 Found 433 hits with Last Name = 'friedlos' and Initial = 'f'
Found 433 hits with Last Name = 'friedlos' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carboxypeptidase G2
(Pseudomonas aeruginosa) | BDBM50171504

((S)-2-{4-[Bis-(2-chloro-ethyl)-amino]-2,3,5,6-tetr...)Show SMILES OC(=O)CC[C@H](NC(=O)c1c(F)c(F)c(N(CCCl)CCCl)c(F)c1F)C(O)=O Show InChI InChI=1S/C16H16Cl2F4N2O5/c17-3-5-24(6-4-18)14-12(21)10(19)9(11(20)13(14)22)15(27)23-7(16(28)29)1-2-8(25)26/h7H,1-6H2,(H,23,27)(H,25,26)(H,28,29)/t7-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against carboxypeptidase G2 from pseudomonas RS16 |
J Med Chem 48: 5321-8 (2005)
Article DOI: 10.1021/jm0502182
BindingDB Entry DOI: 10.7270/Q2DJ5F5C |
More data for this
Ligand-Target Pair | |
Carboxypeptidase G2
(Pseudomonas aeruginosa) | BDBM50171496
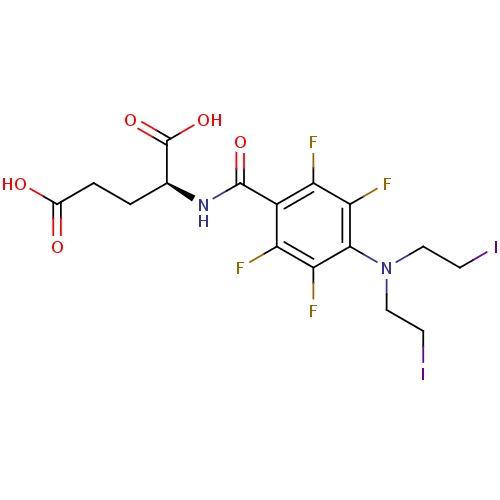
((S)-2-{4-[Bis-(2-iodo-ethyl)-amino]-2,3,5,6-tetraf...)Show SMILES OC(=O)CC[C@H](NC(=O)c1c(F)c(F)c(N(CCI)CCI)c(F)c1F)C(O)=O Show InChI InChI=1S/C16H16F4I2N2O5/c17-10-9(15(27)23-7(16(28)29)1-2-8(25)26)11(18)13(20)14(12(10)19)24(5-3-21)6-4-22/h7H,1-6H2,(H,23,27)(H,25,26)(H,28,29)/t7-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against carboxypeptidase G2 from pseudomonas RS16 |
J Med Chem 48: 5321-8 (2005)
Article DOI: 10.1021/jm0502182
BindingDB Entry DOI: 10.7270/Q2DJ5F5C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480105
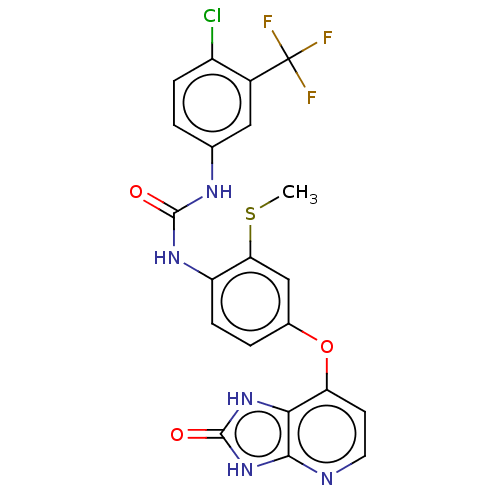
(CHEMBL496201)Show SMILES CSc1cc(Oc2ccnc3[nH]c(=O)[nH]c23)ccc1NC(=O)Nc1ccc(Cl)c(c1)C(F)(F)F Show InChI InChI=1S/C21H15ClF3N5O3S/c1-34-16-9-11(33-15-6-7-26-18-17(15)29-20(32)30-18)3-5-14(16)28-19(31)27-10-2-4-13(22)12(8-10)21(23,24)25/h2-9H,1H3,(H2,27,28,31)(H2,26,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480108
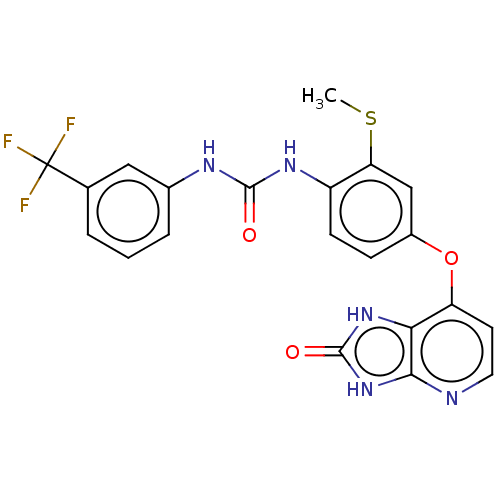
(CHEMBL496009)Show SMILES CSc1cc(Oc2ccnc3[nH]c(=O)[nH]c23)ccc1NC(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C21H16F3N5O3S/c1-33-16-10-13(32-15-7-8-25-18-17(15)28-20(31)29-18)5-6-14(16)27-19(30)26-12-4-2-3-11(9-12)21(22,23)24/h2-10H,1H3,(H2,26,27,30)(H2,25,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50307879
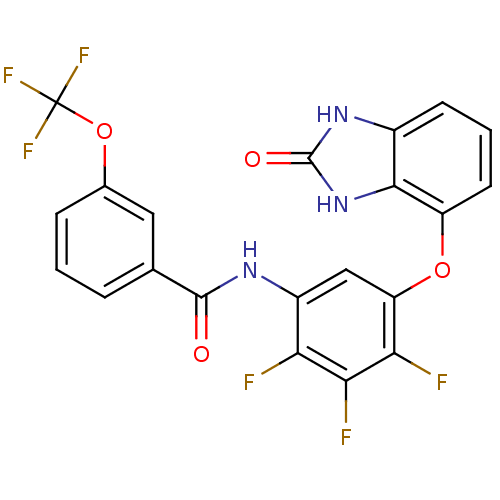
(CHEMBL602123 | N-(2,3,4-trifluoro-5-(2-oxo-2,3-dih...)Show SMILES Fc1c(NC(=O)c2cccc(OC(F)(F)F)c2)cc(Oc2cccc3[nH]c(=O)[nH]c23)c(F)c1F Show InChI InChI=1S/C21H11F6N3O4/c22-15-12(28-19(31)9-3-1-4-10(7-9)34-21(25,26)27)8-14(16(23)17(15)24)33-13-6-2-5-11-18(13)30-20(32)29-11/h1-8H,(H,28,31)(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of cRAF |
J Med Chem 53: 1964-78 (2010)
Article DOI: 10.1021/jm901509a
BindingDB Entry DOI: 10.7270/Q2R211HN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480091
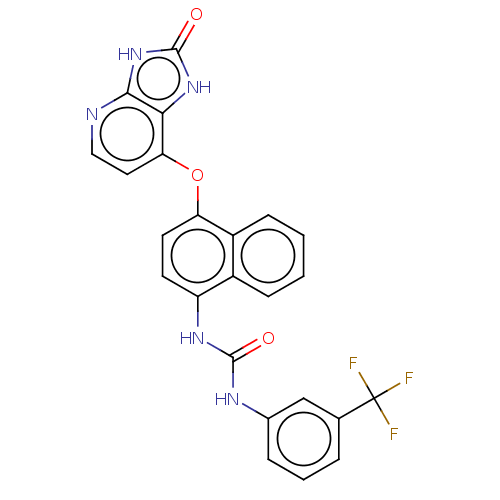
(CHEMBL523753)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2ccc(Oc3ccnc4[nH]c(=O)[nH]c34)c3ccccc23)c1 Show InChI InChI=1S/C24H16F3N5O3/c25-24(26,27)13-4-3-5-14(12-13)29-22(33)30-17-8-9-18(16-7-2-1-6-15(16)17)35-19-10-11-28-21-20(19)31-23(34)32-21/h1-12H,(H2,29,30,33)(H2,28,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480092
