 Found 188 hits with Last Name = 'francisco' and Initial = 'g'
Found 188 hits with Last Name = 'francisco' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50047247

(1,3-Dibutyl-7-(2-oxo-propyl)-3,7-dihydro-purine-2,...)Show InChI InChI=1S/C16H24N4O3/c1-4-6-8-19-14-13(18(11-17-14)10-12(3)21)15(22)20(16(19)23)9-7-5-2/h11H,4-10H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited |
J Med Chem 43: 1223-33 (2000)
Article DOI: 10.1021/jm990558l
BindingDB Entry DOI: 10.7270/Q2NK3HRK |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191378

(CHEMBL212163 | sodium (R,E)-6-((6,8-dihydro-5H-imi...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C/c1cn2CCOCc2n1 |t:3| Show InChI InChI=1S/C13H11N3O4S/c17-11-8(12-16(11)9(6-21-12)13(18)19)3-7-4-15-1-2-20-5-10(15)14-7/h3-4,6,12H,1-2,5H2,(H,18,19)/p-1/b8-3+/t12-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191389
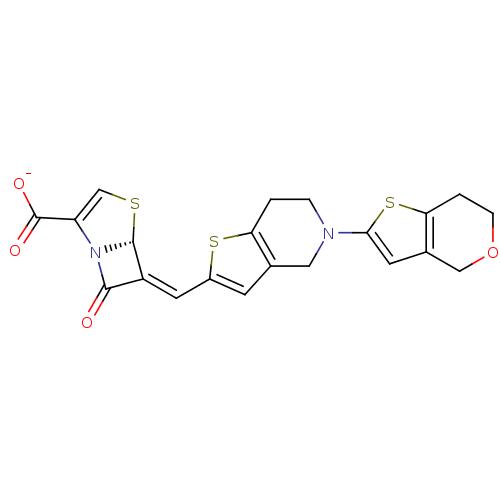
(6-(6,7-dihydro-4H-thieno[3,2-c]pyran-2-ylmethylene...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CN(CCc2s1)c1cc2COCCc2s1 |t:3| Show InChI InChI=1S/C21H18N2O4S3/c24-19-14(20-23(19)15(10-28-20)21(25)26)7-13-5-11-8-22(3-1-16(11)29-13)18-6-12-9-27-4-2-17(12)30-18/h5-7,10,20H,1-4,8-9H2,(H,25,26)/p-1/b14-7-/t20-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191379

((5R)(6Z)-6-(6,7-dihydro-4H-pyrazolo[5,1-c][1,4]-th...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CSCCn2n1 |t:3| Show InChI InChI=1S/C13H11N3O3S2/c17-11-9(12-16(11)10(6-21-12)13(18)19)4-7-3-8-5-20-2-1-15(8)14-7/h3-4,6,12H,1-2,5H2,(H,18,19)/p-1/b9-4-/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191377

((5R),(6Z)-7-oxo-6-(4,5,6,7-tetrahydropyrazolo[1,5-...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CNCCn2n1 |t:3| Show InChI InChI=1S/C13H12N4O3S/c18-11-9(12-17(11)10(6-21-12)13(19)20)4-7-3-8-5-14-1-2-16(8)15-7/h3-4,6,12,14H,1-2,5H2,(H,19,20)/p-1/b9-4-/t12-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191379

((5R)(6Z)-6-(6,7-dihydro-4H-pyrazolo[5,1-c][1,4]-th...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CSCCn2n1 |t:3| Show InChI InChI=1S/C13H11N3O3S2/c17-11-9(12-16(11)10(6-21-12)13(18)19)4-7-3-8-5-20-2-1-15(8)14-7/h3-4,6,12H,1-2,5H2,(H,18,19)/p-1/b9-4-/t12-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191390
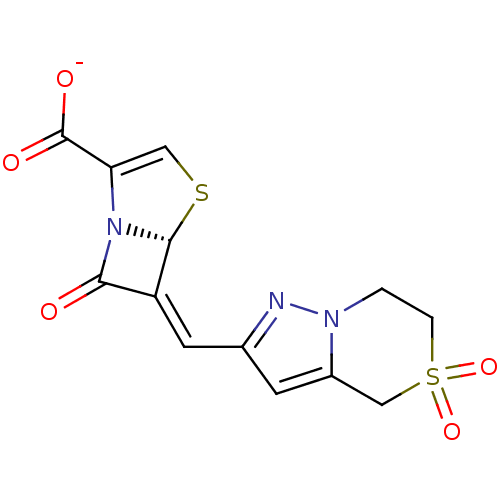
((5R),(6Z)-6-(5,5-dioxo-4,5,6,7-tetrahydro-5'6-pyra...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CS(=O)(=O)CCn2n1 |t:3| Show InChI InChI=1S/C13H11N3O5S2/c17-11-9(12-16(11)10(5-22-12)13(18)19)4-7-3-8-6-23(20,21)2-1-15(8)14-7/h3-5,12H,1-2,6H2,(H,18,19)/p-1/b9-4-/t12-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50473208

