 Found 313 hits with Last Name = 'furuya' and Initial = 't'
Found 313 hits with Last Name = 'furuya' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Voltage-dependent L-type calcium channel subunit alpha-1S
(Rattus norvegicus) | BDBM50226128
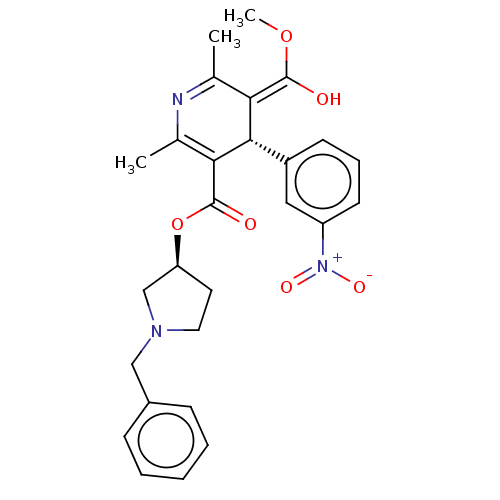
(CHEMBL2093893)Show SMILES Cl.[H][C@@]1(CCN(Cc2ccccc2)C1)OC(=O)C1=C(C)N=C(C)\C(=C(\O)OC)[C@]1([H])c1cccc(c1)[N+]([O-])=O |r,c:18,t:21| Show InChI InChI=1S/C27H29N3O6.ClH/c1-17-23(26(31)35-3)25(20-10-7-11-21(14-20)30(33)34)24(18(2)28-17)27(32)36-22-12-13-29(16-22)15-19-8-5-4-6-9-19;/h4-11,14,22,25,31H,12-13,15-16H2,1-3H3;1H/b26-23+;/t22-,25-;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate |
J Med Chem 29: 2504-11 (1986)
BindingDB Entry DOI: 10.7270/Q2QF8W3R |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1S
(Rattus norvegicus) | BDBM50226129
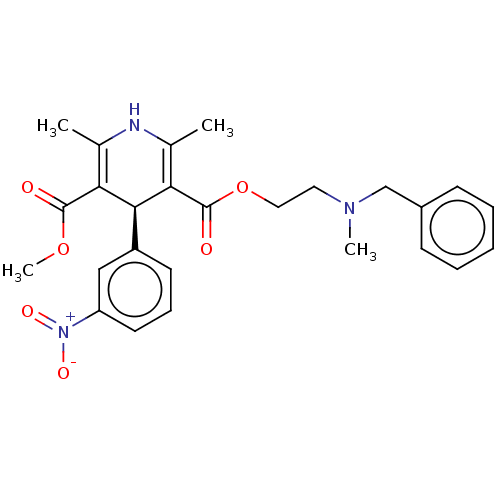
(CHEMBL1314450)Show SMILES COC(=O)C1=C(C)NC(C)=C([C@H]1c1cccc(c1)[N+]([O-])=O)C(=O)OCCN(C)Cc1ccccc1 |c:4,9| Show InChI InChI=1S/C26H29N3O6/c1-17-22(25(30)34-4)24(20-11-8-12-21(15-20)29(32)33)23(18(2)27-17)26(31)35-14-13-28(3)16-19-9-6-5-7-10-19/h5-12,15,24,27H,13-14,16H2,1-4H3/t24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.499 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate |
J Med Chem 29: 2504-11 (1986)
BindingDB Entry DOI: 10.7270/Q2QF8W3R |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1S
(Rattus norvegicus) | BDBM50101815

(CHEBI:7550 | Nicardipine)Show SMILES COC(=O)C1=C(C)NC(C)=C(C1c1cccc(c1)[N+]([O-])=O)C(=O)OCCN(C)Cc1ccccc1 |c:4,9| Show InChI InChI=1S/C26H29N3O6/c1-17-22(25(30)34-4)24(20-11-8-12-21(15-20)29(32)33)23(18(2)27-17)26(31)35-14-13-28(3)16-19-9-6-5-7-10-19/h5-12,15,24,27H,13-14,16H2,1-4H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate |
J Med Chem 29: 2504-11 (1986)
BindingDB Entry DOI: 10.7270/Q2QF8W3R |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1S
(Rattus norvegicus) | BDBM50226130

