 Found 4332 hits with Last Name = 'fox' and Initial = 'b'
Found 4332 hits with Last Name = 'fox' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from alpha4beta2 nAChR (unknown origin) expressed in HEK cell membranes after 4 hrs by liquid scintillation counting ... |
J Nat Prod 81: 1029-1035 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00062
BindingDB Entry DOI: 10.7270/Q2R49TF0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50074627
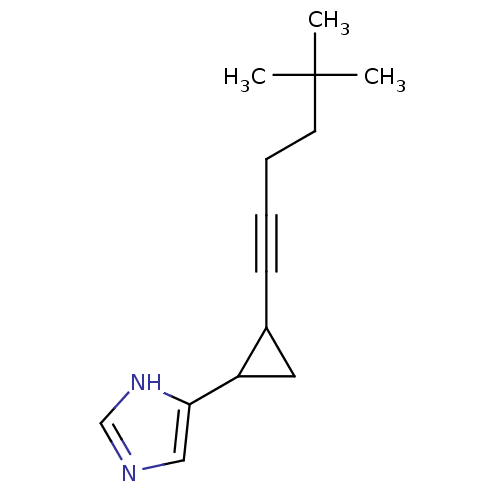
(4-[2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl]-1H-imi...)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing rat histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Dog) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158590
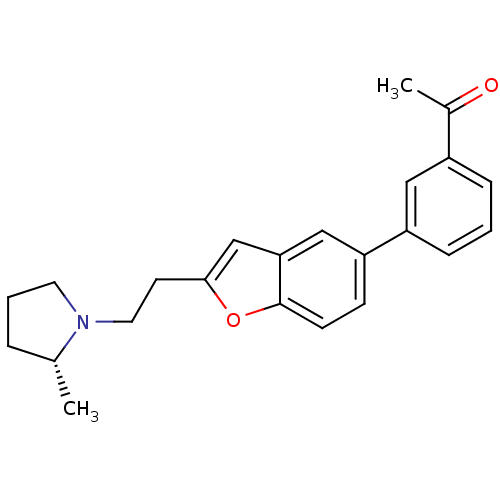
(1-(3-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-b...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1cccc(c1)C(C)=O Show InChI InChI=1S/C23H25NO2/c1-16-5-4-11-24(16)12-10-22-15-21-14-20(8-9-23(21)26-22)19-7-3-6-18(13-19)17(2)25/h3,6-9,13-16H,4-5,10-12H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158588
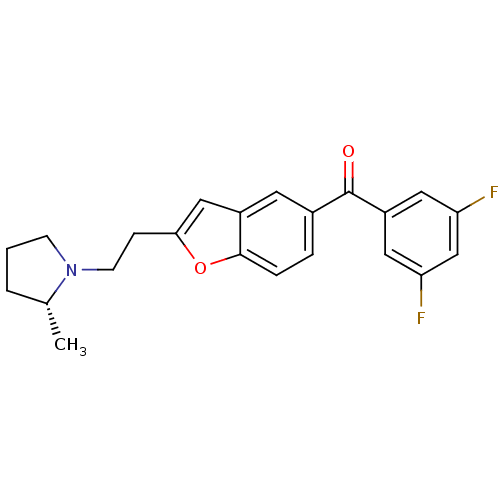
((3,5-Difluoro-phenyl)-{2-[2-((R)-2-methyl-pyrrolid...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)C(=O)c1cc(F)cc(F)c1 Show InChI InChI=1S/C22H21F2NO2/c1-14-3-2-7-25(14)8-6-20-12-16-9-15(4-5-21(16)27-20)22(26)17-10-18(23)13-19(24)11-17/h4-5,9-14H,2-3,6-8H2,1H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174152
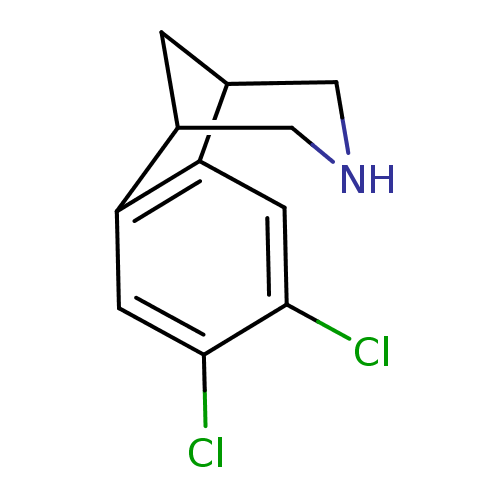
(4,5-Dichloro-10-aza-tricyclo[6.3.1.0*2,7*]dodeca-2...)Show InChI InChI=1S/C11H11Cl2N/c12-10-2-8-6-1-7(5-14-4-6)9(8)3-11(10)13/h2-3,6-7,14H,1,4-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158596
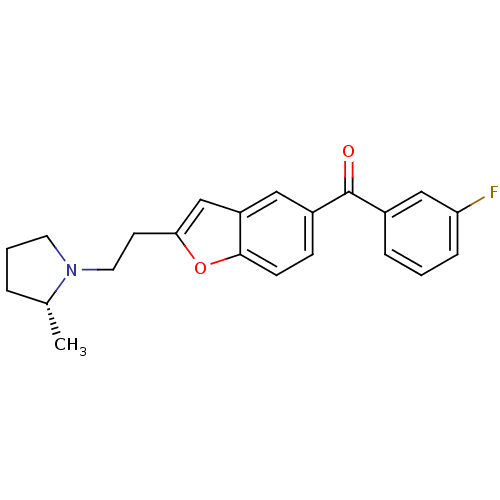
