

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501808 (CHEMBL4465534) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50570460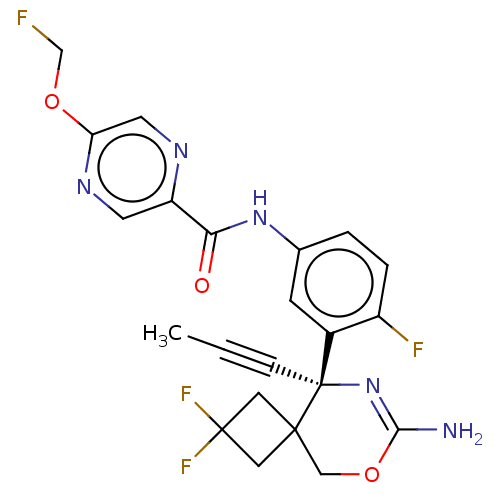 (CHEMBL4854629) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-JNJ962 from BACE1 (unknown origin) expressed in HEK293 cell membrane assessed as inhibition constant by scintillation counting a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01858 BindingDB Entry DOI: 10.7270/Q2GH9NQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501810 (CHEMBL4443968) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501818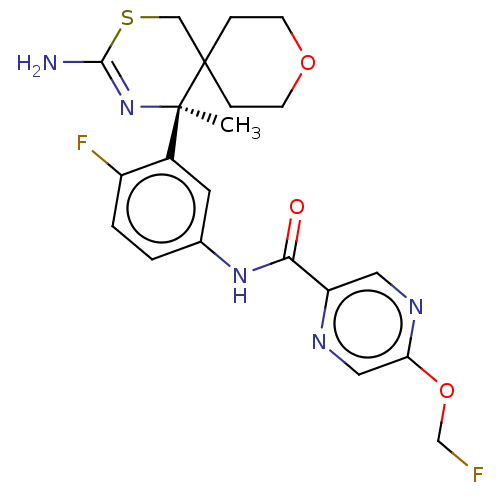 (CHEMBL4583340) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50501818 (CHEMBL4583340) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 479 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-JNJ-962 from BACE2 (unknown origin) expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50570460 (CHEMBL4854629) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-JNJ962 from BACE2 (unknown origin) expressed in HEK293 cell membrane assessed as inhibition constant by scintillation counting a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01858 BindingDB Entry DOI: 10.7270/Q2GH9NQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50501808 (CHEMBL4465534) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-JNJ-962 from BACE2 (unknown origin) expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50501810 (CHEMBL4443968) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-JNJ-962 from BACE2 (unknown origin) expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50157445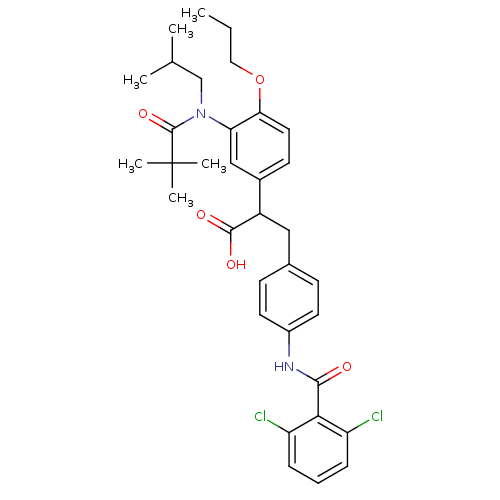 (3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{3-[(2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e... | Bioorg Med Chem Lett 15: 217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.09.089 BindingDB Entry DOI: 10.7270/Q27945Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50157461 (3-{4-[(3,5-Dichloro-pyridine-4-carbonyl)-amino]-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e... | Bioorg Med Chem Lett 15: 217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.09.089 