(CHEMBL558616)Show SMILES Cl.[H][C@]1(CCN(Cc2ccccc2)C1)OC(=O)C1=C(C)N=C(C)\C(=C(\O)OC)[C@]1([H])c1cccc(c1)[N+]([O-])=O |r,c:18,t:21| Show InChI InChI=1S/C27H29N3O6.ClH/c1-17-23(26(31)35-3)25(20-10-7-11-21(14-20)30(33)34)24(18(2)28-17)27(32)36-22-12-13-29(16-22)15-19-8-5-4-6-9-19;/h4-11,14,22,25,31H,12-13,15-16H2,1-3H3;1H/b26-23+;/t22-,25+;/m1./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate |
J Med Chem 29: 2504-11 (1986)
BindingDB Entry DOI: 10.7270/Q2QF8W3R |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1S
(Rattus norvegicus) | BDBM50101817
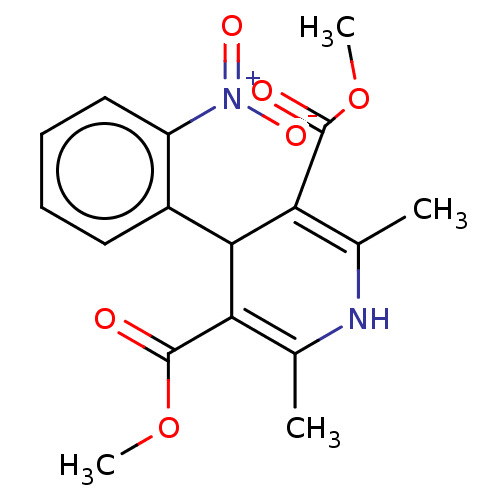
(Adalat | Adalat Cc | Afeditab Cr | BAY-A-1040 | CH...)Show SMILES COC(=O)C1=C(C)NC(C)=C(C1c1ccccc1[N+]([O-])=O)C(=O)OC |c:4,9| Show InChI InChI=1S/C17H18N2O6/c1-9-13(16(20)24-3)15(14(10(2)18-9)17(21)25-4)11-7-5-6-8-12(11)19(22)23/h5-8,15,18H,1-4H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate |
J Med Chem 29: 2504-11 (1986)
BindingDB Entry DOI: 10.7270/Q2QF8W3R |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1S
(Rattus norvegicus) | BDBM50226131
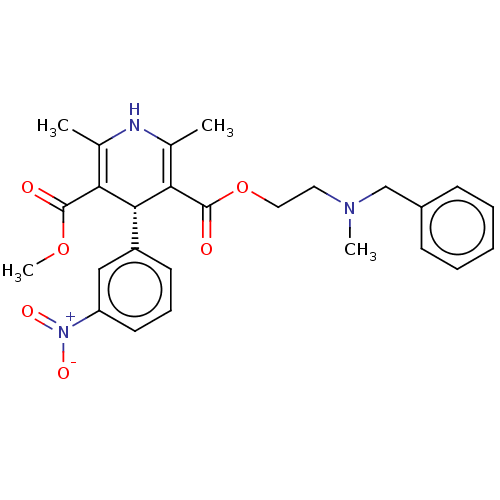
(CHEMBL1598680)Show SMILES COC(=O)C1=C(C)NC(C)=C([C@@H]1c1cccc(c1)[N+]([O-])=O)C(=O)OCCN(C)Cc1ccccc1 |c:4,9| Show InChI InChI=1S/C26H29N3O6/c1-17-22(25(30)34-4)24(20-11-8-12-21(15-20)29(32)33)23(18(2)27-17)26(31)35-14-13-28(3)16-19-9-6-5-7-10-19/h5-12,15,24,27H,13-14,16H2,1-4H3/t24-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate |
J Med Chem 29: 2504-11 (1986)
BindingDB Entry DOI: 10.7270/Q2QF8W3R |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1S
(Rattus norvegicus) | BDBM50226127