((3-Fluoro-phenyl)-{2-[2-((R)-2-methyl-pyrrolidin-1...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)C(=O)c1cccc(F)c1 Show InChI InChI=1S/C22H22FNO2/c1-15-4-3-10-24(15)11-9-20-14-18-12-17(7-8-21(18)26-20)22(25)16-5-2-6-19(23)13-16/h2,5-8,12-15H,3-4,9-11H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174139
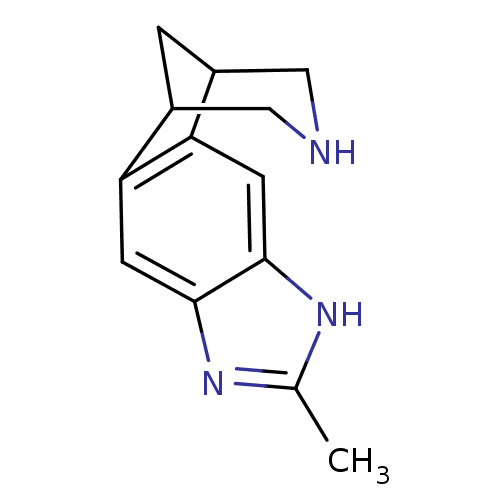
(6-methyl-5,7,13-triazatetracyclo[9.3.1.02,10.04,8]...)Show InChI InChI=1S/C13H15N3/c1-7-15-12-3-10-8-2-9(6-14-5-8)11(10)4-13(12)16-7/h3-4,8-9,14H,2,5-6H2,1H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50166908

(5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...)Show InChI InChI=1S/C13H13N3/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1/h1-2,4-5,8-9,14H,3,6-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hrh3 protein
(RAT) | BDBM50074629
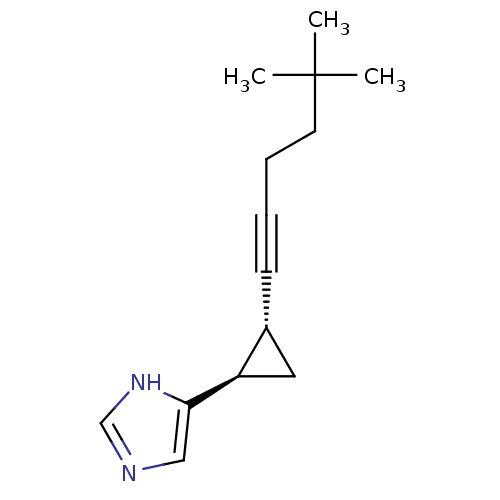
(4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16)/t11-,12-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174145
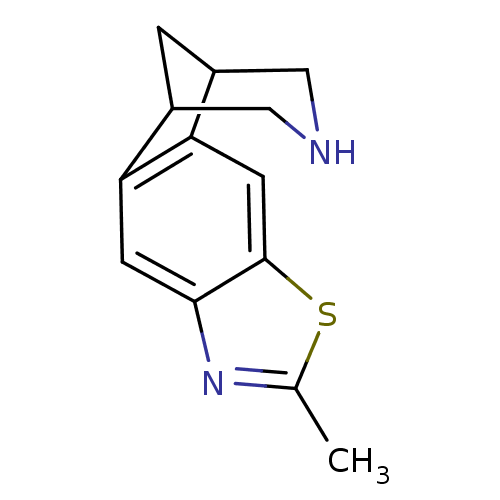
(6-methyl-7-thia-5,13-diazatetracyclo[9.3.1.02,10.0...)Show InChI InChI=1S/C13H14N2S/c1-7-15-12-3-10-8-2-9(6-14-5-8)11(10)4-13(12)16-7/h3-4,8-9,14H,2,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174148
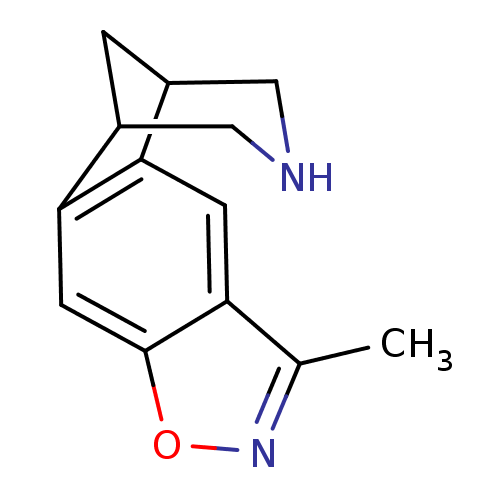
(5-methyl-7-oxa-6,13-diazatetracyclo[9.3.1.02,10.04...)Show InChI InChI=1S/C13H14N2O/c1-7-10-3-11-8-2-9(6-14-5-8)12(11)4-13(10)16-15-7/h3-4,8-9,14H,2,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174154
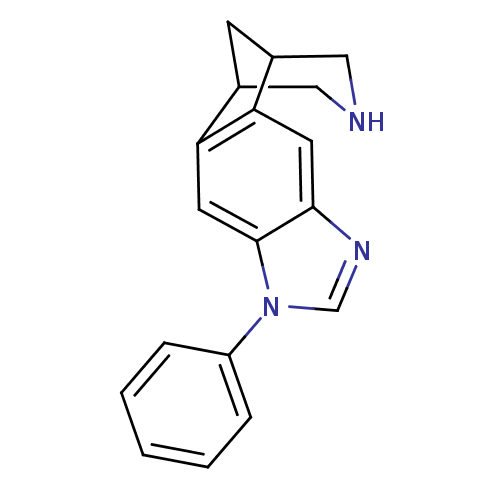
(7-phenyl-5,7,13-triazatetracyclo[9.3.1.02,10.04,8]...)Show InChI InChI=1S/C18H17N3/c1-2-4-14(5-3-1)21-11-20-17-7-15-12-6-13(10-19-9-12)16(15)8-18(17)21/h1-5,7-8,11-13,19H,6,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174156
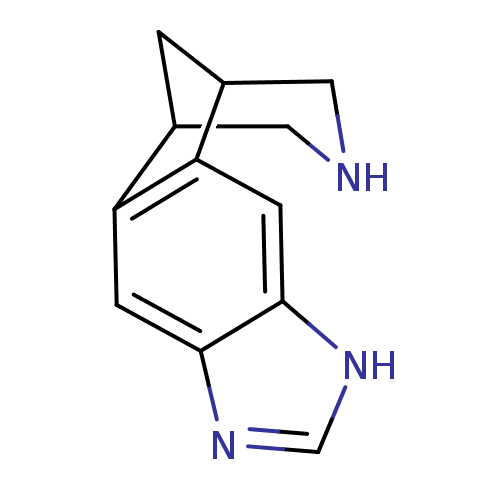
(5,7,13-triazatetracyclo[9.3.1.02,10.04,8]pentadeca...)Show InChI InChI=1S/C12H13N3/c1-7-4-13-5-8(1)10-3-12-11(2-9(7)10)14-6-15-12/h2-3,6-8,13H,1,4-5H2,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174190