(CHEMBL20531)Show SMILES CCCCn1c2ncn(c2c(=O)n(CCCC)c1=O)S(=O)(=O)c1ccc(OC)c(OC)c1 Show InChI InChI=1S/C21H28N4O6S/c1-5-7-11-23-19-18(20(26)24(21(23)27)12-8-6-2)25(14-22-19)32(28,29)15-9-10-16(30-3)17(13-15)31-4/h9-10,13-14H,5-8,11-12H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited |
J Med Chem 43: 1223-33 (2000)
Article DOI: 10.1021/jm990558l
BindingDB Entry DOI: 10.7270/Q2NK3HRK |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50473209
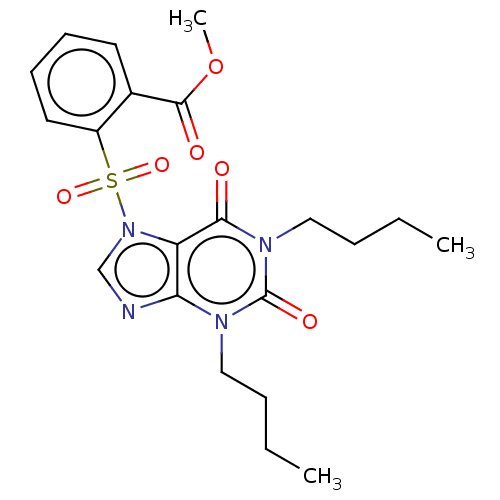
(CHEMBL20958)Show SMILES CCCCn1c2ncn(c2c(=O)n(CCCC)c1=O)S(=O)(=O)c1ccccc1C(=O)OC Show InChI InChI=1S/C21H26N4O6S/c1-4-6-12-23-18-17(19(26)24(21(23)28)13-7-5-2)25(14-22-18)32(29,30)16-11-9-8-10-15(16)20(27)31-3/h8-11,14H,4-7,12-13H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited |
J Med Chem 43: 1223-33 (2000)
Article DOI: 10.1021/jm990558l
BindingDB Entry DOI: 10.7270/Q2NK3HRK |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191386

((5R)(6Z)-7-oxo-6-(4,5,6,7-tetrahydropyrazolo-[1,5-...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CCCCn2n1 |t:3| Show InChI InChI=1S/C14H13N3O3S/c18-12-10(13-17(12)11(7-21-13)14(19)20)6-8-5-9-3-1-2-4-16(9)15-8/h5-7,13H,1-4H2,(H,19,20)/p-1/b10-6-/t13-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191390

((5R),(6Z)-6-(5,5-dioxo-4,5,6,7-tetrahydro-5'6-pyra...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CS(=O)(=O)CCn2n1 |t:3| Show InChI InChI=1S/C13H11N3O5S2/c17-11-9(12-16(11)10(5-22-12)13(18)19)4-7-3-8-6-23(20,21)2-1-15(8)14-7/h3-5,12H,1-2,6H2,(H,18,19)/p-1/b9-4-/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191381

((5R,6Z)-6-{[5-(4-methoxybenzyl)-4,5,6,7-tetrahydro...)Show SMILES COc1ccc(CN2CCc3sc(\C=C4/[C@H]5SC=C(N5C4=O)C([O-])=O)cc3C2)cc1 |c:17| Show InChI InChI=1S/C22H20N2O4S2/c1-28-15-4-2-13(3-5-15)10-23-7-6-19-14(11-23)8-16(30-19)9-17-20(25)24-18(22(26)27)12-29-21(17)24/h2-5,8-9,12,21H,6-7,10-11H2,1H3,(H,26,27)/p-1/b17-9-/t21-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191383

((5R),(6Z)-6-(5,6-dihydro-8H-imidazo[2,1-c]-[1,4]th...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2CCSCc2n1 |t:3| Show InChI InChI=1S/C13H11N3O3S2/c17-11-8(12-16(11)9(5-21-12)13(18)19)3-7-4-15-1-2-20-6-10(15)14-7/h3-5,12H,1-2,6H2,(H,18,19)/p-1/b8-3-/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191377