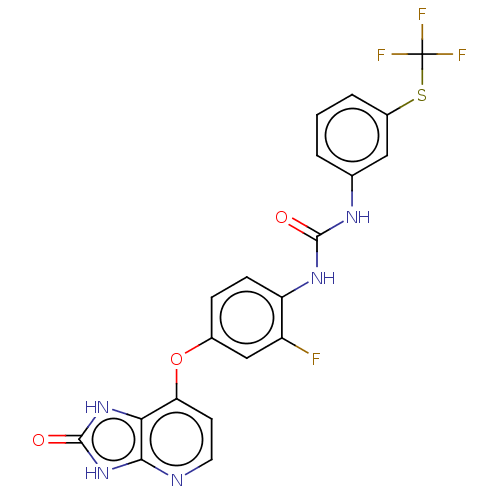
(CHEMBL522072)Show SMILES Fc1cc(Oc2ccnc3[nH]c(=O)[nH]c23)ccc1NC(=O)Nc1cccc(SC(F)(F)F)c1 Show InChI InChI=1S/C20H13F4N5O3S/c21-13-9-11(32-15-6-7-25-17-16(15)28-19(31)29-17)4-5-14(13)27-18(30)26-10-2-1-3-12(8-10)33-20(22,23)24/h1-9H,(H2,26,27,30)(H2,25,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480123
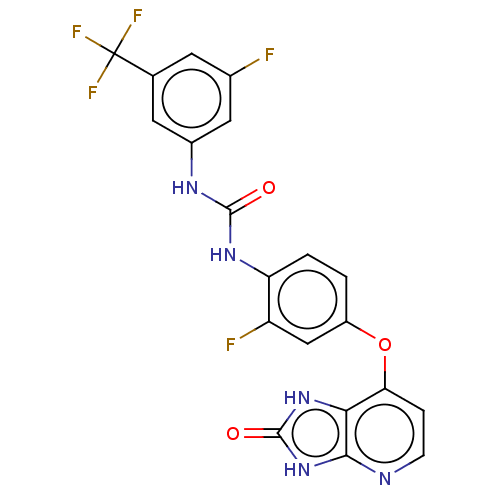
(CHEMBL496612)Show SMILES Fc1cc(NC(=O)Nc2ccc(Oc3ccnc4[nH]c(=O)[nH]c34)cc2F)cc(c1)C(F)(F)F Show InChI InChI=1S/C20H12F5N5O3/c21-10-5-9(20(23,24)25)6-11(7-10)27-18(31)28-14-2-1-12(8-13(14)22)33-15-3-4-26-17-16(15)29-19(32)30-17/h1-8H,(H2,27,28,31)(H2,26,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480112
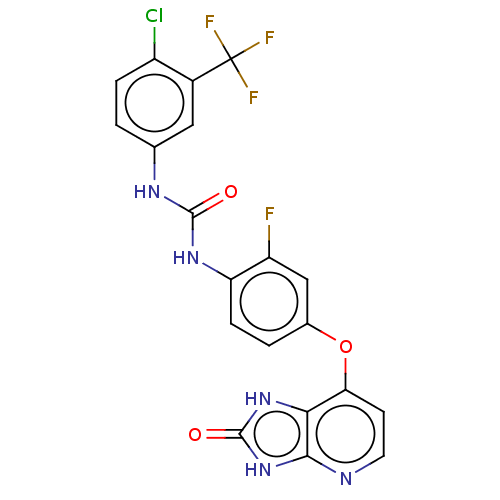
(CHEMBL496020)Show SMILES Fc1cc(Oc2ccnc3[nH]c(=O)[nH]c23)ccc1NC(=O)Nc1ccc(Cl)c(c1)C(F)(F)F Show InChI InChI=1S/C20H12ClF4N5O3/c21-12-3-1-9(7-11(12)20(23,24)25)27-18(31)28-14-4-2-10(8-13(14)22)33-15-5-6-26-17-16(15)29-19(32)30-17/h1-8H,(H2,27,28,31)(H2,26,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480093
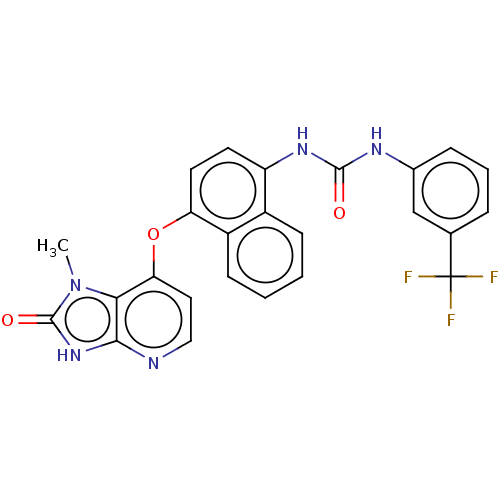
(CHEMBL487717)Show SMILES Cn1c2c(Oc3ccc(NC(=O)Nc4cccc(c4)C(F)(F)F)c4ccccc34)ccnc2[nH]c1=O Show InChI InChI=1S/C25H18F3N5O3/c1-33-21-20(11-12-29-22(21)32-24(33)35)36-19-10-9-18(16-7-2-3-8-17(16)19)31-23(34)30-15-6-4-5-14(13-15)25(26,27)28/h2-13H,1H3,(H,29,32,35)(H2,30,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482016
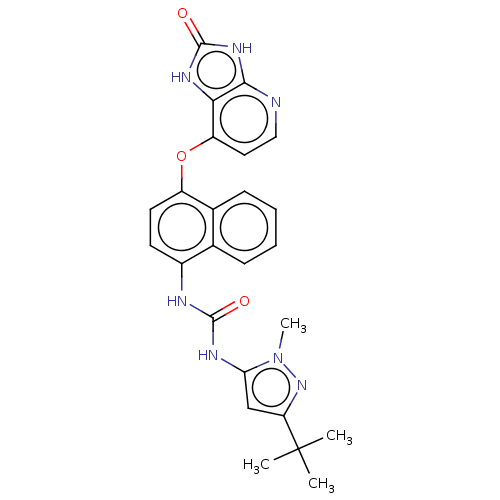
(CHEMBL1091432)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(Oc2ccnc3[nH]c(=O)[nH]c23)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C25H25N7O3/c1-25(2,3)19-13-20(32(4)31-19)28-23(33)27-16-9-10-17(15-8-6-5-7-14(15)16)35-18-11-12-26-22-21(18)29-24(34)30-22/h5-13H,1-4H3,(H2,27,28,33)(H2,26,29,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of human BRAF V600E mutant expressed in baculovirus system |
J Med Chem 53: 2741-56 (2010)
Article DOI: 10.1021/jm900607f
BindingDB Entry DOI: 10.7270/Q2WD43CH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480124
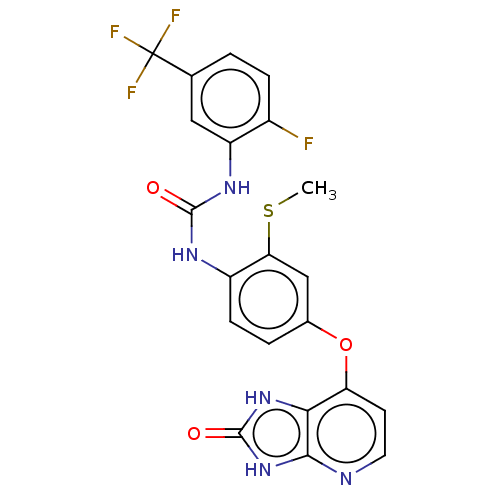
(CHEMBL496202)Show SMILES CSc1cc(Oc2ccnc3[nH]c(=O)[nH]c23)ccc1NC(=O)Nc1cc(ccc1F)C(F)(F)F Show InChI InChI=1S/C21H15F4N5O3S/c1-34-16-9-11(33-15-6-7-26-18-17(15)29-20(32)30-18)3-5-13(16)27-19(31)28-14-8-10(21(23,24)25)2-4-12(14)22/h2-9H,1H3,(H2,27,28,31)(H2,26,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480094
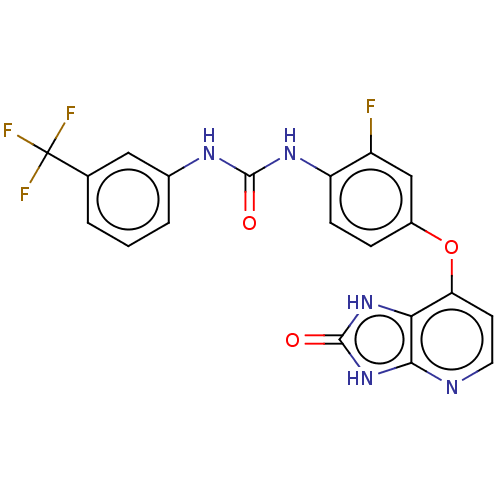
(CHEMBL496414)Show SMILES Fc1cc(Oc2ccnc3[nH]c(=O)[nH]c23)ccc1NC(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C20H13F4N5O3/c21-13-9-12(32-15-6-7-25-17-16(15)28-19(31)29-17)4-5-14(13)27-18(30)26-11-3-1-2-10(8-11)20(22,23)24/h1-9H,(H2,26,27,30)(H2,25,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM29709
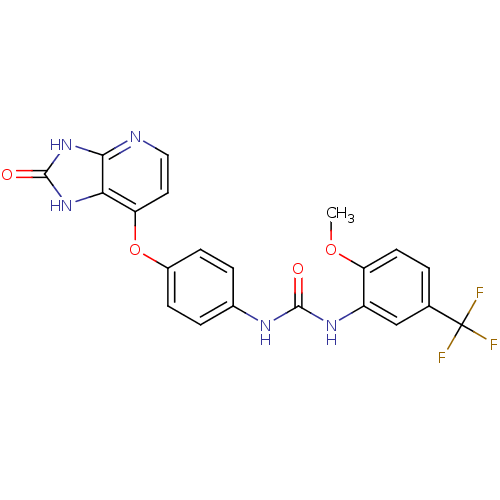
(Pyridoimidazolone, 5i)Show SMILES COc1ccc(cc1NC(=O)Nc1ccc(Oc2ccnc3[nH]c(=O)[nH]c23)cc1)C(F)(F)F Show InChI InChI=1S/C21H16F3N5O4/c1-32-15-7-2-11(21(22,23)24)10-14(15)27-19(30)26-12-3-5-13(6-4-12)33-16-8-9-25-18-17(16)28-20(31)29-18/h2-10H,1H3,(H2,26,27,30)(H2,25,28,29,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.2 | 22 |