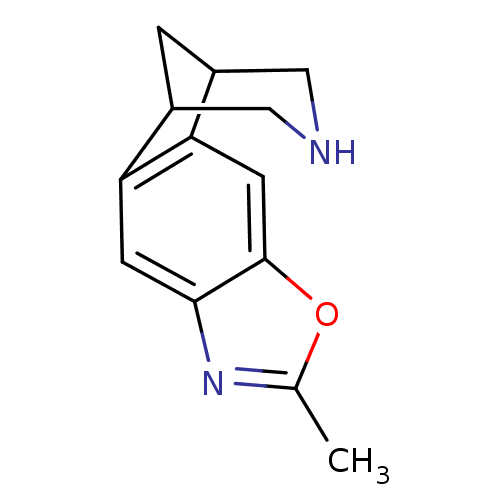
(6-methyl-7-oxa-5,13-diazatetracyclo[9.3.1.02,10.04...)Show InChI InChI=1S/C13H14N2O/c1-7-15-12-3-10-8-2-9(6-14-5-8)11(10)4-13(12)16-7/h3-4,8-9,14H,2,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174170
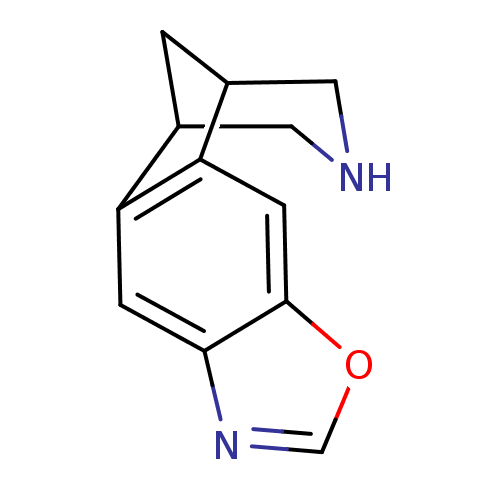
(7-oxa-5,13-diazatetracyclo[9.3.1.02,10.04,8]pentad...)Show InChI InChI=1S/C12H12N2O/c1-7-4-13-5-8(1)10-3-12-11(2-9(7)10)14-6-15-12/h2-3,6-8,13H,1,4-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174141
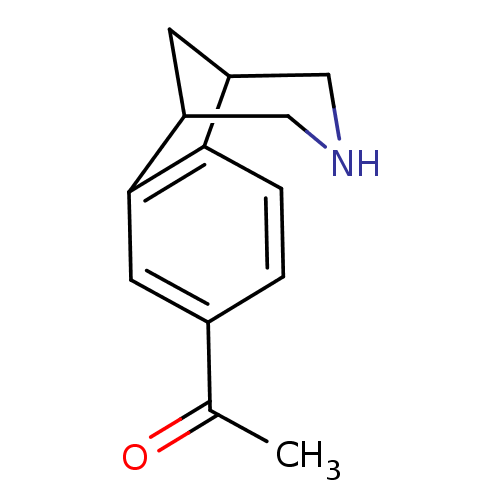
(1-(10-Aza-tricyclo[6.3.1.0*2,7*]dodeca-2(7),3,5-tr...)Show InChI InChI=1S/C13H15NO/c1-8(15)9-2-3-12-10-4-11(7-14-6-10)13(12)5-9/h2-3,5,10-11,14H,4,6-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174163
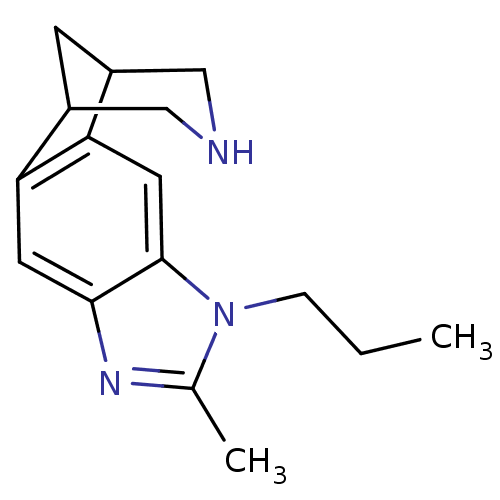
(6-methyl-7-propyl-5,7,13-triazatetracyclo[9.3.1.02...)Show InChI InChI=1S/C16H21N3/c1-3-4-19-10(2)18-15-6-13-11-5-12(9-17-8-11)14(13)7-16(15)19/h6-7,11-12,17H,3-5,8-9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50224191
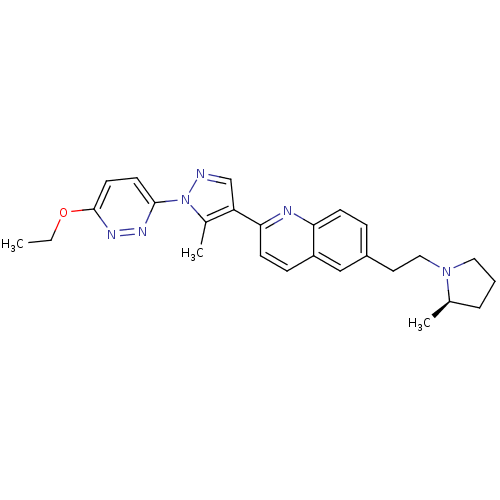
((R)-2-(1-(6-ethoxypyridazin-3-yl)-5-methyl-1H-pyra...)Show SMILES CCOc1ccc(nn1)-n1ncc(c1C)-c1ccc2cc(CCN3CCC[C@H]3C)ccc2n1 Show InChI InChI=1S/C26H30N6O/c1-4-33-26-12-11-25(29-30-26)32-19(3)22(17-27-32)24-10-8-21-16-20(7-9-23(21)28-24)13-15-31-14-5-6-18(31)2/h7-12,16-18H,4-6,13-15H2,1-3H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells |
J Med Chem 50: 5439-48 (2007)
Article DOI: 10.1021/jm0705051
BindingDB Entry DOI: 10.7270/Q25M65G0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158609
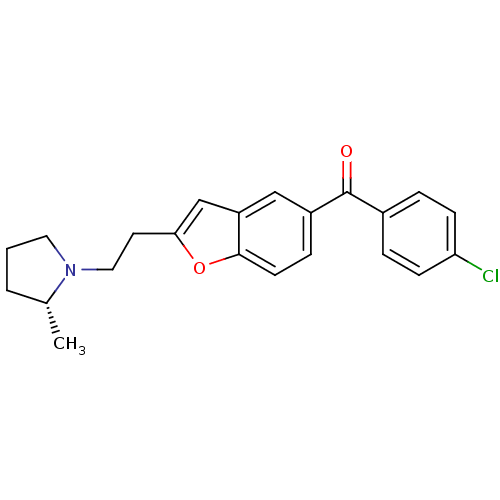
((4-Chloro-phenyl)-{2-[2-((R)-2-methyl-pyrrolidin-1...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H22ClNO2/c1-15-3-2-11-24(15)12-10-20-14-18-13-17(6-9-21(18)26-20)22(25)16-4-7-19(23)8-5-16/h4-9,13-15H,2-3,10-12H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158607