((5R),(6Z)-7-oxo-6-(4,5,6,7-tetrahydropyrazolo[1,5-...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CNCCn2n1 |t:3| Show InChI InChI=1S/C13H12N4O3S/c18-11-9(12-17(11)10(6-21-12)13(19)20)4-7-3-8-5-14-1-2-16(8)15-7/h3-4,6,12,14H,1-2,5H2,(H,19,20)/p-1/b9-4-/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191383

((5R),(6Z)-6-(5,6-dihydro-8H-imidazo[2,1-c]-[1,4]th...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2CCSCc2n1 |t:3| Show InChI InChI=1S/C13H11N3O3S2/c17-11-8(12-16(11)9(5-21-12)13(18)19)3-7-4-15-1-2-20-6-10(15)14-7/h3-5,12H,1-2,6H2,(H,18,19)/p-1/b8-3-/t12-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191386

((5R)(6Z)-7-oxo-6-(4,5,6,7-tetrahydropyrazolo-[1,5-...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CCCCn2n1 |t:3| Show InChI InChI=1S/C14H13N3O3S/c18-12-10(13-17(12)11(7-21-13)14(19)20)6-8-5-9-3-1-2-4-16(9)15-8/h5-7,13H,1-4H2,(H,19,20)/p-1/b10-6-/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50473206

(CHEMBL20951)Show SMILES CCCCn1c2ncn(c2c(=O)n(CCCC)c1=O)S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C20H26N4O4S/c1-3-5-12-22-18-17(19(25)23(20(22)26)13-6-4-2)24(15-21-18)29(27,28)14-16-10-8-7-9-11-16/h7-11,15H,3-6,12-14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited |
J Med Chem 43: 1223-33 (2000)
Article DOI: 10.1021/jm990558l
BindingDB Entry DOI: 10.7270/Q2NK3HRK |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191389

(6-(6,7-dihydro-4H-thieno[3,2-c]pyran-2-ylmethylene...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CN(CCc2s1)c1cc2COCCc2s1 |t:3| Show InChI InChI=1S/C21H18N2O4S3/c24-19-14(20-23(19)15(10-28-20)21(25)26)7-13-5-11-8-22(3-1-16(11)29-13)18-6-12-9-27-4-2-17(12)30-18/h5-7,10,20H,1-4,8-9H2,(H,25,26)/p-1/b14-7-/t20-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191387

((5R),(6Z)-6-(7-methyl-5,6,7,8-tetrahydroimidazo[1,...)Show SMILES CN1CCn2cc(\C=C3/[C@H]4SC=C(N4C3=O)C([O-])=O)nc2C1 |c:11| Show InChI InChI=1S/C14H14N4O3S/c1-16-2-3-17-5-8(15-11(17)6-16)4-9-12(19)18-10(14(20)21)7-22-13(9)18/h4-5,7,13H,2-3,6H2,1H3,(H,20,21)/p-1/b9-4-/t13-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191385
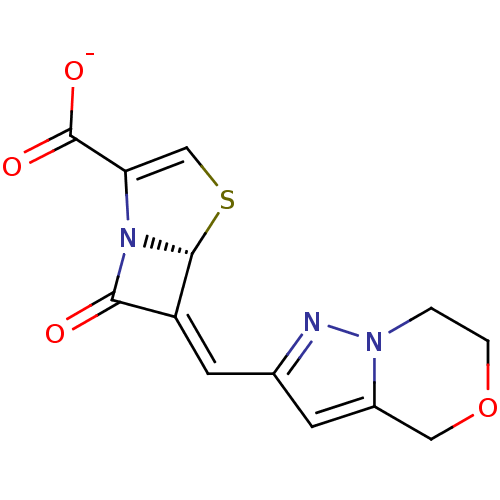
((5R,6Z)-6-(6,7-dihydro-4H-pyrazolo[5,1-c]-[1,4]oxa...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2COCCn2n1 |t:3| Show InChI InChI=1S/C13H11N3O4S/c17-11-9(12-16(11)10(6-21-12)13(18)19)4-7-3-8-5-20-2-1-15(8)14-7/h3-4,6,12H,1-2,5H2,(H,18,19)/p-1/b9-4-/t12-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50473212
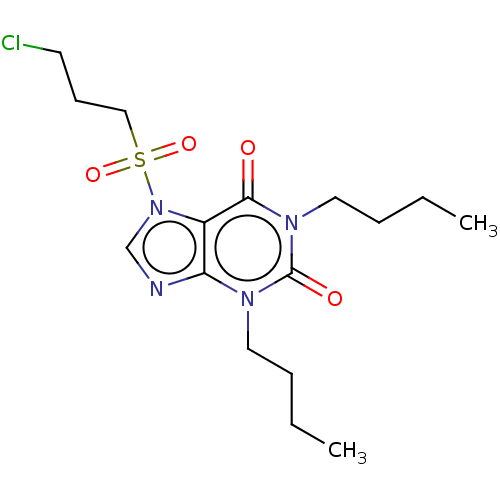
(CHEMBL418740)Show SMILES CCCCn1c2ncn(c2c(=O)n(CCCC)c1=O)S(=O)(=O)CCCCl Show InChI InChI=1S/C16H25ClN4O4S/c1-3-5-9-19-14-13(15(22)20(16(19)23)10-6-4-2)21(12-18-14)26(24,25)11-7-8-17/h12H,3-11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited |
J Med Chem 43: 1223-33 (2000)
Article DOI: 10.1021/jm990558l
BindingDB Entry DOI: 10.7270/Q2NK3HRK |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191380
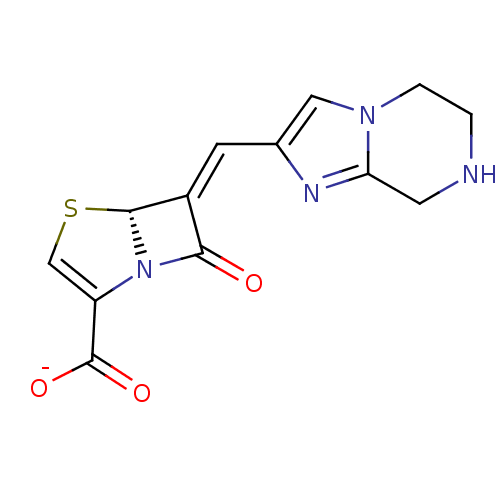
(CHEMBL379440 | sodium (R,E)-7-oxo-6-((5,6,7,8-tetr...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C/c1cn2CCNCc2n1 |t:3| Show InChI InChI=1S/C13H12N4O3S/c18-11-8(12-17(11)9(6-21-12)13(19)20)3-7-5-16-2-1-14-4-10(16)15-7/h3,5-6,12,14H,1-2,4H2,(H,19,20)/p-1/b8-3+/t12-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191378