The Institute of Cancer Research
| Assay Description
The biological activities (IC50s) of the compounds were determined against the B-RAF (V600E) mutant enzyme in vitro. GST-MEK1, B-RAF (V600E), and inh... |
J Med Chem 52: 2255-64 (2009)
Article DOI: 10.1021/jm801509w
BindingDB Entry DOI: 10.7270/Q2PK0DGX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480095
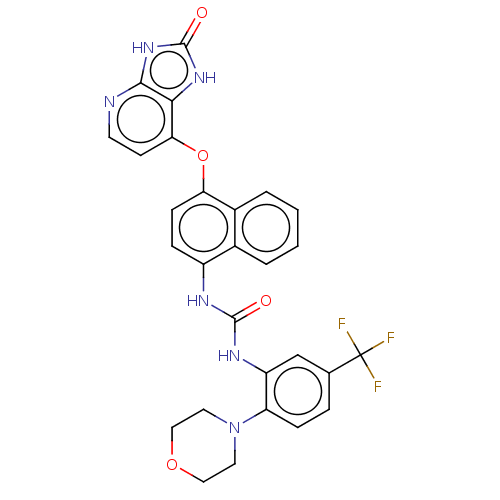
(CHEMBL501686)Show SMILES FC(F)(F)c1ccc(N2CCOCC2)c(NC(=O)Nc2ccc(Oc3ccnc4[nH]c(=O)[nH]c34)c3ccccc23)c1 Show InChI InChI=1S/C28H23F3N6O4/c29-28(30,31)16-5-7-21(37-11-13-40-14-12-37)20(15-16)34-26(38)33-19-6-8-22(18-4-2-1-3-17(18)19)41-23-9-10-32-25-24(23)35-27(39)36-25/h1-10,15H,11-14H2,(H2,33,34,38)(H2,32,35,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM142599

(US8933228, 1)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(Oc2ccnc3[nH]c(=O)[nH]c23)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H29N7O3/c1-18-9-11-19(12-10-18)38-26(17-25(37-38)31(2,3)4)34-29(39)33-22-13-14-23(21-8-6-5-7-20(21)22)41-24-15-16-32-28-27(24)35-30(40)36-28/h5-17H,1-4H3,(H2,33,34,39)(H2,32,35,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 2741-56 (2010)
Article DOI: 10.1021/jm900607f
BindingDB Entry DOI: 10.7270/Q2WD43CH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM29705
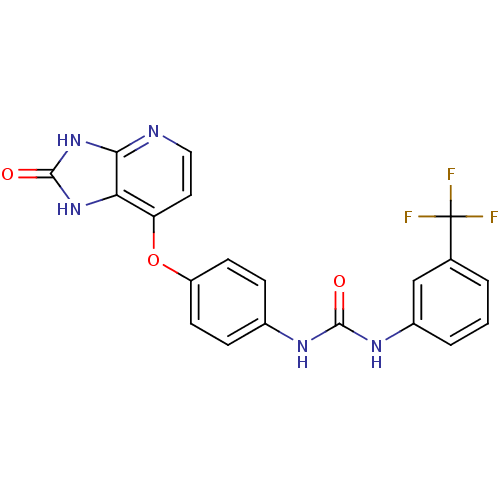
(Pyridoimidazolone, 5e)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2ccc(Oc3ccnc4[nH]c(=O)[nH]c34)cc2)c1 Show InChI InChI=1S/C20H14F3N5O3/c21-20(22,23)11-2-1-3-13(10-11)26-18(29)25-12-4-6-14(7-5-12)31-15-8-9-24-17-16(15)27-19(30)28-17/h1-10H,(H2,25,26,29)(H2,24,27,28,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.2 | 22 |
The Institute of Cancer Research
| Assay Description
The biological activities (IC50s) of the compounds were determined against the B-RAF (V600E) mutant enzyme in vitro. GST-MEK1, B-RAF (V600E), and inh... |
J Med Chem 52: 2255-64 (2009)
Article DOI: 10.1021/jm801509w
BindingDB Entry DOI: 10.7270/Q2PK0DGX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480127
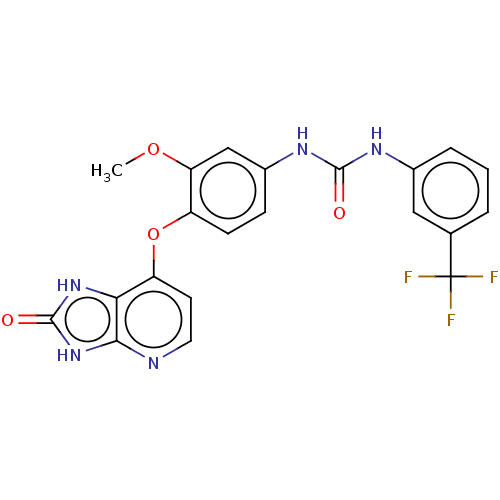
(CHEMBL496203)Show SMILES COc1cc(NC(=O)Nc2cccc(c2)C(F)(F)F)ccc1Oc1ccnc2[nH]c(=O)[nH]c12 Show InChI InChI=1S/C21H16F3N5O4/c1-32-16-10-13(27-19(30)26-12-4-2-3-11(9-12)21(22,23)24)5-6-14(16)33-15-7-8-25-18-17(15)28-20(31)29-18/h2-10H,1H3,(H2,26,27,30)(H2,25,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480096
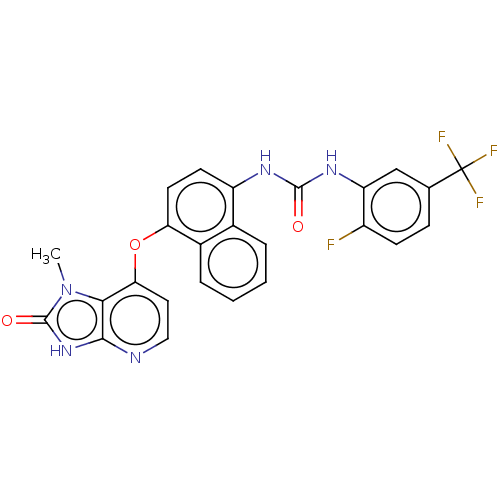
(CHEMBL524068)Show SMILES Cn1c2c(Oc3ccc(NC(=O)Nc4cc(ccc4F)C(F)(F)F)c4ccccc34)ccnc2[nH]c1=O Show InChI InChI=1S/C25H17F4N5O3/c1-34-21-20(10-11-30-22(21)33-24(34)36)37-19-9-8-17(14-4-2-3-5-15(14)19)31-23(35)32-18-12-13(25(27,28)29)6-7-16(18)26/h2-12H,1H3,(H,30,33,36)(H2,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482015
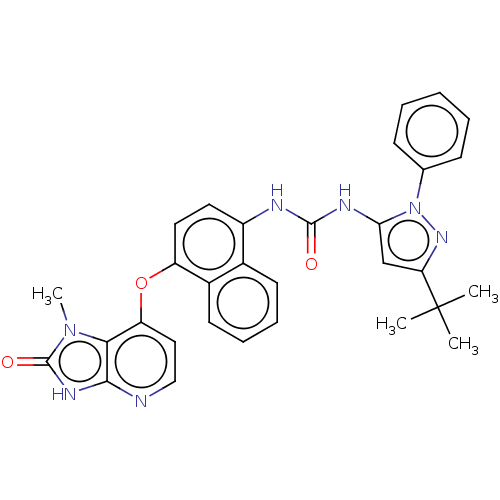