((3-Chloro-phenyl)-{2-[2-((R)-2-methyl-pyrrolidin-1...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)C(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C22H22ClNO2/c1-15-4-3-10-24(15)11-9-20-14-18-12-17(7-8-21(18)26-20)22(25)16-5-2-6-19(23)13-16/h2,5-8,12-15H,3-4,9-11H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50222968

(Cipralisant | GT-2331)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16)/t11-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards rat cortical H3 receptor |
Bioorg Med Chem Lett 13: 1325-8 (2003)
BindingDB Entry DOI: 10.7270/Q2HM59NM |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174144
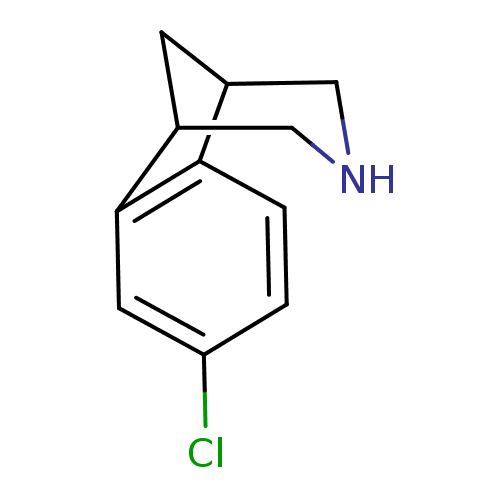
(4-Chloro-10-aza-tricyclo[6.3.1.0*2,7*]dodeca-2(7),...)Show InChI InChI=1S/C11H12ClN/c12-9-1-2-10-7-3-8(6-13-5-7)11(10)4-9/h1-2,4,7-8,13H,3,5-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174147
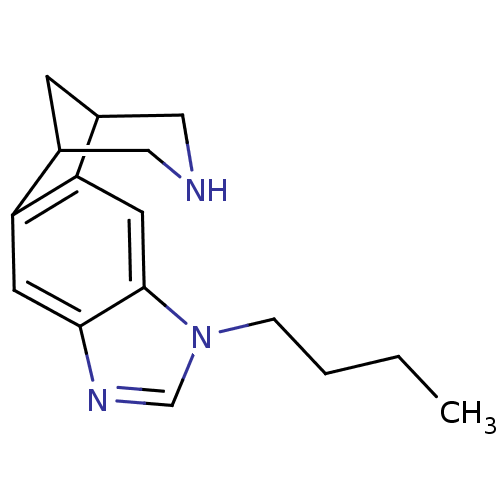
(7-butyl-5,7,13-triazatetracyclo[9.3.1.02,10.04,8]p...)Show InChI InChI=1S/C16H21N3/c1-2-3-4-19-10-18-15-6-13-11-5-12(9-17-8-11)14(13)7-16(15)19/h6-7,10-12,17H,2-5,8-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158603

((4-Fluoro-3-methyl-phenyl)-{2-[2-((R)-2-methyl-pyr...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)C(=O)c1ccc(F)c(C)c1 Show InChI InChI=1S/C23H24FNO2/c1-15-12-17(5-7-21(15)24)23(26)18-6-8-22-19(13-18)14-20(27-22)9-11-25-10-3-4-16(25)2/h5-8,12-14,16H,3-4,9-11H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158592

(CHEMBL368699 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)C(=O)c1ccc(C)cc1 Show InChI InChI=1S/C23H25NO2/c1-16-5-7-18(8-6-16)23(25)19-9-10-22-20(14-19)15-21(26-22)11-13-24-12-3-4-17(24)2/h5-10,14-15,17H,3-4,11-13H2,1-2H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158599