BindingDB Entry DOI: 10.7270/Q27945Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50157458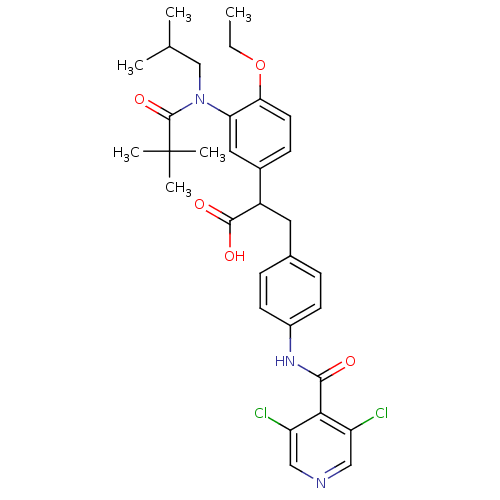 (3-{4-[(3,5-Dichloro-pyridine-4-carbonyl)-amino]-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e... | Bioorg Med Chem Lett 15: 217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.09.089 BindingDB Entry DOI: 10.7270/Q27945Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50505569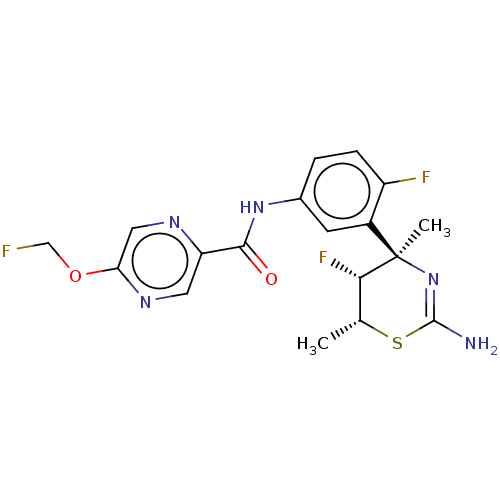 (CHEMBL4557670) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells expressing human wild type amyloid precursor protein assessed as reduction in amyloidbeta40 production inc... | J Med Chem 62: 9331-9337 (2019) Article DOI: 10.1021/acs.jmedchem.9b01140 BindingDB Entry DOI: 10.7270/Q2CJ8HRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50157446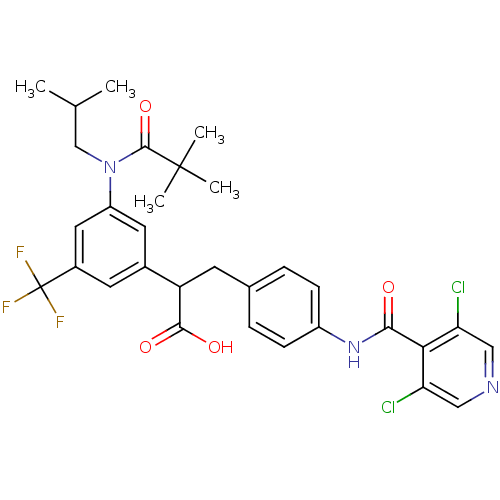 (3-{4-[(3,5-Dichloro-pyridine-4-carbonyl)-amino]-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e... | Bioorg Med Chem Lett 15: 217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.09.089 BindingDB Entry DOI: 10.7270/Q27945Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50157464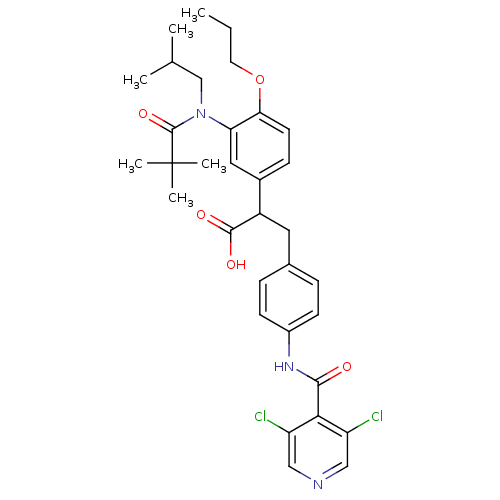 (3-{4-[(3,5-Dichloro-pyridine-4-carbonyl)-amino]-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e... | Bioorg Med Chem Lett 15: 217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.09.089 BindingDB Entry DOI: 10.7270/Q27945Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501811 (CHEMBL4535964) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing APP695 assessed as reduction in Abeta42 level measured after 18 hrs by sandwich ELISA | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432632 (CHEMBL2347211) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing APP695 assessed as reduction in Abeta42 level measured after 18 hrs by sandwich ELISA | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50157470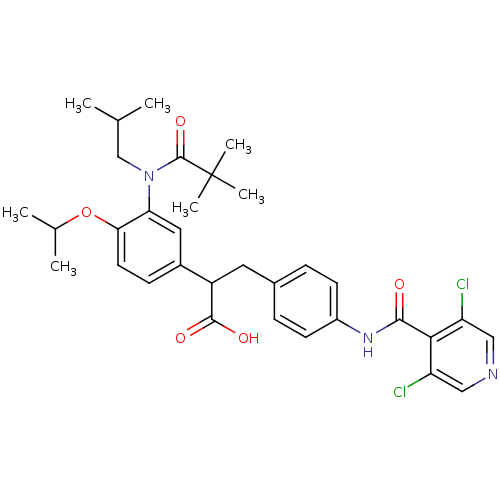 (3-{4-[(3,5-Dichloro-pyridine-4-carbonyl)-amino]-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e... | Bioorg Med Chem Lett 15: 217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.09.089 