(CHEMBL1090977)Show SMILES Cn1c2c(Oc3ccc(NC(=O)Nc4cc(nn4-c4ccccc4)C(C)(C)C)c4ccccc34)ccnc2[nH]c1=O Show InChI InChI=1S/C31H29N7O3/c1-31(2,3)25-18-26(38(36-25)19-10-6-5-7-11-19)34-29(39)33-22-14-15-23(21-13-9-8-12-20(21)22)41-24-16-17-32-28-27(24)37(4)30(40)35-28/h5-18H,1-4H3,(H,32,35,40)(H2,33,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 2741-56 (2010)
Article DOI: 10.1021/jm900607f
BindingDB Entry DOI: 10.7270/Q2WD43CH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM29708
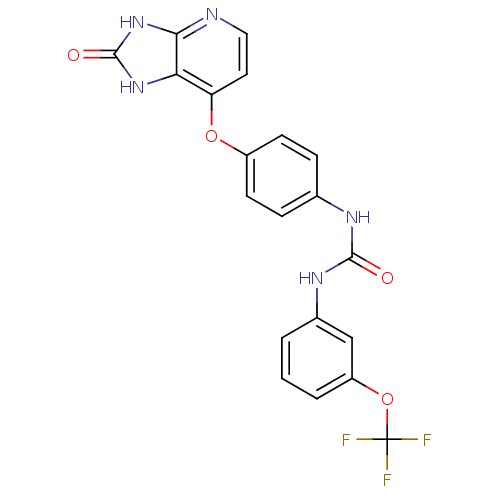
(Pyridoimidazolone, 5h)Show SMILES FC(F)(F)Oc1cccc(NC(=O)Nc2ccc(Oc3ccnc4[nH]c(=O)[nH]c34)cc2)c1 Show InChI InChI=1S/C20H14F3N5O4/c21-20(22,23)32-14-3-1-2-12(10-14)26-18(29)25-11-4-6-13(7-5-11)31-15-8-9-24-17-16(15)27-19(30)28-17/h1-10H,(H2,25,26,29)(H2,24,27,28,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.2 | 22 |
The Institute of Cancer Research
| Assay Description
The biological activities (IC50s) of the compounds were determined against the B-RAF (V600E) mutant enzyme in vitro. GST-MEK1, B-RAF (V600E), and inh... |
J Med Chem 52: 2255-64 (2009)
Article DOI: 10.1021/jm801509w
BindingDB Entry DOI: 10.7270/Q2PK0DGX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480097
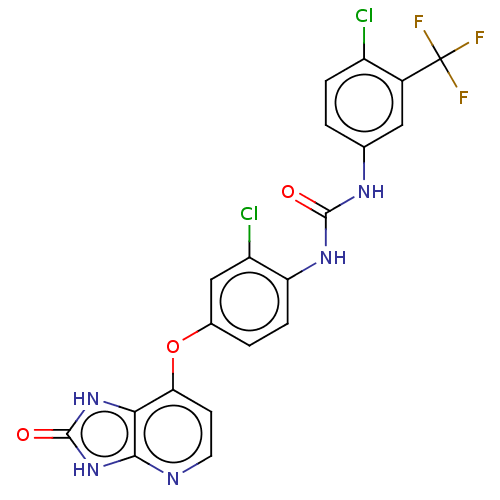
(CHEMBL498025)Show SMILES FC(F)(F)c1cc(NC(=O)Nc2ccc(Oc3ccnc4[nH]c(=O)[nH]c34)cc2Cl)ccc1Cl Show InChI InChI=1S/C20H12Cl2F3N5O3/c21-12-3-1-9(7-11(12)20(23,24)25)27-18(31)28-14-4-2-10(8-13(14)22)33-15-5-6-26-17-16(15)29-19(32)30-17/h1-8H,(H2,27,28,31)(H2,26,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM29715
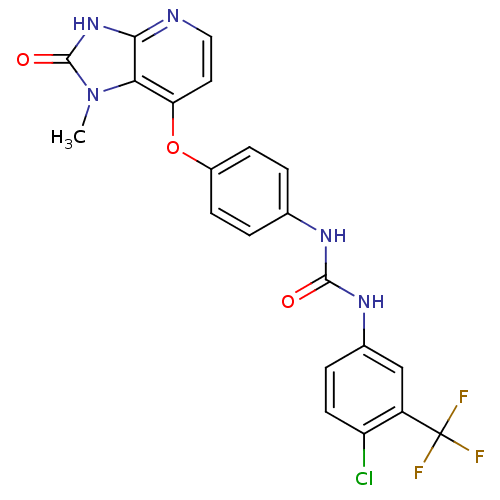
(Pyridoimidazolone, 5o)Show SMILES Cn1c2c(Oc3ccc(NC(=O)Nc4ccc(Cl)c(c4)C(F)(F)F)cc3)ccnc2[nH]c1=O Show InChI InChI=1S/C21H15ClF3N5O3/c1-30-17-16(8-9-26-18(17)29-20(30)32)33-13-5-2-11(3-6-13)27-19(31)28-12-4-7-15(22)14(10-12)21(23,24)25/h2-10H,1H3,(H,26,29,32)(H2,27,28,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.2 | 22 |
The Institute of Cancer Research
| Assay Description
The biological activities (IC50s) of the compounds were determined against the B-RAF (V600E) mutant enzyme in vitro. GST-MEK1, B-RAF (V600E), and inh... |
J Med Chem 52: 2255-64 (2009)
Article DOI: 10.1021/jm801509w
BindingDB Entry DOI: 10.7270/Q2PK0DGX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480098

(CHEMBL496185)Show SMILES FC(F)(F)c1cc(NC(=O)Nc2ccc(Oc3ccnc4[nH]c(=O)[nH]c34)cc2C(F)(F)F)ccc1Cl Show InChI InChI=1S/C21H12ClF6N5O3/c22-13-3-1-9(7-11(13)20(23,24)25)30-18(34)31-14-4-2-10(8-12(14)21(26,27)28)36-15-5-6-29-17-16(15)32-19(35)33-17/h1-8H,(H2,30,31,34)(H2,29,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM29701
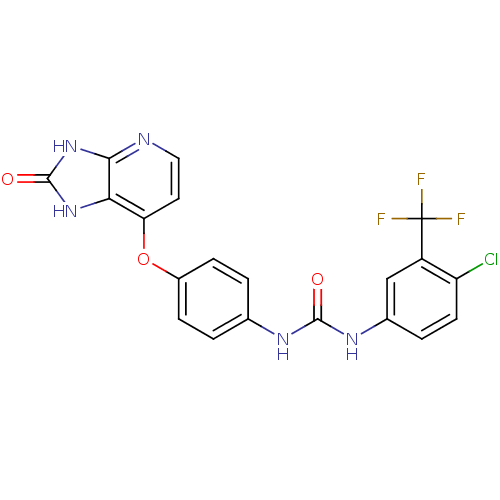
(Pyridoimidazolone, 5a)Show SMILES FC(F)(F)c1cc(NC(=O)Nc2ccc(Oc3ccnc4[nH]c(=O)[nH]c34)cc2)ccc1Cl Show InChI InChI=1S/C20H13ClF3N5O3/c21-14-6-3-11(9-13(14)20(22,23)24)27-18(30)26-10-1-4-12(5-2-10)32-15-7-8-25-17-16(15)28-19(31)29-17/h1-9H,(H2,26,27,30)(H2,25,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480116
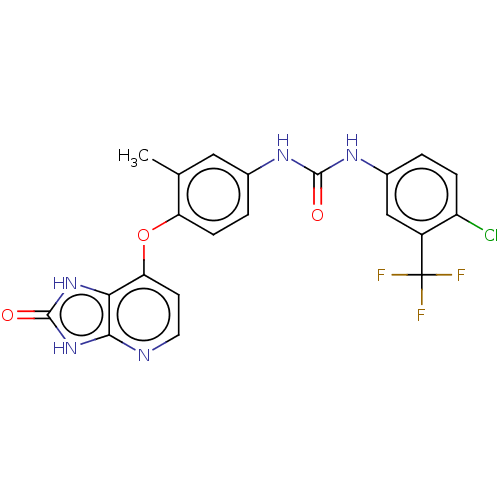