(CHEMBL542169)Show SMILES Cl.[H][C@]1(CCN(Cc2ccccc2)C1)OC(=O)C1=C(C)N=C(C)\C(=C(\O)OC)[C@@]1([H])c1cccc(c1)[N+]([O-])=O |r,c:18,t:21| Show InChI InChI=1S/C27H29N3O6.ClH/c1-17-23(26(31)35-3)25(20-10-7-11-21(14-20)30(33)34)24(18(2)28-17)27(32)36-22-12-13-29(16-22)15-19-8-5-4-6-9-19;/h4-11,14,22,25,31H,12-13,15-16H2,1-3H3;1H/b26-23+;/t22-,25-;/m1./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate |
J Med Chem 29: 2504-11 (1986)
BindingDB Entry DOI: 10.7270/Q2QF8W3R |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1S
(Rattus norvegicus) | BDBM50226126
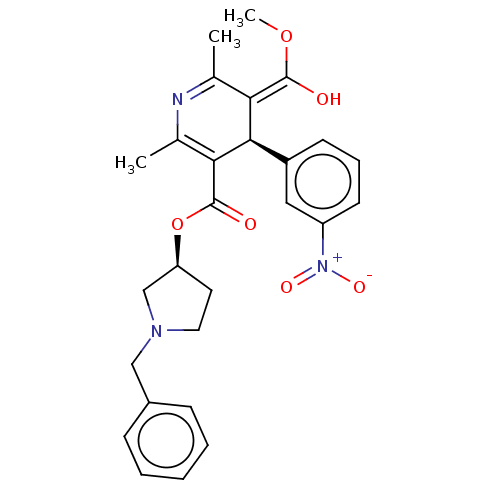
(CHEMBL553553)Show SMILES Cl.[H][C@@]1(CCN(Cc2ccccc2)C1)OC(=O)C1=C(C)N=C(C)\C(=C(\O)OC)[C@@]1([H])c1cccc(c1)[N+]([O-])=O |r,c:18,t:21| Show InChI InChI=1S/C27H29N3O6.ClH/c1-17-23(26(31)35-3)25(20-10-7-11-21(14-20)30(33)34)24(18(2)28-17)27(32)36-22-12-13-29(16-22)15-19-8-5-4-6-9-19;/h4-11,14,22,25,31H,12-13,15-16H2,1-3H3;1H/b26-23+;/t22-,25+;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate |
J Med Chem 29: 2504-11 (1986)
BindingDB Entry DOI: 10.7270/Q2QF8W3R |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-6/beta-8
(Homo sapiens (Human)) | BDBM297773

(US10118929, Compound A1)Show SMILES OC(=O)CC(N1CCN(CCCCNc2nc3ccccc3[nH]2)C1=O)c1cccc(c1)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
SciFluor Life Sciences, Inc.
US Patent
| Assay Description
The optimized protocol was validated by employing reference compounds such as Cilengitide (+Vβ3/αVβ5−VN interaction) and CWHM12 ... |
US Patent US10118929 (2018)
BindingDB Entry DOI: 10.7270/Q2V126WM |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM606867

(US11685738, Compound A3)Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1ccc(OC(F)F)nc1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50200120

(CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115826
BindingDB Entry DOI: 10.7270/Q2C2513C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM606865

(US11685738, Compound A4)Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1cnc2ccc(F)cc2c1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM606917
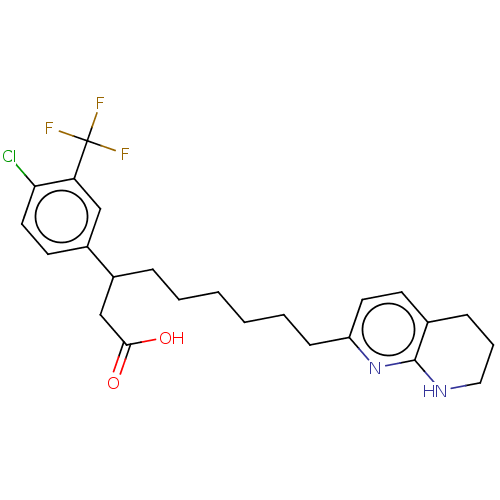
(US11685738, Compound A23)Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1ccc(Cl)c(c1)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM606955

(US11685738, Compound A21-2)Show SMILES OC(=O)C[C@H](CCCCCCc1ccc2CCCNc2n1)c1cccc(c1)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM606865