(CHEMBL212163 | sodium (R,E)-6-((6,8-dihydro-5H-imi...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C/c1cn2CCOCc2n1 |t:3| Show InChI InChI=1S/C13H11N3O4S/c17-11-8(12-16(11)9(6-21-12)13(18)19)3-7-4-15-1-2-20-5-10(15)14-7/h3-4,6,12H,1-2,5H2,(H,18,19)/p-1/b8-3+/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191388

((5R)(6Z)-6-(6,7-5H-dihydropyrazolo[5,1-b]-oxazin-2...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2OCCCn2n1 |t:3| Show InChI InChI=1S/C13H11N3O4S/c17-11-8(12-16(11)9(6-21-12)13(18)19)4-7-5-10-15(14-7)2-1-3-20-10/h4-6,12H,1-3H2,(H,18,19)/p-1/b8-4-/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50076683

(CHEMBL6533 | Sodium; (2S,3S,5R,6R)-6-hydroxymethyl...)Show SMILES C[C@]1(Cn2ccnn2)[C@@H](N2[C@@H]([C@H](CO)C2=O)S1(=O)=O)C([O-])=O Show InChI InChI=1S/C11H14N4O6S/c1-11(5-14-3-2-12-13-14)7(10(18)19)15-8(17)6(4-16)9(15)22(11,20)21/h2-3,6-7,9,16H,4-5H2,1H3,(H,18,19)/p-1/t6-,7+,9-,11+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase |
Bioorg Med Chem Lett 9: 997-1002 (1999)
BindingDB Entry DOI: 10.7270/Q2PK0FBJ |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191387

((5R),(6Z)-6-(7-methyl-5,6,7,8-tetrahydroimidazo[1,...)Show SMILES CN1CCn2cc(\C=C3/[C@H]4SC=C(N4C3=O)C([O-])=O)nc2C1 |c:11| Show InChI InChI=1S/C14H14N4O3S/c1-16-2-3-17-5-8(15-11(17)6-16)4-9-12(19)18-10(14(20)21)7-22-13(9)18/h4-5,7,13H,2-3,6H2,1H3,(H,20,21)/p-1/b9-4-/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191380

