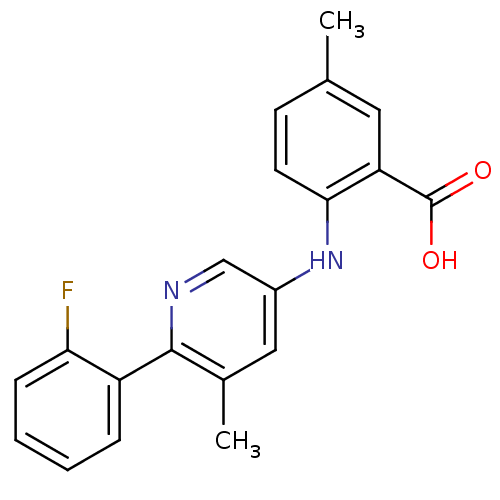 (US8536165, 77) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221907 (US9315463, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102477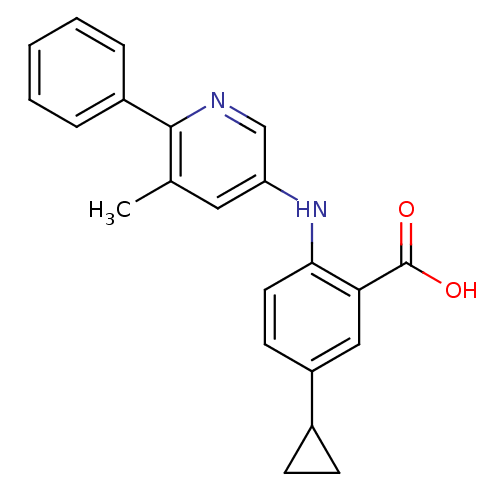 (US8536165, 74) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221898 (US9315463, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221901 (US9315463, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102483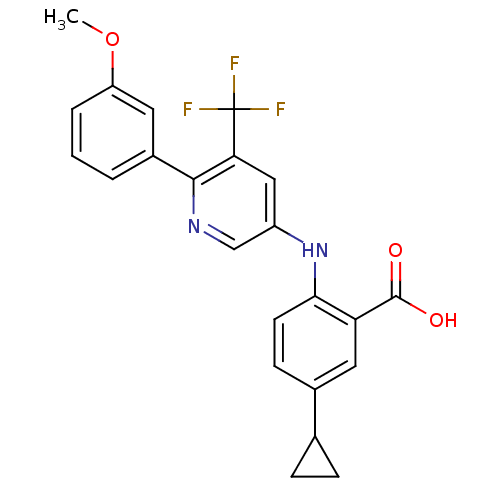 (US8536165, 82) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102497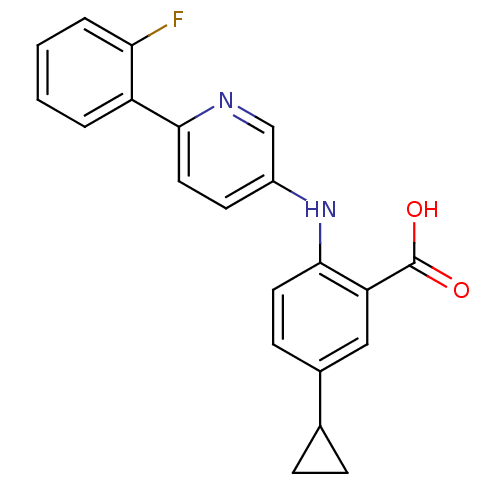 (US8536165, 107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221900 (US9315463, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM221911 (US9315463, 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A. US Patent | Assay Description The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102493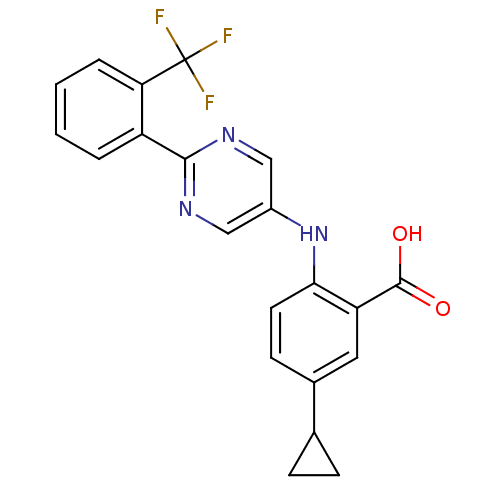 (US8536165, 97) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102490 (US8536165, 94) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221909 (US9315463, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102479 (US8536165, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102492 (US8536165, 96) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102454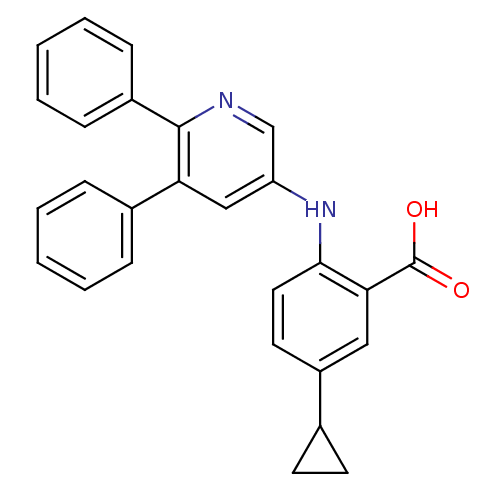 (US8536165, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102469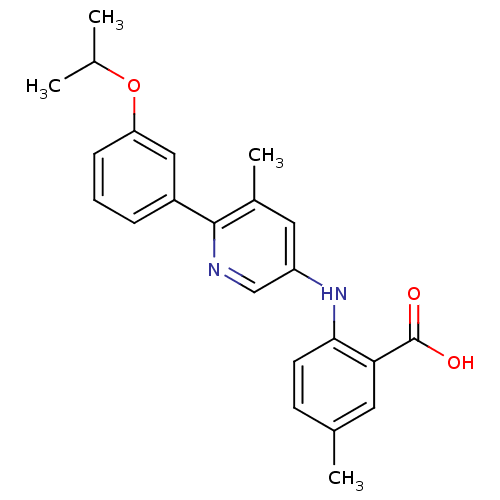 (US8536165, 