(US11685738, Compound A4)Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1cnc2ccc(F)cc2c1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-8
(Homo sapiens (Human)) | BDBM606947
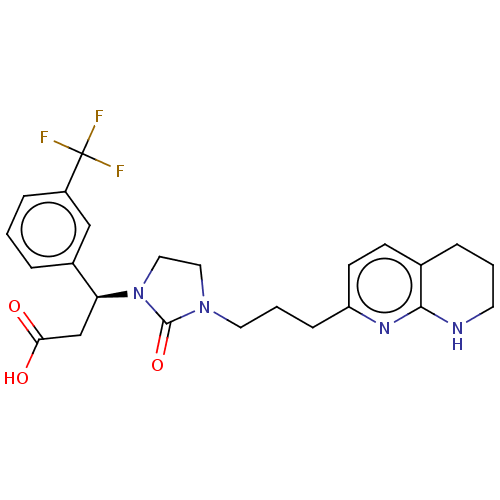
(US11685738, Compound A15s)Show SMILES OC(=O)C[C@H](N1CCN(CCCc2ccc3CCCNc3n2)C1=O)c1cccc(c1)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM606954
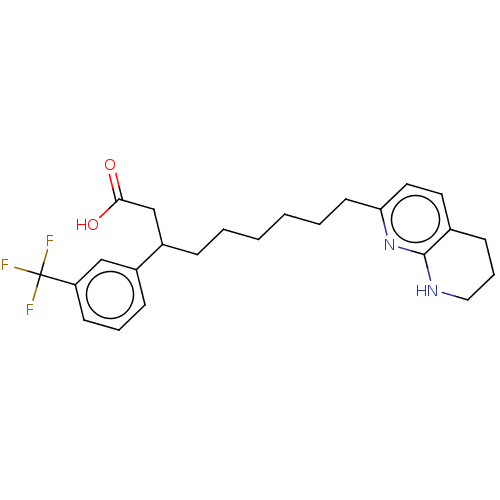
(US11685738, Compound A21 | acid )Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1cccc(c1)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7.64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM197726
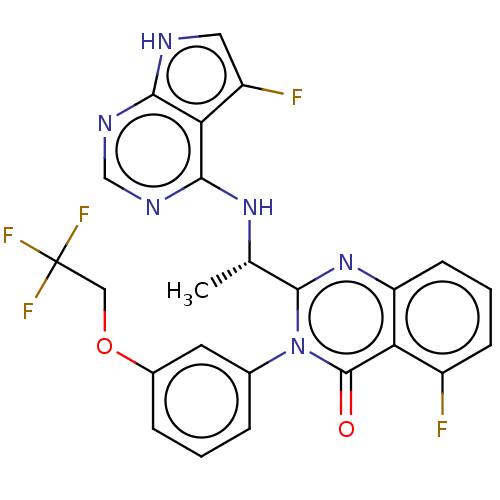
(US9216985, 17A)Show SMILES C[C@H](Nc1ncnc2[nH]cc(F)c12)c1nc2cccc(F)c2c(=O)n1-c1cccc(OCC(F)(F)F)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
SciFluor Life Sciences, Inc.
US Patent
| Assay Description
The ability of the compounds of the present invention to inhibit the activity of four PI3K isoforms, PI3Kα, PI3Kβ, PI3Kγ, and PI3K&d... |
US Patent US9216985 (2015)
BindingDB Entry DOI: 10.7270/Q22V2DZ4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM197718

(US9216985, 1A)Show SMILES CC[C@H](Nc1ncnc2[nH]ccc12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
SciFluor Life Sciences, Inc.
US Patent
| Assay Description
The ability of the compounds of the present invention to inhibit the activity of four PI3K isoforms, PI3Kα, PI3Kβ, PI3Kγ, and PI3K&d... |
US Patent US9216985 (2015)
BindingDB Entry DOI: 10.7270/Q22V2DZ4 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM606883

(US11685738, Compound A6)Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1cnc2cc(F)c(F)cc2c1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM606864