(CHEMBL379440 | sodium (R,E)-7-oxo-6-((5,6,7,8-tetr...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C/c1cn2CCNCc2n1 |t:3| Show InChI InChI=1S/C13H12N4O3S/c18-11-8(12-17(11)9(6-21-12)13(19)20)3-7-5-16-2-1-14-4-10(16)15-7/h3,5-6,12,14H,1-2,4H2,(H,19,20)/p-1/b8-3+/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50473211
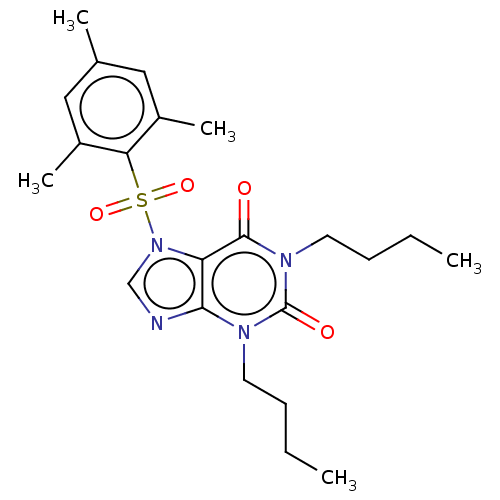
(CHEMBL282923)Show SMILES CCCCn1c2ncn(c2c(=O)n(CCCC)c1=O)S(=O)(=O)c1c(C)cc(C)cc1C Show InChI InChI=1S/C22H30N4O4S/c1-6-8-10-24-20-18(21(27)25(22(24)28)11-9-7-2)26(14-23-20)31(29,30)19-16(4)12-15(3)13-17(19)5/h12-14H,6-11H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited |
J Med Chem 43: 1223-33 (2000)
Article DOI: 10.1021/jm990558l
BindingDB Entry DOI: 10.7270/Q2NK3HRK |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191388

((5R)(6Z)-6-(6,7-5H-dihydropyrazolo[5,1-b]-oxazin-2...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2OCCCn2n1 |t:3| Show InChI InChI=1S/C13H11N3O4S/c17-11-8(12-16(11)9(6-21-12)13(18)19)4-7-5-10-15(14-7)2-1-3-20-10/h4-6,12H,1-3H2,(H,18,19)/p-1/b8-4-/t12-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191385

((5R,6Z)-6-(6,7-dihydro-4H-pyrazolo[5,1-c]-[1,4]oxa...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2COCCn2n1 |t:3| Show InChI InChI=1S/C13H11N3O4S/c17-11-9(12-16(11)10(6-21-12)13(18)19)4-7-3-8-5-20-2-1-15(8)14-7/h3-4,6,12H,1-2,5H2,(H,18,19)/p-1/b9-4-/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Imipenem-hydrolyzing beta-lactamase
(Enterobacter cloacae) | BDBM50191378

(CHEMBL212163 | sodium (R,E)-6-((6,8-dihydro-5H-imi...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C/c1cn2CCOCc2n1 |t:3| Show InChI InChI=1S/C13H11N3O4S/c17-11-8(12-16(11)9(6-21-12)13(18)19)3-7-4-15-1-2-20-5-10(15)14-7/h3-4,6,12H,1-2,5H2,(H,18,19)/p-1/b8-3+/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae Imi1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Bacteroides fragilis) | BDBM50191383

((5R),(6Z)-6-(5,6-dihydro-8H-imidazo[2,1-c]-[1,4]th...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2CCSCc2n1 |t:3| Show InChI InChI=1S/C13H11N3O3S2/c17-11-8(12-16(11)9(5-21-12)13(18)19)3-7-4-15-1-2-20-6-10(15)14-7/h3-5,12H,1-2,6H2,(H,18,19)/p-1/b8-3-/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Bacteroides fragilis CcrA |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50076678
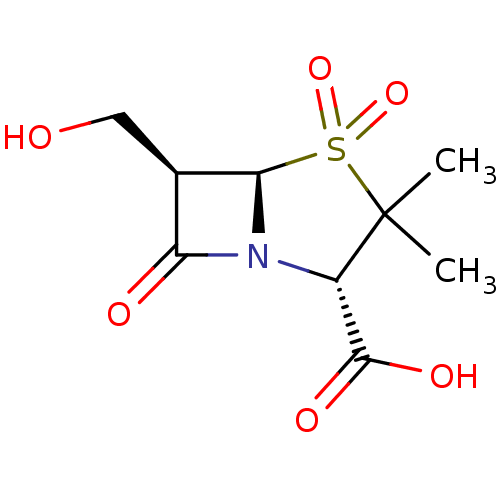
((2S,5R,6R)-6-Hydroxymethyl-3,3-dimethyl-4,4,7-trio...)Show SMILES CC1(C)[C@@H](N2[C@@H]([C@H](CO)C2=O)S1(=O)=O)C(O)=O Show InChI InChI=1S/C9H13NO6S/c1-9(2)5(8(13)14)10-6(12)4(3-11)7(10)17(9,15)16/h4-5,7,11H,3H2,1-2H3,(H,13,14)/t4-,5+,7-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase |
Bioorg Med Chem Lett 9: 991-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TB163Q |
More data for this
Ligand-Target Pair | |
Imipenem-hydrolyzing beta-lactamase
(Enterobacter cloacae) | BDBM50191389