(CHEMBL522724)Show SMILES Cc1cc(NC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)ccc1Oc1ccnc2[nH]c(=O)[nH]c12 Show InChI InChI=1S/C21H15ClF3N5O3/c1-10-8-11(27-19(31)28-12-2-4-14(22)13(9-12)21(23,24)25)3-5-15(10)33-16-6-7-26-18-17(16)29-20(32)30-18/h2-9H,1H3,(H2,27,28,31)(H2,26,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM29701

(Pyridoimidazolone, 5a)Show SMILES FC(F)(F)c1cc(NC(=O)Nc2ccc(Oc3ccnc4[nH]c(=O)[nH]c34)cc2)ccc1Cl Show InChI InChI=1S/C20H13ClF3N5O3/c21-14-6-3-11(9-13(14)20(22,23)24)27-18(30)26-10-1-4-12(5-2-10)32-15-7-8-25-17-16(15)28-19(31)29-17/h1-9H,(H2,26,27,30)(H2,25,28,29,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.2 | 22 |
The Institute of Cancer Research
| Assay Description
The biological activities (IC50s) of the compounds were determined against the B-RAF (V600E) mutant enzyme in vitro. GST-MEK1, B-RAF (V600E), and inh... |
J Med Chem 52: 2255-64 (2009)
Article DOI: 10.1021/jm801509w
BindingDB Entry DOI: 10.7270/Q2PK0DGX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480099

(CHEMBL498459)Show SMILES Cn1c2c(Oc3ccc(NC(=O)Nc4ccc(Cl)c(c4)C(F)(F)F)c4ccccc34)ccnc2[nH]c1=O Show InChI InChI=1S/C25H17ClF3N5O3/c1-34-21-20(10-11-30-22(21)33-24(34)36)37-19-9-8-18(14-4-2-3-5-15(14)19)32-23(35)31-13-6-7-17(26)16(12-13)25(27,28)29/h2-12H,1H3,(H,30,33,36)(H2,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480100

(CHEMBL487718)Show SMILES Cn1c2c(Oc3ccc(NC(=O)Nc4cccc(SC(F)(F)F)c4)c4ccccc34)ccnc2[nH]c1=O Show InChI InChI=1S/C25H18F3N5O3S/c1-33-21-20(11-12-29-22(21)32-24(33)35)36-19-10-9-18(16-7-2-3-8-17(16)19)31-23(34)30-14-5-4-6-15(13-14)37-25(26,27)28/h2-13H,1H3,(H,29,32,35)(H2,30,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480115

(CHEMBL496019)Show SMILES COc1cc(NC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)ccc1Oc1ccnc2[nH]c(=O)[nH]c12 Show InChI InChI=1S/C21H15ClF3N5O4/c1-33-16-9-11(28-19(31)27-10-2-4-13(22)12(8-10)21(23,24)25)3-5-14(16)34-15-6-7-26-18-17(15)29-20(32)30-18/h2-9H,1H3,(H2,27,28,31)(H2,26,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM142601
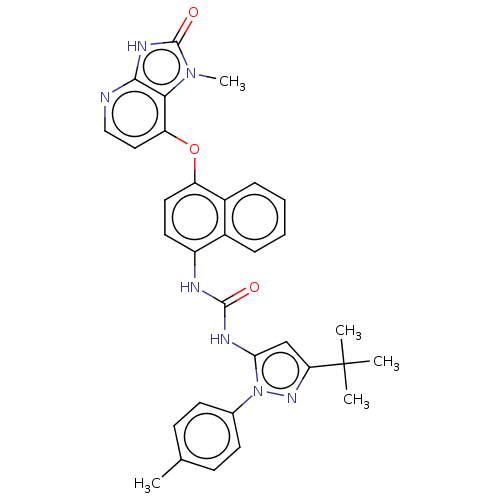
(US8933228, 3)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(Oc2ccnc3[nH]c(=O)n(C)c23)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C32H31N7O3/c1-19-10-12-20(13-11-19)39-27(18-26(37-39)32(2,3)4)35-30(40)34-23-14-15-24(22-9-7-6-8-21(22)23)42-25-16-17-33-29-28(25)38(5)31(41)36-29/h6-18H,1-5H3,(H,33,36,41)(H2,34,35,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 2741-56 (2010)
Article DOI: 10.1021/jm900607f
BindingDB Entry DOI: 10.7270/Q2WD43CH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM29710
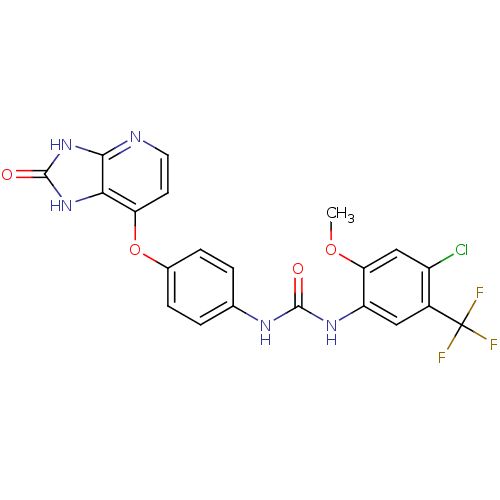
(Pyridoimidazolone, 5j)Show SMILES COc1cc(Cl)c(cc1NC(=O)Nc1ccc(Oc2ccnc3[nH]c(=O)[nH]c23)cc1)C(F)(F)F Show InChI InChI=1S/C21H15ClF3N5O4/c1-33-16-9-13(22)12(21(23,24)25)8-14(16)28-19(31)27-10-2-4-11(5-3-10)34-15-6-7-26-18-17(15)29-20(32)30-18/h2-9H,1H3,(H2,27,28,31)(H2,26,29,30,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.2 | 22 |
The Institute of Cancer Research
| Assay Description
The biological activities (IC50s) of the compounds were determined against the B-RAF (V600E) mutant enzyme in vitro. GST-MEK1, B-RAF (V600E), and inh... |