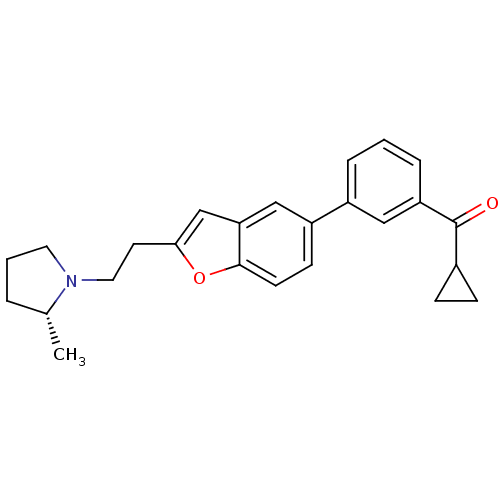
(CHEMBL369502 | Cyclopropyl-(3-{2-[2-((R)-2-methyl-...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1cccc(c1)C(=O)C1CC1 Show InChI InChI=1S/C25H27NO2/c1-17-4-3-12-26(17)13-11-23-16-22-15-20(9-10-24(22)28-23)19-5-2-6-21(14-19)25(27)18-7-8-18/h2,5-6,9-10,14-18H,3-4,7-8,11-13H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from alpha3beta4 nAChR (unknown origin) expressed in HEK cell membranes after 4 hrs by liquid scintillation counting ... |
J Nat Prod 81: 1029-1035 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00062
BindingDB Entry DOI: 10.7270/Q2R49TF0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158605
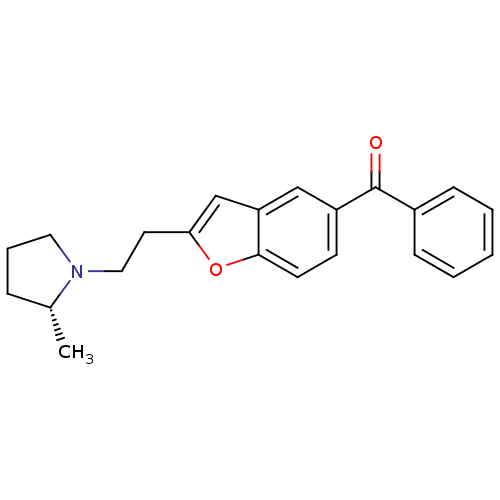
(CHEMBL362662 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)C(=O)c1ccccc1 Show InChI InChI=1S/C22H23NO2/c1-16-6-5-12-23(16)13-11-20-15-19-14-18(9-10-21(19)25-20)22(24)17-7-3-2-4-8-17/h2-4,7-10,14-16H,5-6,11-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50143282
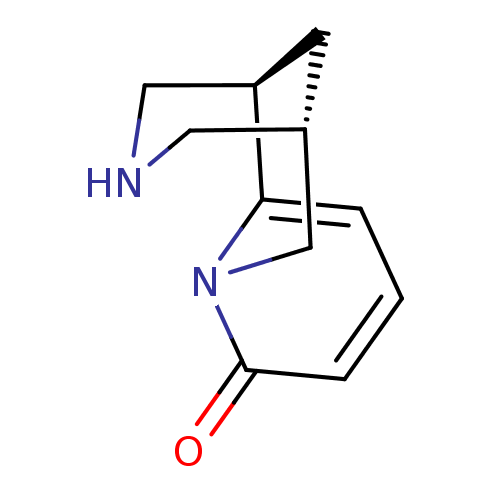
((-)-cytisine | (1R,5S)-1,2,3,4,5,6-Hexahydro-1,5-m...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2/t8?,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50143282

((-)-cytisine | (1R,5S)-1,2,3,4,5,6-Hexahydro-1,5-m...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2/t8?,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against nicotinic acetylcholine receptor alpha4-beta2 in human HEK293 cells using [3H]- nicotine as radioligand |
Bioorg Med Chem Lett 15: 2974-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.036
BindingDB Entry DOI: 10.7270/Q25Q4WWB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158593
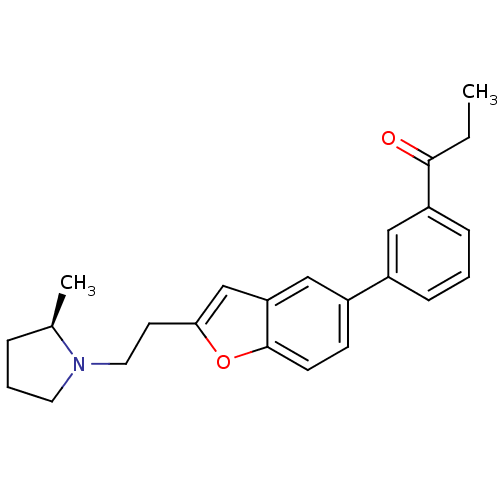