(6-(6,7-dihydro-4H-thieno[3,2-c]pyran-2-ylmethylene...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CN(CCc2s1)c1cc2COCCc2s1 |t:3| Show InChI InChI=1S/C21H18N2O4S3/c24-19-14(20-23(19)15(10-28-20)21(25)26)7-13-5-11-8-22(3-1-16(11)29-13)18-6-12-9-27-4-2-17(12)30-18/h5-7,10,20H,1-4,8-9H2,(H,25,26)/p-1/b14-7-/t20-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae Imi1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50191382
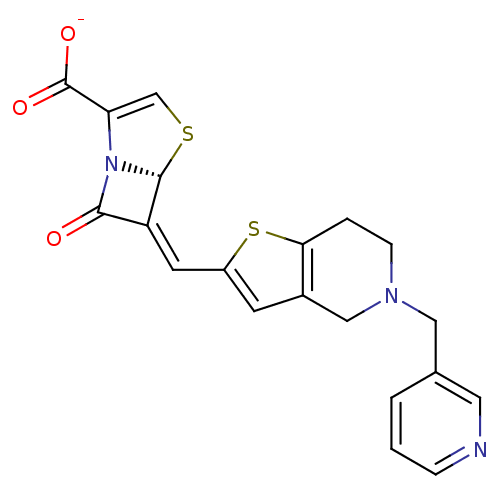
((5R,6Z)-7-oxo-6-{[5-(pyridin-3-ylmethyl)-4,5,6,7-t...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CN(Cc3cccnc3)CCc2s1 |t:3| Show InChI InChI=1S/C20H17N3O3S2/c24-18-15(19-23(18)16(11-27-19)20(25)26)7-14-6-13-10-22(5-3-17(13)28-14)9-12-2-1-4-21-8-12/h1-2,4,6-8,11,19H,3,5,9-10H2,(H,25,26)/p-1/b15-7-/t19-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Bacteroides fragilis) | BDBM50191389

(6-(6,7-dihydro-4H-thieno[3,2-c]pyran-2-ylmethylene...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CN(CCc2s1)c1cc2COCCc2s1 |t:3| Show InChI InChI=1S/C21H18N2O4S3/c24-19-14(20-23(19)15(10-28-20)21(25)26)7-13-5-11-8-22(3-1-16(11)29-13)18-6-12-9-27-4-2-17(12)30-18/h5-7,10,20H,1-4,8-9H2,(H,25,26)/p-1/b14-7-/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Bacteroides fragilis CcrA |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191381

((5R,6Z)-6-{[5-(4-methoxybenzyl)-4,5,6,7-tetrahydro...)Show SMILES COc1ccc(CN2CCc3sc(\C=C4/[C@H]5SC=C(N5C4=O)C([O-])=O)cc3C2)cc1 |c:17| Show InChI InChI=1S/C22H20N2O4S2/c1-28-15-4-2-13(3-5-15)10-23-7-6-19-14(11-23)8-16(30-19)9-17-20(25)24-18(22(26)27)12-29-21(17)24/h2-5,8-9,12,21H,6-7,10-11H2,1H3,(H,26,27)/p-1/b17-9-/t21-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Imipenem-hydrolyzing beta-lactamase
(Enterobacter cloacae) | BDBM50191379

((5R)(6Z)-6-(6,7-dihydro-4H-pyrazolo[5,1-c][1,4]-th...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CSCCn2n1 |t:3| Show InChI InChI=1S/C13H11N3O3S2/c17-11-9(12-16(11)10(6-21-12)13(18)19)4-7-3-8-5-20-2-1-15(8)14-7/h3-4,6,12H,1-2,5H2,(H,18,19)/p-1/b9-4-/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae Imi1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50191382