BindingDB Entry DOI: 10.7270/Q27945Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501814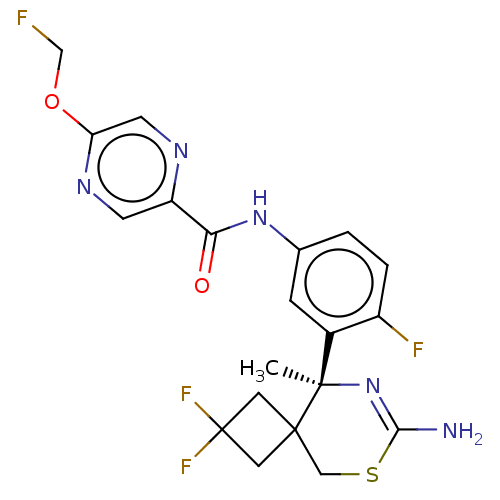 (CHEMBL4592239) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing APP695 assessed as reduction in Abeta42 level measured after 18 hrs by sandwich ELISA | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501806 (CHEMBL4473080) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using Mca-APP Swedish Lys-Met/Asn-Leu mutant-Dnp quencher as substrate by fluorescence assay | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50157475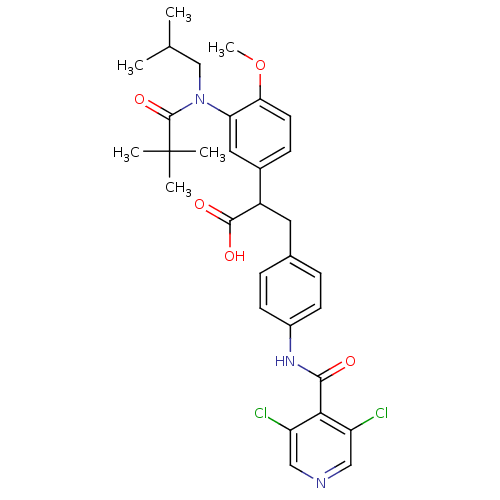 (3-{4-[(3,5-Dichloro-pyridine-4-carbonyl)-amino]-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e... | Bioorg Med Chem Lett 15: 217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.09.089 BindingDB Entry DOI: 10.7270/Q27945Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501816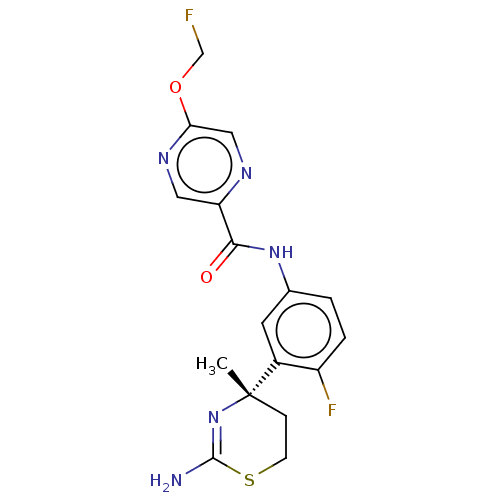 (CHEMBL4568202) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing APP695 assessed as reduction in Abeta42 level measured after 18 hrs by sandwich ELISA | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM47353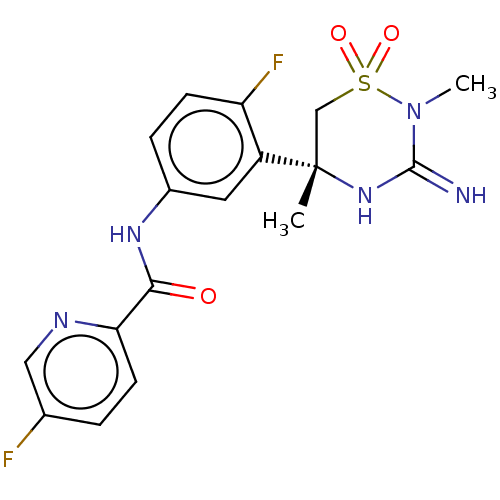 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE2 (unknown origin) using APP harboring Swedish Lys/Met mutant-derived peptide as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01858 BindingDB Entry DOI: 10.7270/Q2GH9NQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM47353 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using APP harboring Swedish Lys/Met mutant-derived peptide as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01858 BindingDB Entry DOI: 10.7270/Q2GH9NQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501813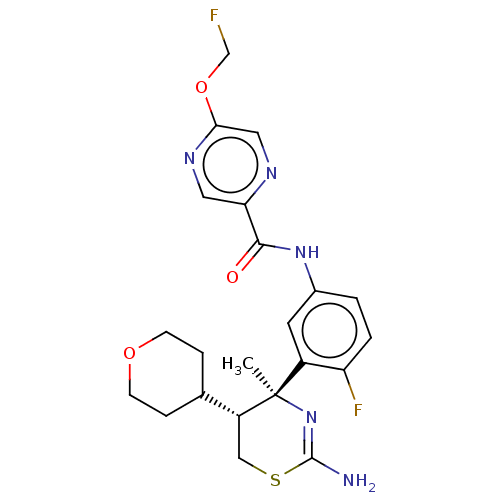 (CHEMBL4587362) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing APP695 assessed as reduction in Abeta42 level measured after 18 hrs by sandwich ELISA | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50157447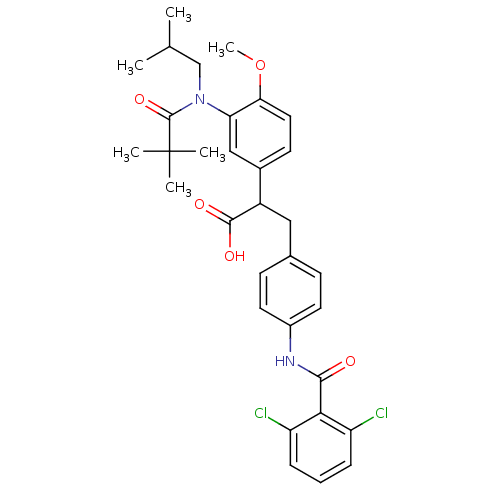 (3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{3-[(2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e... | Bioorg