J Med Chem 52: 2255-64 (2009)
Article DOI: 10.1021/jm801509w
BindingDB Entry DOI: 10.7270/Q2PK0DGX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482014
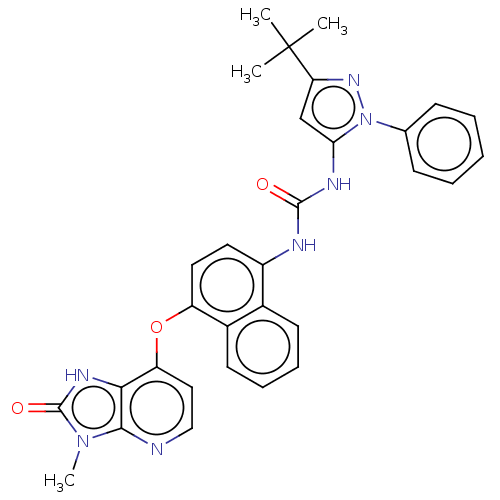
(CHEMBL1090980)Show SMILES Cn1c2nccc(Oc3ccc(NC(=O)Nc4cc(nn4-c4ccccc4)C(C)(C)C)c4ccccc34)c2[nH]c1=O Show InChI InChI=1S/C31H29N7O3/c1-31(2,3)25-18-26(38(36-25)19-10-6-5-7-11-19)34-29(39)33-22-14-15-23(21-13-9-8-12-20(21)22)41-24-16-17-32-28-27(24)35-30(40)37(28)4/h5-18H,1-4H3,(H,35,40)(H2,33,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 2741-56 (2010)
Article DOI: 10.1021/jm900607f
BindingDB Entry DOI: 10.7270/Q2WD43CH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM29723

(Pyridoimidazolone, 5w)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2ccc(Sc3ccnc4[nH]c(=O)[nH]c34)cc2)c1 Show InChI InChI=1S/C20H14F3N5O2S/c21-20(22,23)11-2-1-3-13(10-11)26-18(29)25-12-4-6-14(7-5-12)31-15-8-9-24-17-16(15)27-19(30)28-17/h1-10H,(H2,25,26,29)(H2,24,27,28,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.2 | 22 |
The Institute of Cancer Research
| Assay Description
The biological activities (IC50s) of the compounds were determined against the B-RAF (V600E) mutant enzyme in vitro. GST-MEK1, B-RAF (V600E), and inh... |
J Med Chem 52: 2255-64 (2009)
Article DOI: 10.1021/jm801509w
BindingDB Entry DOI: 10.7270/Q2PK0DGX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM29722
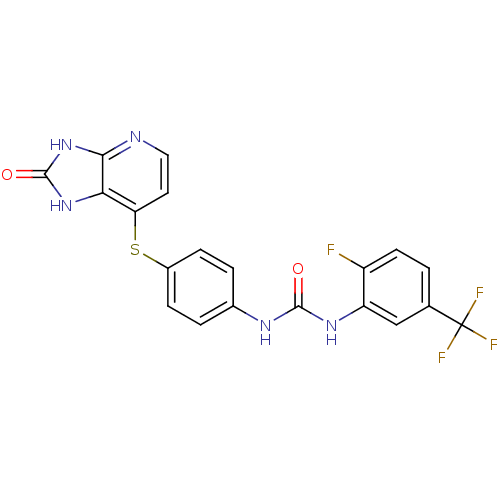
(Pyridoimidazolone, 5v)Show SMILES Fc1ccc(cc1NC(=O)Nc1ccc(Sc2ccnc3[nH]c(=O)[nH]c23)cc1)C(F)(F)F Show InChI InChI=1S/C20H13F4N5O2S/c21-13-6-1-10(20(22,23)24)9-14(13)27-18(30)26-11-2-4-12(5-3-11)32-15-7-8-25-17-16(15)28-19(31)29-17/h1-9H,(H2,26,27,30)(H2,25,28,29,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | 7.2 | 22 |
The Institute of Cancer Research
| Assay Description
The biological activities (IC50s) of the compounds were determined against the B-RAF (V600E) mutant enzyme in vitro. GST-MEK1, B-RAF (V600E), and inh... |
J Med Chem 52: 2255-64 (2009)
Article DOI: 10.1021/jm801509w
BindingDB Entry DOI: 10.7270/Q2PK0DGX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480101
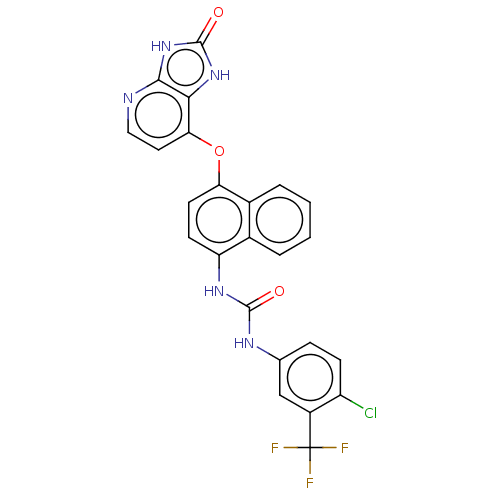
(CHEMBL521725)Show SMILES FC(F)(F)c1cc(NC(=O)Nc2ccc(Oc3ccnc4[nH]c(=O)[nH]c34)c3ccccc23)ccc1Cl Show InChI InChI=1S/C24H15ClF3N5O3/c25-16-6-5-12(11-15(16)24(26,27)28)30-22(34)31-17-7-8-18(14-4-2-1-3-13(14)17)36-19-9-10-29-21-20(19)32-23(35)33-21/h1-11H,(H2,30,31,34)(H2,29,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180334

(2-(3,4-Methylenedioxyphenylamino)-6-(3-acetamidoph...)Show InChI InChI=1S/C19H16N4O3/c1-12(24)21-14-4-2-3-13(7-14)16-9-20-10-19(23-16)22-15-5-6-17-18(8-15)26-11-25-17/h2-10H,11H2,1H3,(H,21,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482021

(CHEMBL1089706)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(Oc2ccnc3[nH]c(=O)[nH]c23)cc1)C(C)(C)C Show InChI InChI=1S/C21H23N7O3/c1-21(2,3)15-11-16(28(4)27-15)24-19(29)23-12-5-7-13(8-6-12)31-14-9-10-22-18-17(14)25-20(30)26-18/h5-11H,1-4H3,(H2,23,24,29)(H2,22,25,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of human BRAF V600E mutant expressed in baculovirus system |