((5R,6Z)-7-oxo-6-{[5-(pyridin-3-ylmethyl)-4,5,6,7-t...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CN(Cc3cccnc3)CCc2s1 |t:3| Show InChI InChI=1S/C20H17N3O3S2/c24-18-15(19-23(18)16(11-27-19)20(25)26)7-14-6-13-10-22(5-3-17(13)28-14)9-12-2-1-4-21-8-12/h1-2,4,6-8,11,19H,3,5,9-10H2,(H,25,26)/p-1/b15-7-/t19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50076681
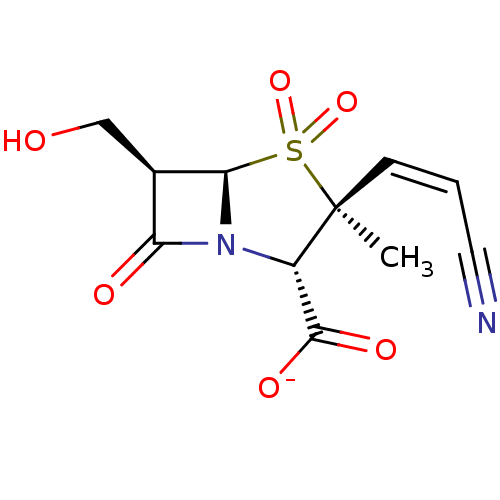
(CHEMBL6469 | Sodium; (2S,3S,5R,6R)-3-((Z)-2-cyano-...)Show SMILES C[C@]1(\C=C/C#N)[C@@H](N2[C@@H]([C@H](CO)C2=O)S1(=O)=O)C([O-])=O Show InChI InChI=1S/C11H12N2O6S/c1-11(3-2-4-12)7(10(16)17)13-8(15)6(5-14)9(13)20(11,18)19/h2-3,6-7,9,14H,5H2,1H3,(H,16,17)/p-1/b3-2-/t6-,7+,9-,11+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase |
Bioorg Med Chem Lett 9: 997-1002 (1999)
BindingDB Entry DOI: 10.7270/Q2PK0FBJ |
More data for this
Ligand-Target Pair | |
Imipenem-hydrolyzing beta-lactamase
(Enterobacter cloacae) | BDBM50191385

((5R,6Z)-6-(6,7-dihydro-4H-pyrazolo[5,1-c]-[1,4]oxa...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2COCCn2n1 |t:3| Show InChI InChI=1S/C13H11N3O4S/c17-11-9(12-16(11)10(6-21-12)13(18)19)4-7-3-8-5-20-2-1-15(8)14-7/h3-4,6,12H,1-2,5H2,(H,18,19)/p-1/b9-4-/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae Imi1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Imipenem-hydrolyzing beta-lactamase
(Enterobacter cloacae) | BDBM50191387

((5R),(6Z)-6-(7-methyl-5,6,7,8-tetrahydroimidazo[1,...)Show SMILES CN1CCn2cc(\C=C3/[C@H]4SC=C(N4C3=O)C([O-])=O)nc2C1 |c:11| Show InChI InChI=1S/C14H14N4O3S/c1-16-2-3-17-5-8(15-11(17)6-16)4-9-12(19)18-10(14(20)21)7-22-13(9)18/h4-5,7,13H,2-3,6H2,1H3,(H,20,21)/p-1/b9-4-/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae Imi1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50473210

(CHEMBL20822)Show SMILES CCCCn1c2ncn(c2c(=O)n(CCCC)c1=O)S(=O)(=O)c1cc(C)c(Cl)cc1C Show InChI InChI=1S/C21H27ClN4O4S/c1-5-7-9-24-19-18(20(27)25(21(24)28)10-8-6-2)26(13-23-19)31(29,30)17-12-14(3)16(22)11-15(17)4/h11-13H,5-10H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited |
J Med Chem 43: 1223-33 (2000)
Article DOI: 10.1021/jm990558l
BindingDB Entry DOI: 10.7270/Q2NK3HRK |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50473207
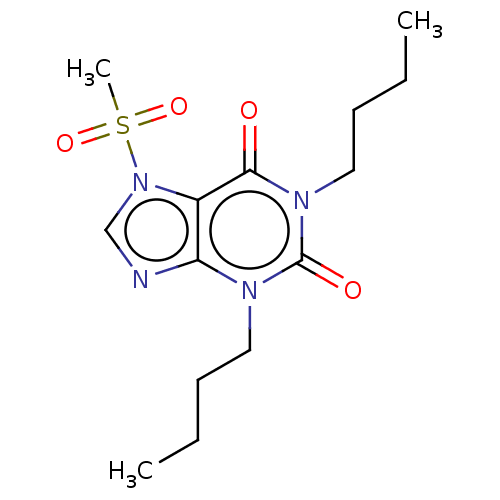
(CHEMBL418371)Show InChI InChI=1S/C14H22N4O4S/c1-4-6-8-16-12-11(18(10-15-12)23(3,21)22)13(19)17(14(16)20)9-7-5-2/h10H,4-9H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited |
J Med Chem 43: 1223-33 (2000)
Article DOI: 10.1021/jm990558l
BindingDB Entry DOI: 10.7270/Q2NK3HRK |
More data for this
Ligand-Target Pair | |
Imipenem-hydrolyzing beta-lactamase
(Enterobacter cloacae) | BDBM50191381