(US11685738, Compound A1)Show SMILES OC(=O)C[C@H](N1CCN(CCCc2ccc3CCCNc3n2)C1=O)c1ccc(OC(F)F)nc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM606866
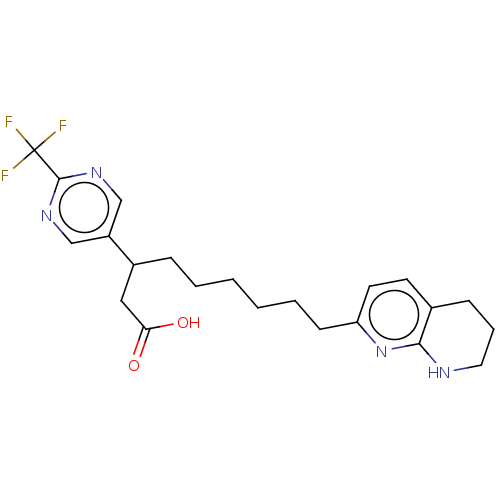
(US11685738, Compound A2)Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1cnc(nc1)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM197724
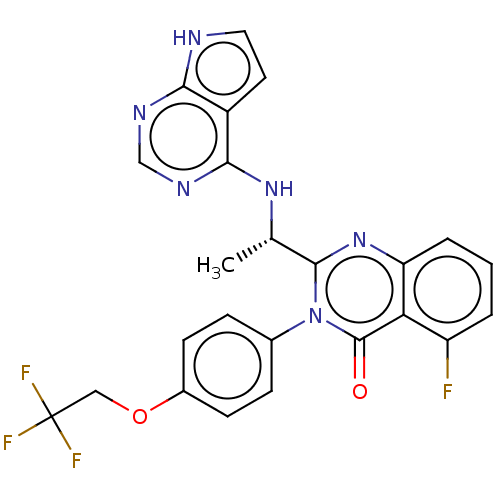
(US9216985, 15A)Show SMILES C[C@H](Nc1ncnc2[nH]ccc12)c1nc2cccc(F)c2c(=O)n1-c1ccc(OCC(F)(F)F)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
SciFluor Life Sciences, Inc.
US Patent
| Assay Description
The ability of the compounds of the present invention to inhibit the activity of four PI3K isoforms, PI3Kα, PI3Kβ, PI3Kγ, and PI3K&d... |
US Patent US9216985 (2015)
BindingDB Entry DOI: 10.7270/Q22V2DZ4 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM606947

(US11685738, Compound A15s)Show SMILES OC(=O)C[C@H](N1CCN(CCCc2ccc3CCCNc3n2)C1=O)c1cccc(c1)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 9.28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50134778

((S)-3-(6-Methoxy-pyridin-3-yl)-3-{2-oxo-3-[3-(5,6,...)Show SMILES COc1ccc(cn1)[C@H](CC(O)=O)N1CCN(CCCc2ccc3CCCNc3n2)C1=O Show InChI InChI=1S/C23H29N5O4/c1-32-20-9-7-17(15-25-20)19(14-21(29)30)28-13-12-27(23(28)31)11-3-5-18-8-6-16-4-2-10-24-22(16)26-18/h6-9,15,19H,2-5,10-14H2,1H3,(H,24,26)(H,29,30)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 9.57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM606881
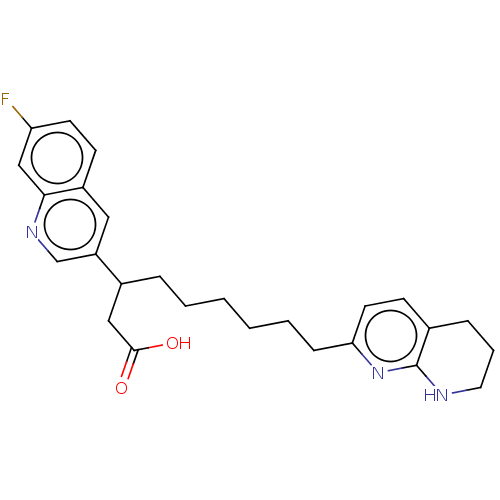
(US11685738, Compound A5)Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1cnc2cc(F)ccc2c1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 9.97 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM197728

(US9216985, 20A)Show SMILES C[C@H](Nc1ncnc2[nH]ccc12)c1nc2cccc(Cl)c2c(=O)n1-c1cccc(OCC(F)(F)F)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
SciFluor Life Sciences, Inc.
US Patent
| Assay Description
The ability of the compounds of the present invention to inhibit the activity of four PI3K isoforms, PI3Kα, PI3Kβ, PI3Kγ, and PI3K&d... |
US Patent US9216985 (2015)
BindingDB Entry DOI: 10.7270/Q22V2DZ4 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM606866

(US11685738, Compound A2)Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1cnc(nc1)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 11.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM606884

(US11685738, Compound A7)Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1cnc2cc(ccc2c1)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 11.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-8
(Homo sapiens (Human)) | BDBM606864