J Med Chem 53: 2741-56 (2010)
Article DOI: 10.1021/jm900607f
BindingDB Entry DOI: 10.7270/Q2WD43CH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50481997
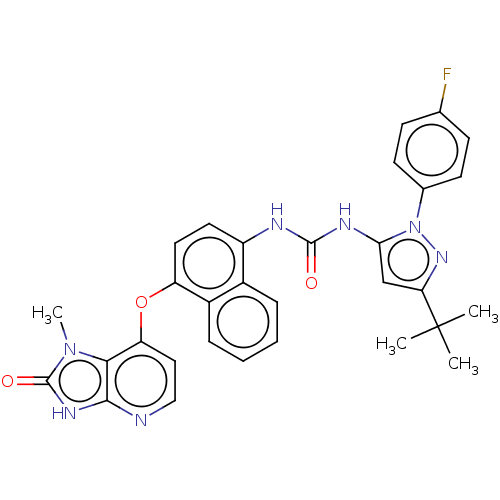
(CHEMBL1090976)Show SMILES Cn1c2c(Oc3ccc(NC(=O)Nc4cc(nn4-c4ccc(F)cc4)C(C)(C)C)c4ccccc34)ccnc2[nH]c1=O Show InChI InChI=1S/C31H28FN7O3/c1-31(2,3)25-17-26(39(37-25)19-11-9-18(32)10-12-19)35-29(40)34-22-13-14-23(21-8-6-5-7-20(21)22)42-24-15-16-33-28-27(24)38(4)30(41)36-28/h5-17H,1-4H3,(H,33,36,41)(H2,34,35,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 2741-56 (2010)
Article DOI: 10.1021/jm900607f
BindingDB Entry DOI: 10.7270/Q2WD43CH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM142600
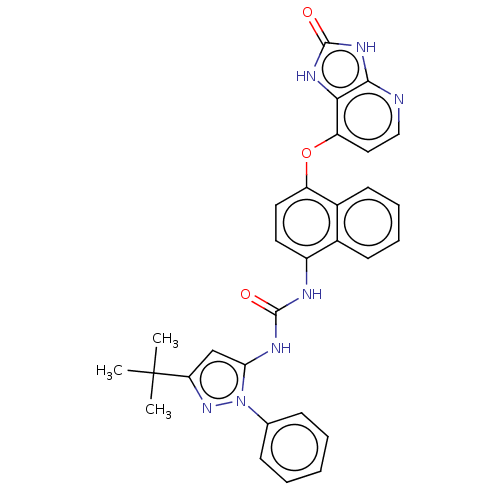
(US8933228, 2)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(Oc3ccnc4[nH]c(=O)[nH]c34)c3ccccc23)n(n1)-c1ccccc1 Show InChI InChI=1S/C30H27N7O3/c1-30(2,3)24-17-25(37(36-24)18-9-5-4-6-10-18)33-28(38)32-21-13-14-22(20-12-8-7-11-19(20)21)40-23-15-16-31-27-26(23)34-29(39)35-27/h4-17H,1-3H3,(H2,32,33,38)(H2,31,34,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 2741-56 (2010)
Article DOI: 10.1021/jm900607f
BindingDB Entry DOI: 10.7270/Q2WD43CH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482017
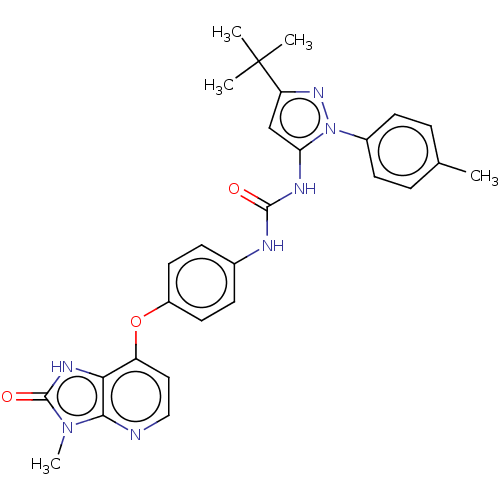
(CHEMBL1091430)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(Oc2ccnc3n(C)c(=O)[nH]c23)cc1)C(C)(C)C Show InChI InChI=1S/C28H29N7O3/c1-17-6-10-19(11-7-17)35-23(16-22(33-35)28(2,3)4)31-26(36)30-18-8-12-20(13-9-18)38-21-14-15-29-25-24(21)32-27(37)34(25)5/h6-16H,1-5H3,(H,32,37)(H2,30,31,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 2741-56 (2010)
Article DOI: 10.1021/jm900607f
BindingDB Entry DOI: 10.7270/Q2WD43CH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480121
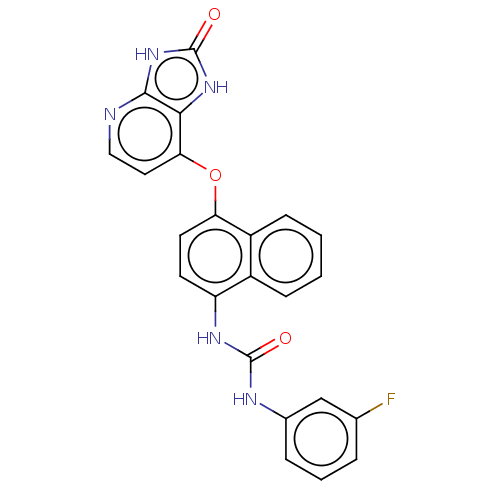
(CHEMBL526838)Show SMILES Fc1cccc(NC(=O)Nc2ccc(Oc3ccnc4[nH]c(=O)[nH]c34)c3ccccc23)c1 Show InChI InChI=1S/C23H16FN5O3/c24-13-4-3-5-14(12-13)26-22(30)27-17-8-9-18(16-7-2-1-6-15(16)17)32-19-10-11-25-21-20(19)28-23(31)29-21/h1-12H,(H2,26,27,30)(H2,25,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480102
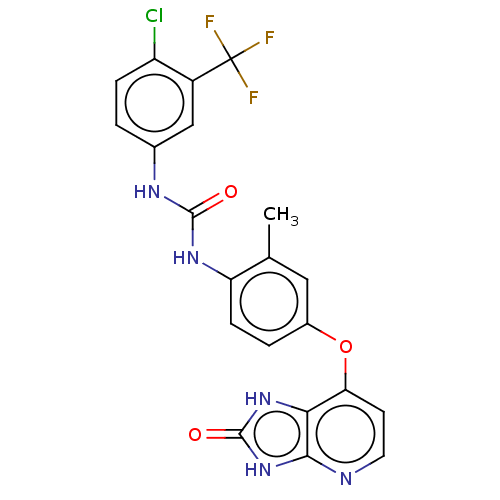
(CHEMBL524801)Show SMILES Cc1cc(Oc2ccnc3[nH]c(=O)[nH]c23)ccc1NC(=O)Nc1ccc(Cl)c(c1)C(F)(F)F Show InChI InChI=1S/C21H15ClF3N5O3/c1-10-8-12(33-16-6-7-26-18-17(16)29-20(32)30-18)3-5-15(10)28-19(31)27-11-2-4-14(22)13(9-11)21(23,24)25/h2-9H,1H3,(H2,27,28,31)(H2,26,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480107

(CHEMBL496081)Show SMILES Cn1c2c(Oc3ccc(NC(=O)Nc4cccc(c4)C(C)(C)C)c4ccccc34)ccnc2[nH]c1=O Show InChI InChI=1S/C28H27N5O3/c1-28(2,3)17-8-7-9-18(16-17)30-26(34)31-21-12-13-22(20-11-6-5-10-19(20)21)36-23-14-15-29-25-24(23)33(4)27(35)32-25/h5-16H,1-4H3,(H,29,32,35)(H2,30,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50480103

