

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227831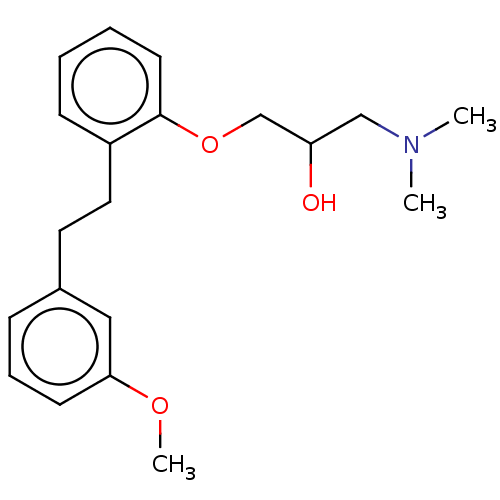 (CHEMBL52242) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227791 (CHEMBL53815) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227978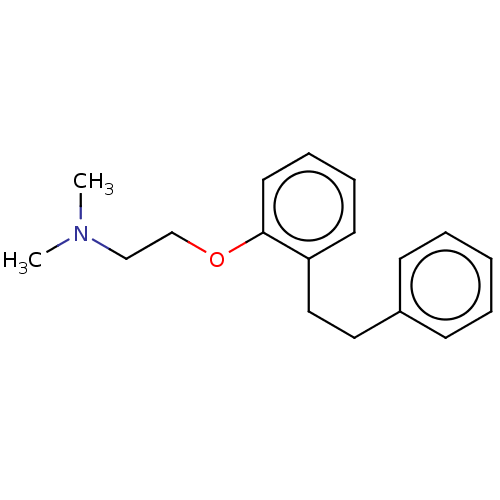 (CHEMBL53316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50228190 (CHEMBL54263) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227787 (CHEMBL1743933) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227837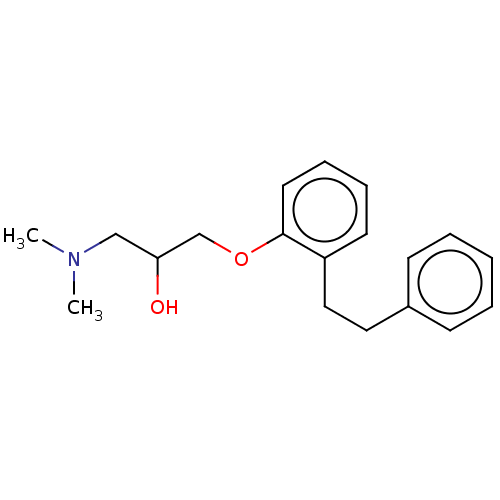 (CHEMBL52131) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50093789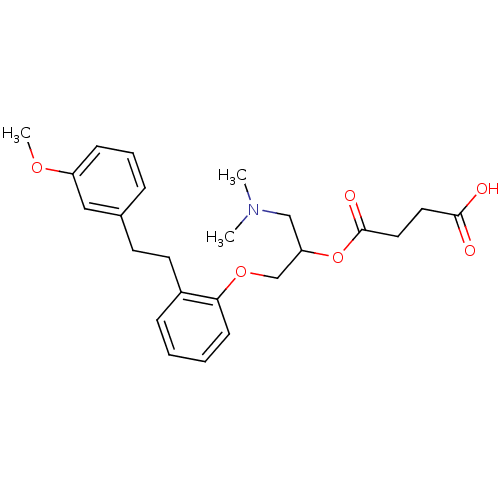 (CHEMBL541829 | Succinic acid mono-(2-dimethylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227788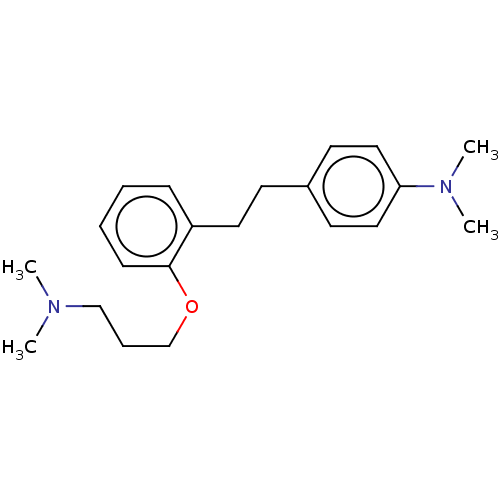 (CHEMBL1743936) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227974 (CHEMBL298554) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227976 (CHEMBL299389) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227653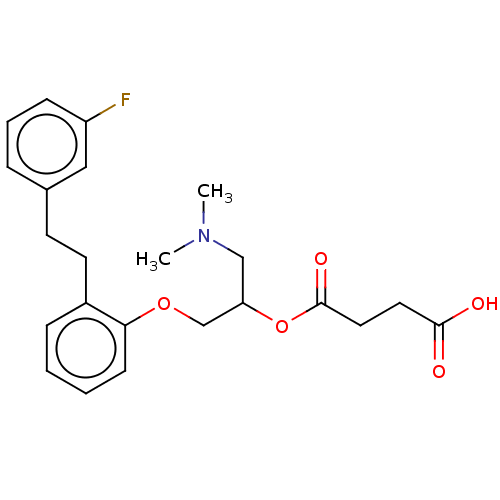 (CHEMBL299207) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227977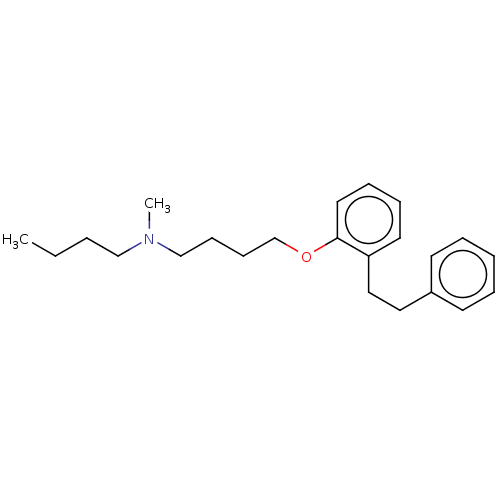 (CHEMBL300631) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227790 (CHEMBL53344) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227789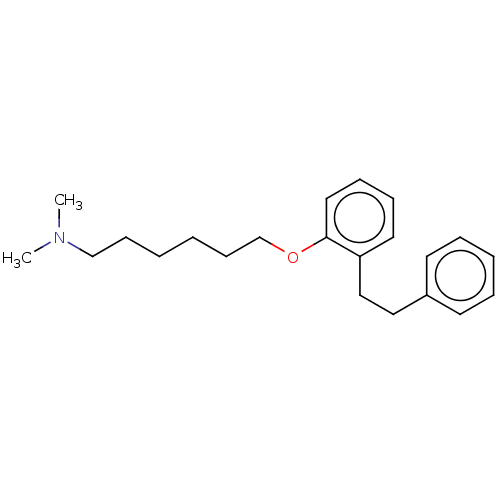 (CHEMBL412653) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227832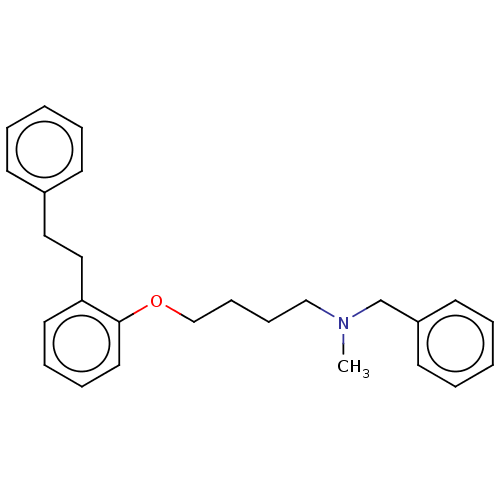 (CHEMBL54101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 198 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50228189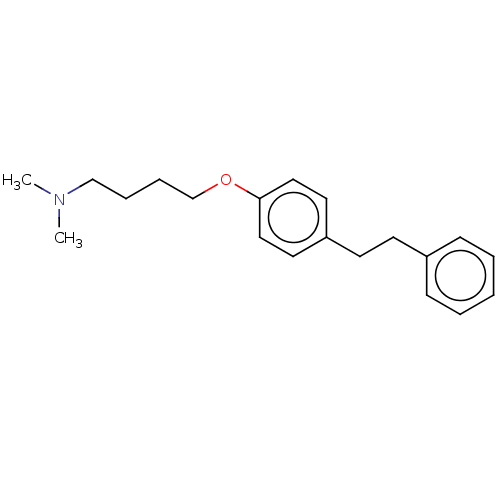 (CHEMBL52710) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 416 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50228052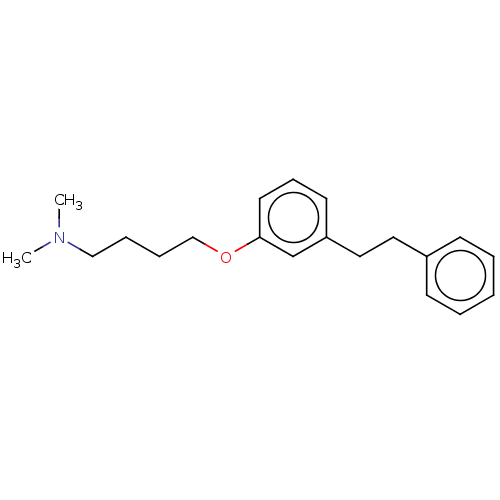 (CHEMBL52982) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 517 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227831 (CHEMBL52242) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210107 (7-chloro-3-(4-chlorophenylsulfonyl)quinazoline-2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058973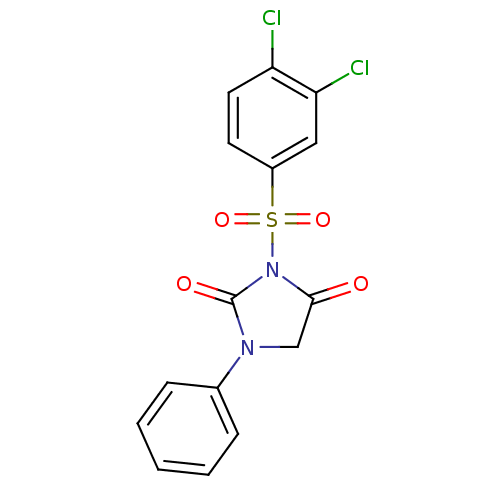 (3-(3,4-Dichloro-benzenesulfonyl)-1-phenyl-imidazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058984 (3-(3,4-Dichloro-benzenesulfonyl)-1-(3,4-dimethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058990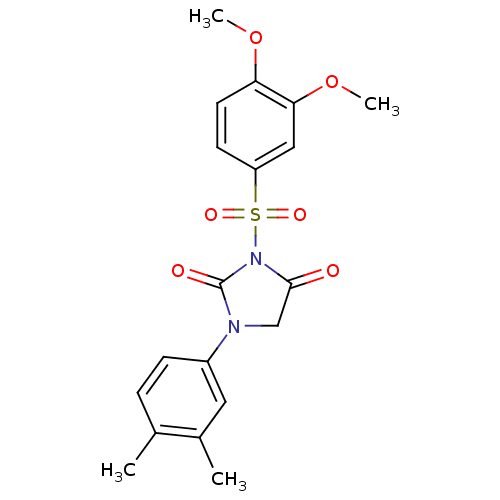 (3-(3,4-Dimethoxy-benzenesulfonyl)-1-(3,4-dimethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210098 (6-(4,5-dichloro-2-methoxybenzyl)-4-(4-chlorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058996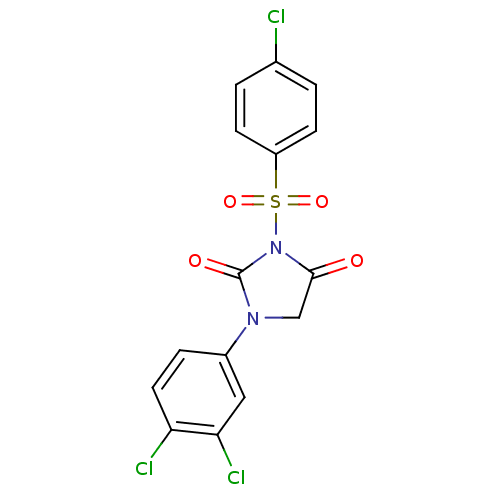 (3-(4-Chloro-benzenesulfonyl)-1-(3,4-dichloro-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210116 (6-(5-fluoro-2-methoxybenzyl)-4-(4-chlorophenylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210120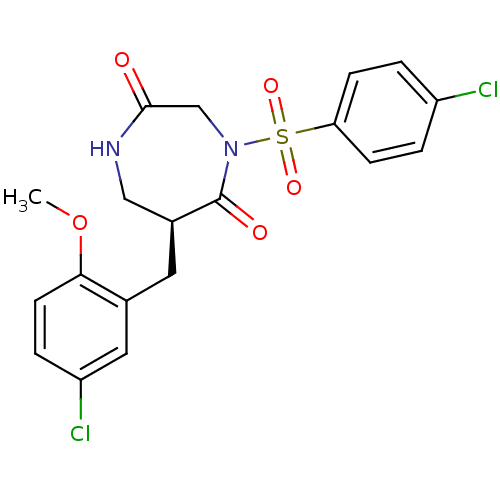 ((S)-6-(5-chloro-2-methoxybenzyl)-4-(4-chlorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210094 (3-((1-(4-chlorophenylsulfonyl)-3,7-dioxo-1,4-diaze...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50059007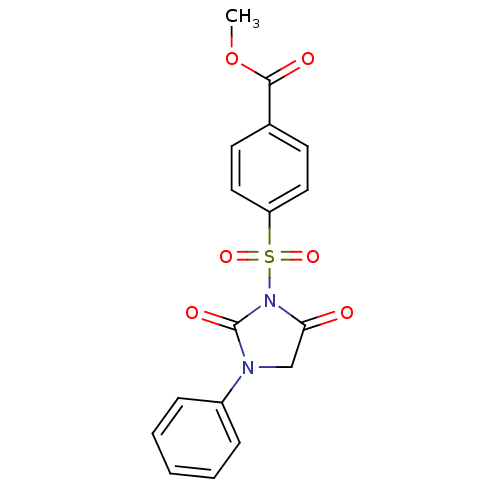 (4-(2,5-Dioxo-3-phenyl-imidazolidine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058964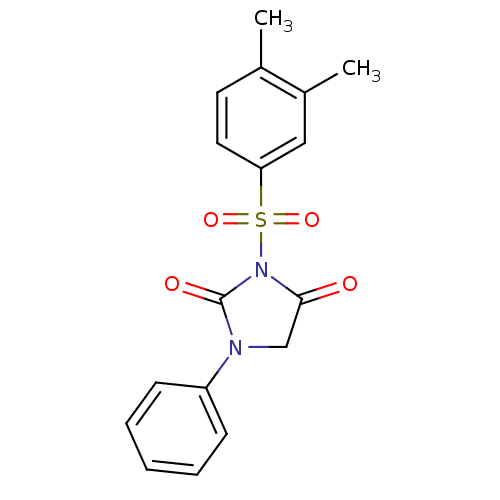 (3-(3,4-Dimethyl-benzenesulfonyl)-1-phenyl-imidazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227791 (CHEMBL53815) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100740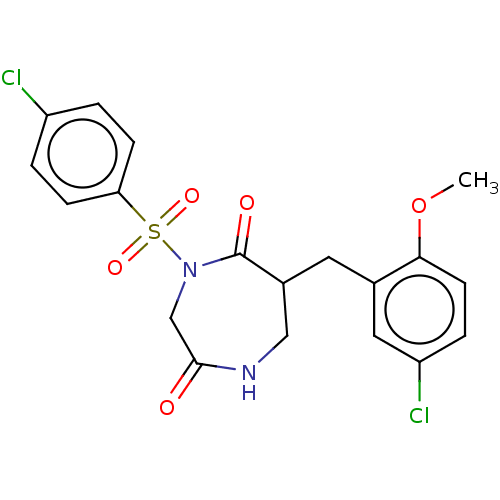 (CHEMBL391608 | US8507714, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058969 (3-(4-Chloro-benzenesulfonyl)-1-(3,4-dimethyl-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210099 (CHEMBL246964 | rac-2q) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058972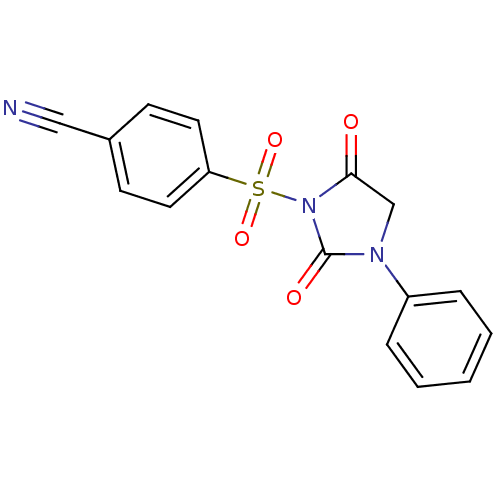 (4-(2,5-Dioxo-3-phenyl-imidazolidine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210096 (2-(4-chloro-2-((1-(4-chlorophenylsulfonyl)-3,7-dio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM87059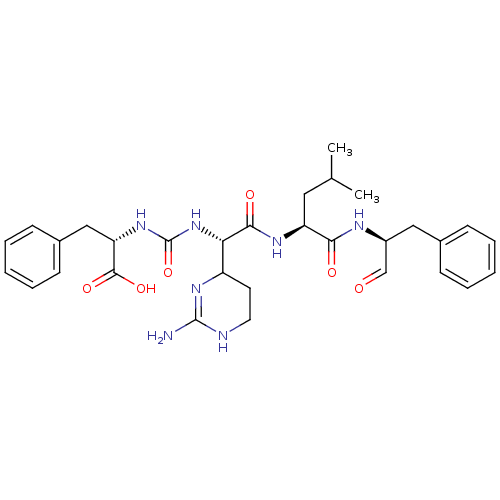 (CHEMBL247767 | Chymostatin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058977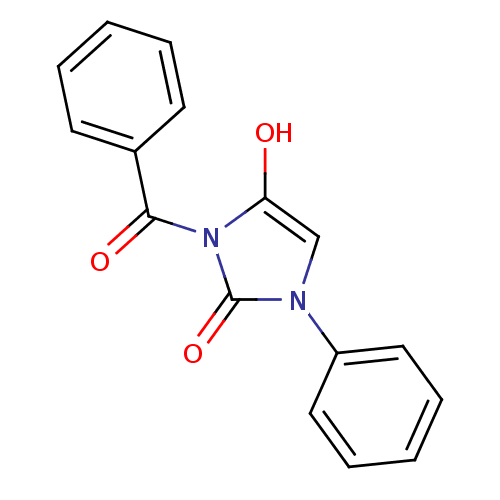 (3-Benzoyl-1-phenyl-imidazolidine-2,4-dione | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50093789 (CHEMBL541829 | Succinic acid mono-(2-dimethylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227788 (CHEMBL1743936) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227837 (CHEMBL52131) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058999 (3-(3,4-Dimethyl-benzenesulfonyl)-1-(3,4-dimethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227787 (CHEMBL1743933) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50059002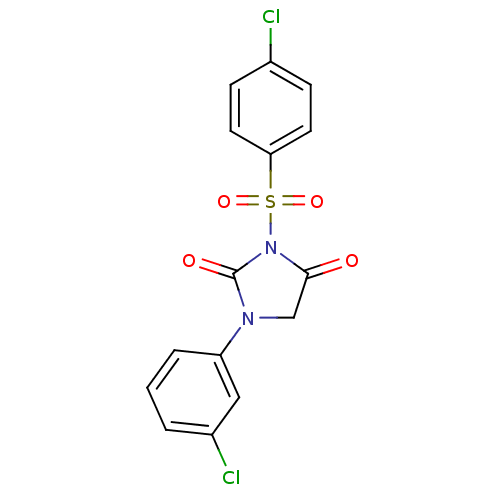 (3-(4-Chloro-benzenesulfonyl)-1-(3-chloro-phenyl)-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058975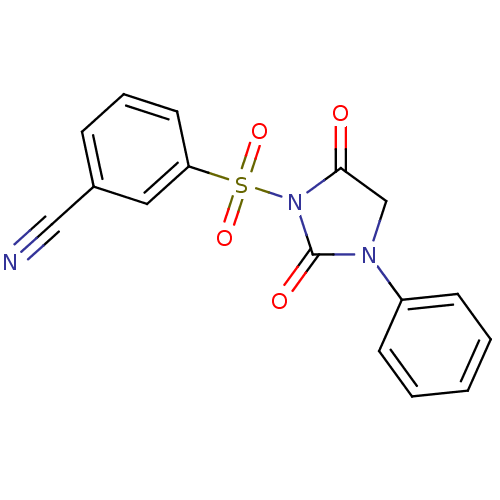 (3-(2,5-Dioxo-3-phenyl-imidazolidine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058994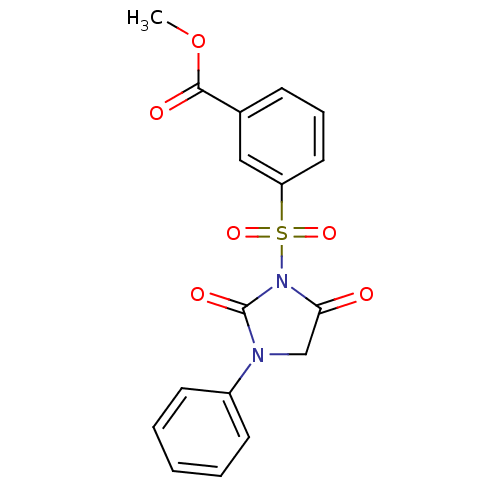 (3-(2,5-Dioxo-3-phenyl-imidazolidine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210113 (6-(5-chloro-2-ethoxybenzyl)-4-(4-chlorophenylsulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227978 (CHEMBL53316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210097 (6-(2-chlorobenzyl)-4-(4-chlorophenylsulfonyl)-1,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50059014 (3-(3,4-Dimethoxy-benzenesulfonyl)-1-phenyl-imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50228190 (CHEMBL54263) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 217 total ) | Next | Last >> |