(1-(3-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-b...)Show SMILES CCC(=O)c1cccc(c1)-c1ccc2oc(CCN3CCC[C@H]3C)cc2c1 Show InChI InChI=1S/C24H27NO2/c1-3-23(26)20-8-4-7-18(14-20)19-9-10-24-21(15-19)16-22(27-24)11-13-25-12-5-6-17(25)2/h4,7-10,14-17H,3,5-6,11-13H2,1-2H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50224192
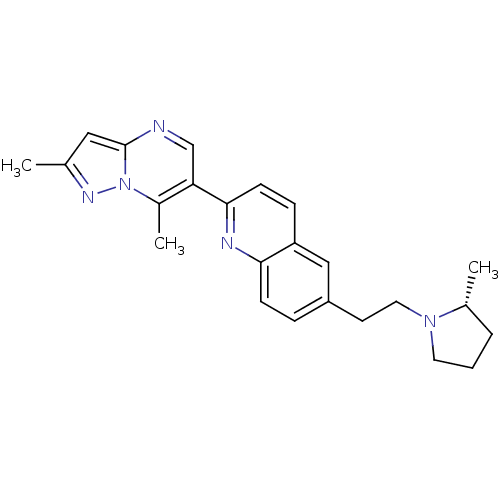
((R)-2-(2,7-dimethylpyrazolo[1,5-a]pyrimidin-6-yl)-...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1cnc2cc(C)nn2c1C Show InChI InChI=1S/C24H27N5/c1-16-13-24-25-15-21(18(3)29(24)27-16)23-9-7-20-14-19(6-8-22(20)26-23)10-12-28-11-4-5-17(28)2/h6-9,13-15,17H,4-5,10-12H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells |
J Med Chem 50: 5439-48 (2007)
Article DOI: 10.1021/jm0705051
BindingDB Entry DOI: 10.7270/Q25M65G0 |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50074629

(4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16)/t11-,12-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158604
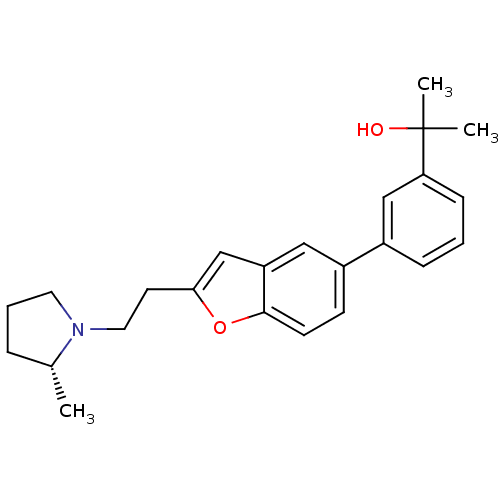
(1-(3-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-b...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1cccc(c1)C(C)(C)O Show InChI InChI=1S/C24H29NO2/c1-17-6-5-12-25(17)13-11-22-16-20-14-19(9-10-23(20)27-22)18-7-4-8-21(15-18)24(2,3)26/h4,7-10,14-17,26H,5-6,11-13H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50224187
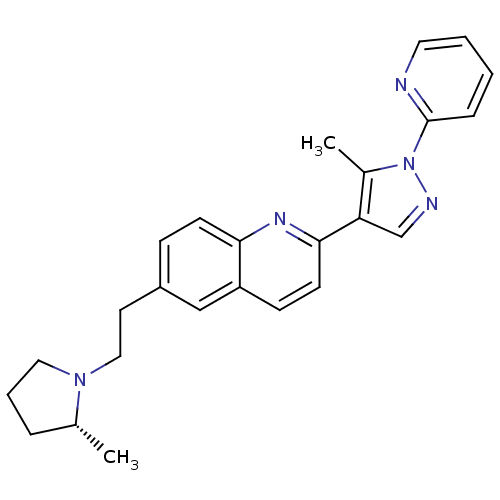
((R)-2-(5-methyl-1-(pyridin-2-yl)-1H-pyrazol-4-yl)-...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1cnn(c1C)-c1ccccn1 Show InChI InChI=1S/C25H27N5/c1-18-6-5-14-29(18)15-12-20-8-10-23-21(16-20)9-11-24(28-23)22-17-27-30(19(22)2)25-7-3-4-13-26-25/h3-4,7-11,13,16-18H,5-6,12,14-15H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells |
J Med Chem 50: 5439-48 (2007)
Article DOI: 10.1021/jm0705051
BindingDB Entry DOI: 10.7270/Q25M65G0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158598