(CHEMBL498112)Show SMILES Fc1ccc(cc1NC(=O)Nc1ccc(Oc2ccnc3[nH]c(=O)[nH]c23)c2ccccc12)C(F)(F)F Show InChI InChI=1S/C24H15F4N5O3/c25-15-6-5-12(24(26,27)28)11-17(15)31-22(34)30-16-7-8-18(14-4-2-1-3-13(14)16)36-19-9-10-29-21-20(19)32-23(35)33-21/h1-11H,(H2,30,31,34)(H2,29,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant |
J Med Chem 52: 3881-91 (2009)
Article DOI: 10.1021/jm900242c
BindingDB Entry DOI: 10.7270/Q2Z03BZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482013
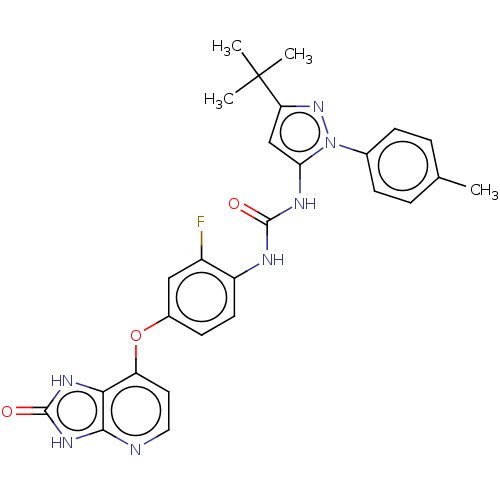
(CHEMBL1091849)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(Oc2ccnc3[nH]c(=O)[nH]c23)cc1F)C(C)(C)C Show InChI InChI=1S/C27H26FN7O3/c1-15-5-7-16(8-6-15)35-22(14-21(34-35)27(2,3)4)31-25(36)30-19-10-9-17(13-18(19)28)38-20-11-12-29-24-23(20)32-26(37)33-24/h5-14H,1-4H3,(H2,30,31,36)(H2,29,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 2741-56 (2010)
Article DOI: 10.1021/jm900607f
BindingDB Entry DOI: 10.7270/Q2WD43CH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM29724

(Pyridoimidazolone, 5x)Show SMILES FC(F)(F)c1cc(NC(=O)Nc2ccc(Sc3ccnc4[nH]c(=O)[nH]c34)cc2)ccc1Cl Show InChI InChI=1S/C20H13ClF3N5O2S/c21-14-6-3-11(9-13(14)20(22,23)24)27-18(30)26-10-1-4-12(5-2-10)32-15-7-8-25-17-16(15)28-19(31)29-17/h1-9H,(H2,26,27,30)(H2,25,28,29,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.2 | 22 |
The Institute of Cancer Research
| Assay Description
The biological activities (IC50s) of the compounds were determined against the B-RAF (V600E) mutant enzyme in vitro. GST-MEK1, B-RAF (V600E), and inh... |
J Med Chem 52: 2255-64 (2009)
Article DOI: 10.1021/jm801509w
BindingDB Entry DOI: 10.7270/Q2PK0DGX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482019
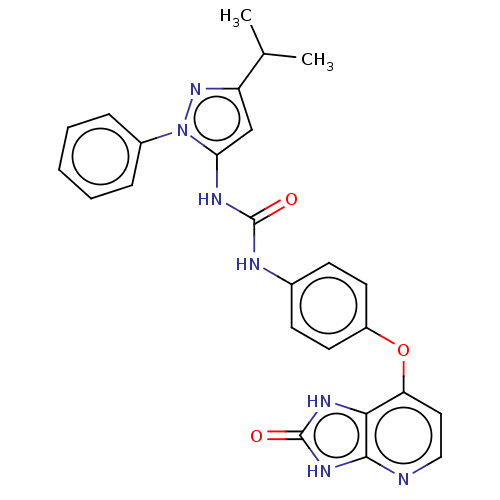
(CHEMBL1092523)Show SMILES CC(C)c1cc(NC(=O)Nc2ccc(Oc3ccnc4[nH]c(=O)[nH]c34)cc2)n(n1)-c1ccccc1 Show InChI InChI=1S/C25H23N7O3/c1-15(2)19-14-21(32(31-19)17-6-4-3-5-7-17)28-24(33)27-16-8-10-18(11-9-16)35-20-12-13-26-23-22(20)29-25(34)30-23/h3-15H,1-2H3,(H2,27,28,33)(H2,26,29,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of human BRAF V600E mutant expressed in baculovirus system |
J Med Chem 53: 2741-56 (2010)
Article DOI: 10.1021/jm900607f
BindingDB Entry DOI: 10.7270/Q2WD43CH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50307879

(CHEMBL602123 | N-(2,3,4-trifluoro-5-(2-oxo-2,3-dih...)Show SMILES Fc1c(NC(=O)c2cccc(OC(F)(F)F)c2)cc(Oc2cccc3[nH]c(=O)[nH]c23)c(F)c1F Show InChI InChI=1S/C21H11F6N3O4/c22-15-12(28-19(31)9-3-1-4-10(7-9)34-21(25,26)27)8-14(16(23)17(15)24)33-13-6-2-5-11-18(13)30-20(32)29-11/h1-8H,(H,28,31)(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of wild type BRAF |
J Med Chem 53: 1964-78 (2010)
Article DOI: 10.1021/jm901509a
BindingDB Entry DOI: 10.7270/Q2R211HN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | 7.2 | 22 |
The Institute of Cancer Research
| Assay Description
The biological activities (IC50s) of the compounds were determined against the B-RAF (V600E) mutant enzyme in vitro. GST-MEK1, B-RAF (V600E), and inh... |
J Med Chem 52: 2255-64 (2009)
Article DOI: 10.1021/jm801509w
BindingDB Entry DOI: 10.7270/Q2PK0DGX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data