Med Chem Lett 15: 217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.09.089 BindingDB Entry DOI: 10.7270/Q27945Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501809 (CHEMBL4463201) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing APP695 assessed as reduction in Abeta42 level measured after 18 hrs by sandwich ELISA | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501806 (CHEMBL4473080) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing APP695 assessed as reduction in Abeta42 level measured after 18 hrs by sandwich ELISA | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50157459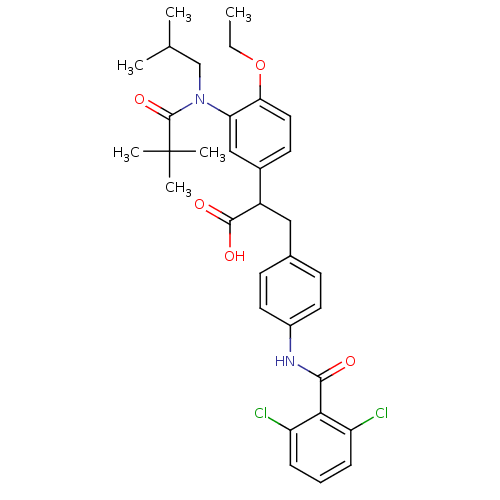 (3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{3-[(2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e... | Bioorg Med Chem Lett 15: 217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.09.089 BindingDB Entry DOI: 10.7270/Q27945Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501811 (CHEMBL4535964) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using Mca-APP Swedish Lys-Met/Asn-Leu mutant-Dnp quencher as substrate by fluorescence assay | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501813 (CHEMBL4587362) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using Mca-APP Swedish Lys-Met/Asn-Leu mutant-Dnp quencher as substrate by fluorescence assay | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501809 (CHEMBL4463201) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using Mca-APP Swedish Lys-Met/Asn-Leu mutant-Dnp quencher as substrate by fluorescence assay | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50157469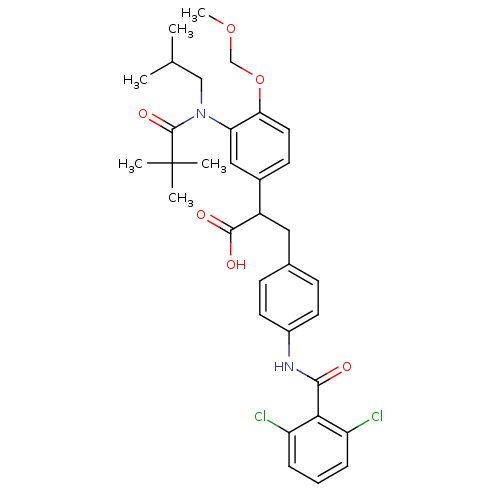 (3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{3-[(2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e... | Bioorg Med Chem Lett 15: 217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.09.089 BindingDB Entry DOI: 10.7270/Q27945Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501808 (CHEMBL4465534) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using Mca-APP Swedish Lys-Met/Asn-Leu mutant-Dnp quencher as substrate by fluorescence assay | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501810 (CHEMBL4443968) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing APP695 assessed as reduction in Abeta42 level measured after 18 hrs by sandwich ELISA | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501812 (CHEMBL4451133) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing APP695 assessed as reduction in Abeta42 level measured after 18 hrs by sandwich ELISA | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50432632 (CHEMBL2347211) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using Mca-APP Swedish Lys-Met/Asn-Leu mutant-Dnp quencher as substrate by fluorescence assay | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049128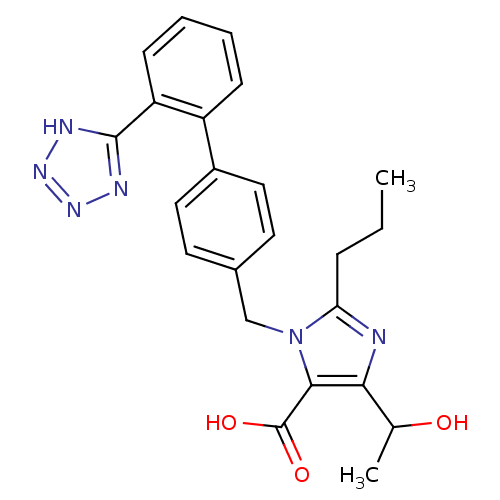 (5-(1-Hydroxy-ethyl)-2-propyl-3-[2'-(2H-tetrazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501816 (CHEMBL4568202) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using Mca-APP Swedish Lys-Met/Asn-Leu mutant-Dnp quencher as substrate by fluorescence assay | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50157466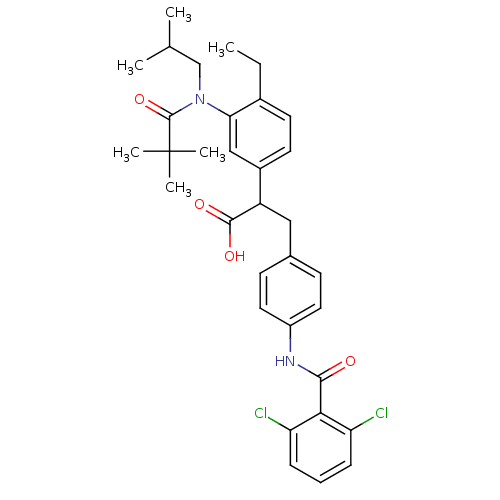 (3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{3-[(2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaken Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e... | Bioorg Med Chem Lett 15: 217-20 (2004) Article DOI: 10.1016/j.bmcl.2004.09.089 BindingDB Entry DOI: 10.7270/Q27945Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50505569 (CHEMBL4557670) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 incubated for 3 hrs using APP derived peptide as substrate by HTRF assay | J Med Chem 62: 9331-9337 (2019) Article DOI: 10.1021/acs.jmedchem.9b01140 BindingDB Entry DOI: 10.7270/Q2CJ8HRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049118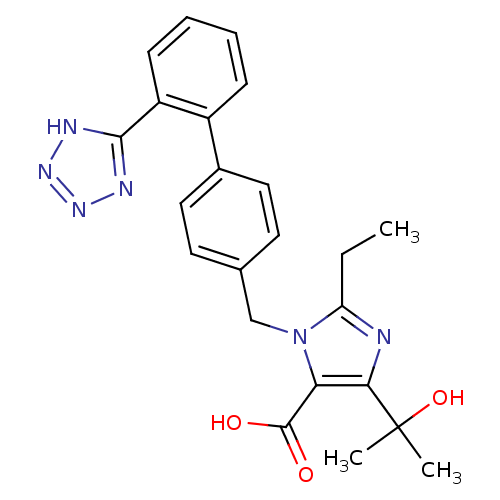 (2-Ethyl-5-(1-hydroxy-1-methyl-ethyl)-3-[2'-(2H-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501819 (CHEMBL4561732) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing APP695 assessed as reduction in Abeta42 level measured after 18 hrs by sandwich ELISA | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501814 (CHEMBL4592239) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using Mca-APP Swedish Lys-Met/Asn-Leu mutant-Dnp quencher as substrate by fluorescence assay | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501818 (CHEMBL4583340) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing APP695 assessed as reduction in Abeta42 level measured after 18 hrs by sandwich ELISA | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049123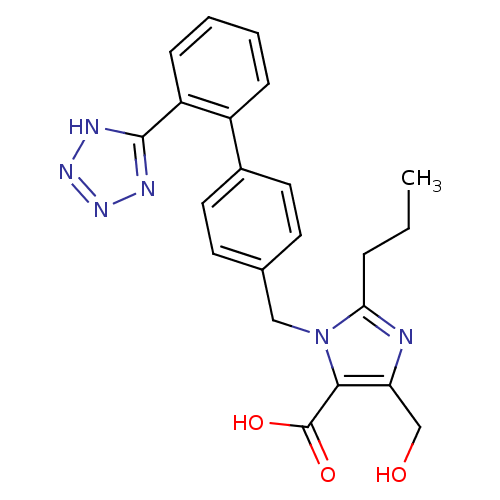 (5-Hydroxymethyl-2-propyl-3-[2'-(2H-tetrazol-5-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50570460 (CHEMBL4854629) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing wild type APP assessed as reduction in amyloid beta 42 level incubated for 18 hrs by AlphaLISA A... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01858 BindingDB Entry DOI: 10.7270/Q2GH9NQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Mus musculus (Mouse)) | BDBM50570460 (CHEMBL4854629) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE2 in mouse MIN6 cells expressing wild type TMEM27 assessed as reduction in TMEM27 secretion by MSD electrochemiluminescence assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01858 BindingDB Entry DOI: 10.7270/Q2GH9NQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50505569 (CHEMBL4557670) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) by HTRF assay | J Med Chem 62: 9331-9337 (2019) Article DOI: 10.1021/acs.jmedchem.9b01140 BindingDB Entry DOI: 10.7270/Q2CJ8HRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501817 (CHEMBL4536643) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 (1 to 454 residues) (unknown origin) using Mca-APP Swedish Lys-Met/Asn-Leu mutant-Dnp quencher as substrate by fluorescence assay | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50501807 (CHEMBL4563915) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SKNBE2 cells expressing APP695 assessed as reduction in Abeta42 level measured after 18 hrs by sandwich ELISA | J Med Chem 62: 5080-5095 (2019) Article DOI: 10.1021/acs.jmedchem.9b00309 BindingDB Entry DOI: 10.7270/Q22J6G35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 341 total ) | Next | Last >> |