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102494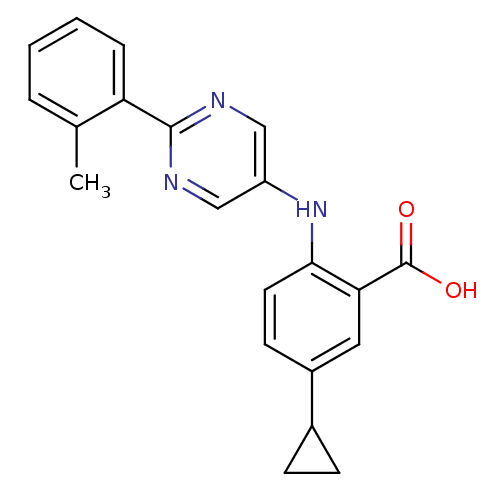 (US8536165, 98) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102491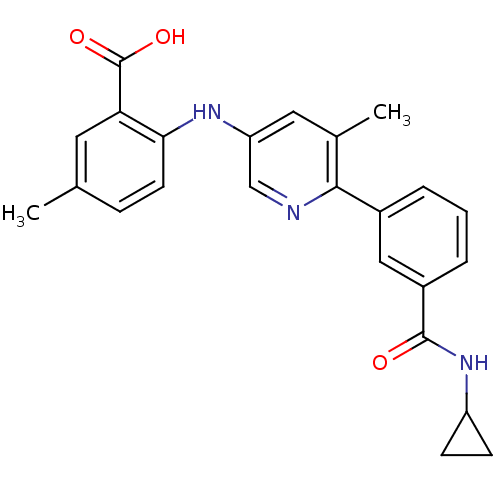 (US8536165, 95) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102473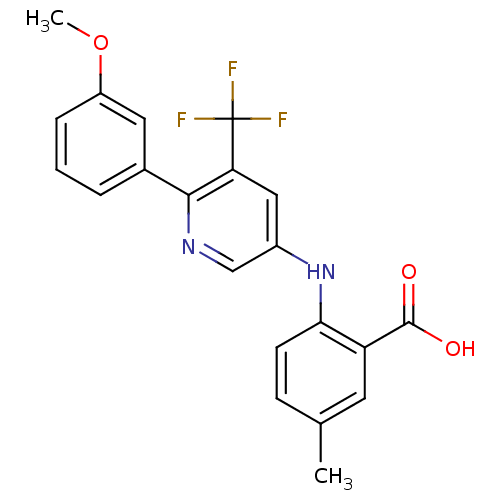 (US8536165, 67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM221906 (US9315463, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | 25 |
Almirall, S.A. US Patent | Assay Description The study of binding to human adrenergic beta1 and beta2 receptors was performed using commercial membranes prepared from Sf9 cells where they are ov... | US Patent US9315463 (2016) BindingDB Entry DOI: 10.7270/Q24X56MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102470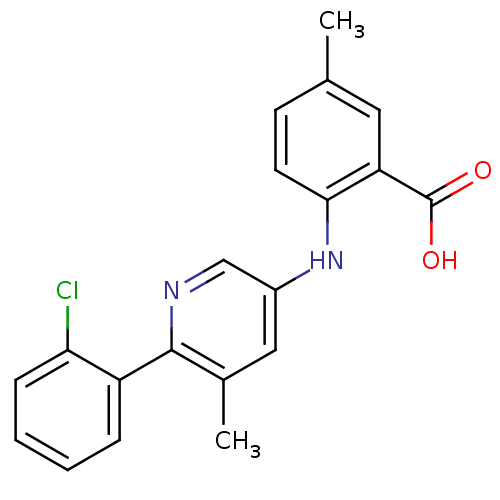 (US8536165, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102450 (US8536165, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102465 (US8536165, 54) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM102487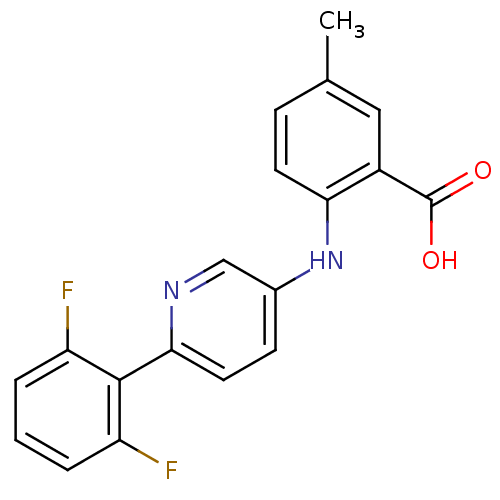 (US8536165, 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Almirall, S.A. US Patent | Assay Description Inhibition of human DHODH activity assay: DHODH activity and its inhibition were studied using a chromogen reduction assay with DCIP (2,6-dichloroph... | US Patent US8536165 (2013) BindingDB Entry DOI: 10.7270/Q2C24V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 154 total ) | Next | Last >> |