(CHEMBL178950 | Cyclopropyl-(4-{2-[2-((R)-2-methyl-...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1ccc(cc1)C(=O)C1CC1 Show InChI InChI=1S/C25H27NO2/c1-17-3-2-13-26(17)14-12-23-16-22-15-21(10-11-24(22)28-23)18-4-6-19(7-5-18)25(27)20-8-9-20/h4-7,10-11,15-17,20H,2-3,8-9,12-14H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50465459

(CHEMBL4290888)Show SMILES [H][C@@]12CC[C@@]11Oc3nc(Cl)ccc3[C@@]1([H])CN2 |r| Show InChI InChI=1S/C11H11ClN2O/c12-9-2-1-6-7-5-13-8-3-4-11(7,8)15-10(6)14-9/h1-2,7-8,13H,3-5H2/t7-,8-,11+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from alpha4beta2 nAChR (unknown origin) expressed in HEK cell membranes after 4 hrs by liquid scintillation counting ... |
J Nat Prod 81: 1029-1035 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00062
BindingDB Entry DOI: 10.7270/Q2R49TF0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158610
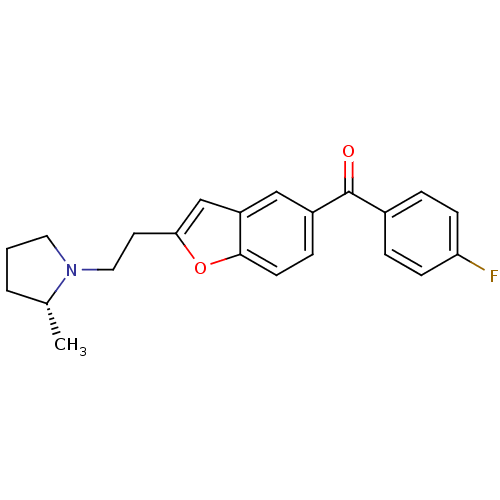
((4-Fluoro-phenyl)-{2-[2-((R)-2-methyl-pyrrolidin-1...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)C(=O)c1ccc(F)cc1 Show InChI InChI=1S/C22H22FNO2/c1-15-3-2-11-24(15)12-10-20-14-18-13-17(6-9-21(18)26-20)22(25)16-4-7-19(23)8-5-16/h4-9,13-15H,2-3,10-12H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158602
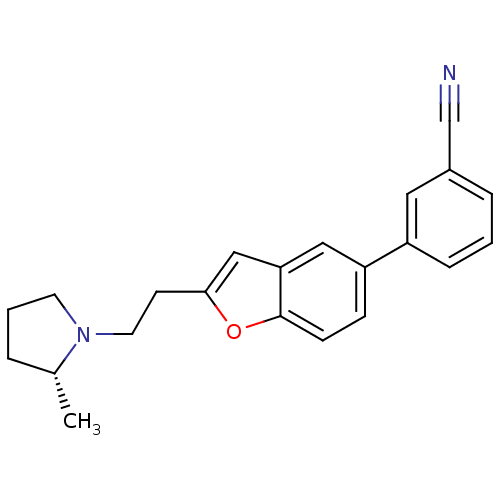
(3-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-benz...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1cccc(c1)C#N Show InChI InChI=1S/C22H22N2O/c1-16-4-3-10-24(16)11-9-21-14-20-13-19(7-8-22(20)25-21)18-6-2-5-17(12-18)15-23/h2,5-8,12-14,16H,3-4,9-11H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174158
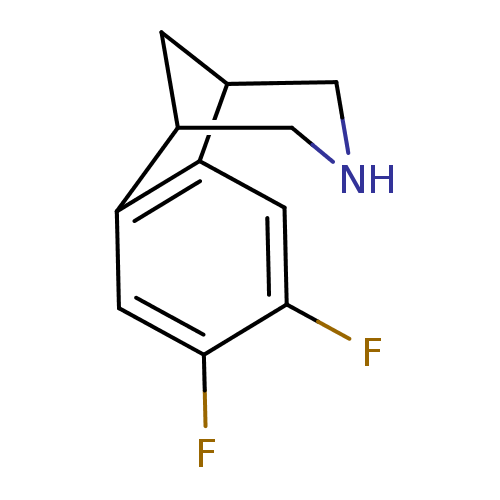
(4,5-Difluoro-10-aza-tricyclo[6.3.1.0*2,7*]dodeca-2...)Show InChI InChI=1S/C11H11F2N/c12-10-2-8-6-1-7(5-14-4-6)9(8)3-11(10)13/h2-3,6-7,14H,1,4-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174142
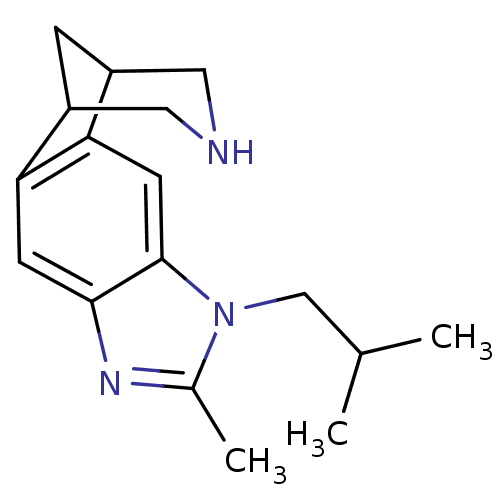