((5R,6Z)-6-{[5-(4-methoxybenzyl)-4,5,6,7-tetrahydro...)Show SMILES COc1ccc(CN2CCc3sc(\C=C4/[C@H]5SC=C(N5C4=O)C([O-])=O)cc3C2)cc1 |c:17| Show InChI InChI=1S/C22H20N2O4S2/c1-28-15-4-2-13(3-5-15)10-23-7-6-19-14(11-23)8-16(30-19)9-17-20(25)24-18(22(26)27)12-29-21(17)24/h2-5,8-9,12,21H,6-7,10-11H2,1H3,(H,26,27)/p-1/b17-9-/t21-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae Imi1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Imipenem-hydrolyzing beta-lactamase
(Enterobacter cloacae) | BDBM50191380

(CHEMBL379440 | sodium (R,E)-7-oxo-6-((5,6,7,8-tetr...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C/c1cn2CCNCc2n1 |t:3| Show InChI InChI=1S/C13H12N4O3S/c18-11-8(12-17(11)9(6-21-12)13(19)20)3-7-5-16-2-1-14-4-10(16)15-7/h3,5-6,12,14H,1-2,4H2,(H,19,20)/p-1/b8-3+/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae Imi1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Bacteroides fragilis) | BDBM50191385

((5R,6Z)-6-(6,7-dihydro-4H-pyrazolo[5,1-c]-[1,4]oxa...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2COCCn2n1 |t:3| Show InChI InChI=1S/C13H11N3O4S/c17-11-9(12-16(11)10(6-21-12)13(18)19)4-7-3-8-5-20-2-1-15(8)14-7/h3-4,6,12H,1-2,5H2,(H,18,19)/p-1/b9-4-/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Bacteroides fragilis CcrA |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50403733
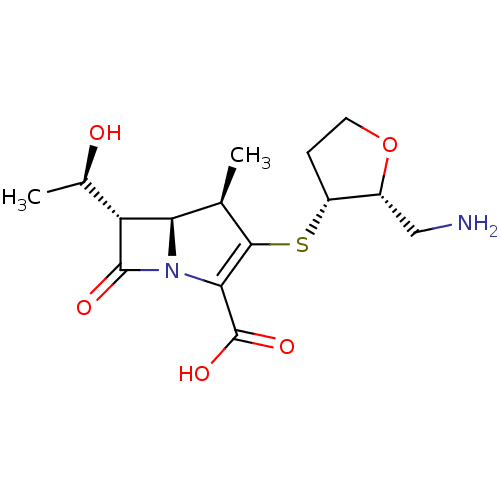
(CHEMBL1206880 | CL-191121)Show SMILES C[C@@H](O)[C@@H]1[C@H]2[C@@H](C)C(S[C@@H]3CCO[C@@H]3CN)=C(N2C1=O)C(O)=O |c:16| Show InChI InChI=1S/C15H22N2O5S/c1-6-11-10(7(2)18)14(19)17(11)12(15(20)21)13(6)23-9-3-4-22-8(9)5-16/h6-11,18H,3-5,16H2,1-2H3,(H,20,21)/t6-,7-,8-,9-,10-,11-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase |
Bioorg Med Chem Lett 9: 991-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TB163Q |
More data for this
Ligand-Target Pair | |
Imipenem-hydrolyzing beta-lactamase
(Enterobacter cloacae) | BDBM50191382

((5R,6Z)-7-oxo-6-{[5-(pyridin-3-ylmethyl)-4,5,6,7-t...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CN(Cc3cccnc3)CCc2s1 |t:3| Show InChI InChI=1S/C20H17N3O3S2/c24-18-15(19-23(18)16(11-27-19)20(25)26)7-14-6-13-10-22(5-3-17(13)28-14)9-12-2-1-4-21-8-12/h1-2,4,6-8,11,19H,3,5,9-10H2,(H,25,26)/p-1/b15-7-/t19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae Imi1 |
J Med Chem 49: 4623-37 (2006)
Article DOI: 10.1021/jm060021p
BindingDB Entry DOI: 10.7270/Q2TX3F1B |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50076680

(CHEMBL6461 | Ro-48-1220 | Sodium; (2S,3R,5R)-3-((Z...)Show SMILES C[C@]1(\C=C\C#N)[C@@H](N2[C@@H](CC2=O)S1(=O)=O)C([O-])=O Show InChI InChI=1S/C10H10N2O5S/c1-10(3-2-4-11)8(9(14)15)12-6(13)5-7(12)18(10,16)17/h2-3,7-8H,5H2,1H3,(H,14,15)/p-1/b3-2+/t7-,8+,10+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase |
Bioorg Med Chem Lett 9: 997-1002 (1999)
BindingDB Entry DOI: 10.7270/Q2PK0FBJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data