(7-isobutyl-6-methyl-5,7,13-triazatetracyclo[9.3.1....)Show InChI InChI=1S/C17H23N3/c1-10(2)9-20-11(3)19-16-5-14-12-4-13(8-18-7-12)15(14)6-17(16)20/h5-6,10,12-13,18H,4,7-9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50224189
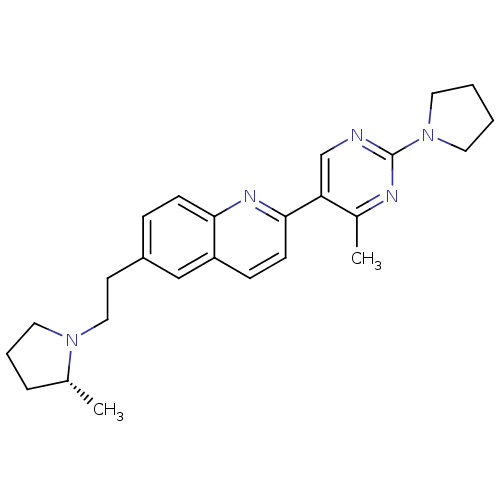
((R)-2-(4-methyl-2-(pyrrolidin-1-yl)pyrimidin-5-yl)...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1cnc(nc1C)N1CCCC1 Show InChI InChI=1S/C25H31N5/c1-18-6-5-14-29(18)15-11-20-7-9-23-21(16-20)8-10-24(28-23)22-17-26-25(27-19(22)2)30-12-3-4-13-30/h7-10,16-18H,3-6,11-15H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells |
J Med Chem 50: 5439-48 (2007)
Article DOI: 10.1021/jm0705051
BindingDB Entry DOI: 10.7270/Q25M65G0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174176
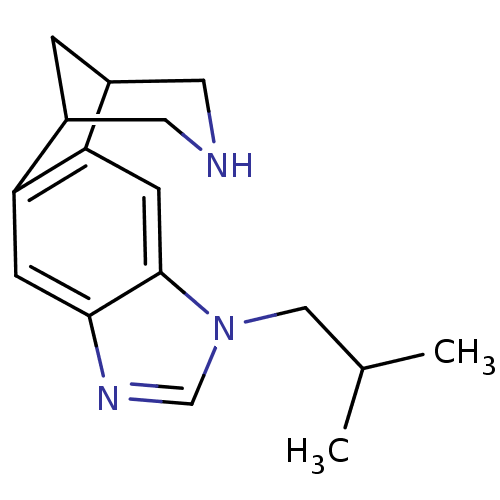
(7-isobutyl-5,7,13-triazatetracyclo[9.3.1.02,10.04,...)Show InChI InChI=1S/C16H21N3/c1-10(2)8-19-9-18-15-4-13-11-3-12(7-17-6-11)14(13)5-16(15)19/h4-5,9-12,17H,3,6-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174180
(6,7-dimethyl-5,7,13-triazatetracyclo[9.3.1.02,10.0...)Show InChI InChI=1S/C14H17N3/c1-8-16-13-4-11-9-3-10(7-15-6-9)12(11)5-14(13)17(8)2/h4-5,9-10,15H,3,6-7H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174183
(4-Trifluoromethyl-10-aza-tricyclo[6.3.1.0*2,7*]dod...)Show InChI InChI=1S/C12H12F3N/c13-12(14,15)9-1-2-10-7-3-8(6-16-5-7)11(10)4-9/h1-2,4,7-8,16H,3,5-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50224188
((R)-2-methyl-3-(6-(2-(2-methylpyrrolidin-1-yl)ethy...)Show SMILES C[C@@H]1CCCN1CCc1ccc2nc(ccc2c1)-c1cc2cccnc2nc1C Show InChI InChI=1S/C25H26N4/c1-17-5-4-13-29(17)14-11-19-7-9-23-20(15-19)8-10-24(28-23)22-16-21-6-3-12-26-25(21)27-18(22)2/h3,6-10,12,15-17H,4-5,11,13-14H2,1-2H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells |
J Med Chem 50: 5439-48 (2007)
Article DOI: 10.1021/jm0705051
BindingDB Entry DOI: 10.7270/Q25M65G0 |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data