(US11685738, Compound A1)Show SMILES OC(=O)C[C@H](N1CCN(CCCc2ccc3CCCNc3n2)C1=O)c1ccc(OC(F)F)nc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 13.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 5
(Homo sapiens (Human)) | BDBM50334268

(CHEMBL1642655 | CHEMBL2205637 | N-(6-(1H-imidazol-...)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)Nc1cn2cc(ccc2n1)-n1ccnc1 Show InChI InChI=1S/C21H21N5O/c1-21(2,3)16-6-4-15(5-7-16)20(27)24-18-13-26-12-17(8-9-19(26)23-18)25-11-10-22-14-25/h4-14H,1-3H3,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ASK1 assessed as phosphorylated fluorescent peptide by mobility shift assay |
Bioorg Med Chem 19: 486-9 (2011)
Article DOI: 10.1016/j.bmc.2010.11.004
BindingDB Entry DOI: 10.7270/Q2JH3MGM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM606917

(US11685738, Compound A23)Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1ccc(Cl)c(c1)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 14.8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 5
(Homo sapiens (Human)) | BDBM50334269

(4-tert-butyl-N-(imidazo[1,2-a]quinolin-2-yl)benzam...)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)Nc1cn2c(ccc3ccccc23)n1 Show InChI InChI=1S/C22H21N3O/c1-22(2,3)17-11-8-16(9-12-17)21(26)24-19-14-25-18-7-5-4-6-15(18)10-13-20(25)23-19/h4-14H,1-3H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ASK1 assessed as phosphorylated fluorescent peptide by mobility shift assay |
Bioorg Med Chem 19: 486-9 (2011)
Article DOI: 10.1016/j.bmc.2010.11.004
BindingDB Entry DOI: 10.7270/Q2JH3MGM |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM606919

(US11685738, Compound A28 | acid )Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1cc(ccc1F)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 16.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM606918

(US11685738, Compound A24 | acid )Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1cc(Cl)cc(c1)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 17.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-8
(Homo sapiens (Human)) | BDBM50134778

((S)-3-(6-Methoxy-pyridin-3-yl)-3-{2-oxo-3-[3-(5,6,...)Show SMILES COc1ccc(cn1)[C@H](CC(O)=O)N1CCN(CCCc2ccc3CCCNc3n2)C1=O Show InChI InChI=1S/C23H29N5O4/c1-32-20-9-7-17(15-25-20)19(14-21(29)30)28-13-12-27(23(28)31)11-3-5-18-8-6-16-4-2-10-24-22(16)26-18/h6-9,15,19H,2-5,10-14H2,1H3,(H,24,26)(H,29,30)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 17.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM606867

(US11685738, Compound A3)Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1ccc(OC(F)F)nc1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 18.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 5
(Homo sapiens (Human)) | BDBM50334270

(2-methyl-2-(4-(6-(thiophen-3-yl)imidazo[1,2-a]pyri...)Show SMILES CC(C)(C(O)=O)c1ccc(cc1)C(=O)Nc1cn2cc(ccc2n1)-c1ccsc1 Show InChI InChI=1S/C22H19N3O3S/c1-22(2,21(27)28)17-6-3-14(4-7-17)20(26)24-18-12-25-11-15(5-8-19(25)23-18)16-9-10-29-13-16/h3-13H,1-2H3,(H,24,26)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ASK1 assessed as phosphorylated fluorescent peptide by mobility shift assay |
Bioorg Med Chem 19: 486-9 (2011)
Article DOI: 10.1016/j.bmc.2010.11.004
BindingDB Entry DOI: 10.7270/Q2JH3MGM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM197720

(US9216985, 6A)Show SMILES CC[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(F)c2c(=O)n1-c1cccc(CC(F)(F)F)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SciFluor Life Sciences, Inc.
US Patent
| Assay Description
The ability of the compounds of the present invention to inhibit the activity of four PI3K isoforms, PI3Kα, PI3Kβ, PI3Kγ, and PI3K&d... |
US Patent US9216985 (2015)
BindingDB Entry DOI: 10.7270/Q22V2DZ4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM197722

(US9216985, 10A)Show SMILES CC[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(F)c2c(=O)n1-c1cccc(OCC(F)(F)F)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SciFluor Life Sciences, Inc.
US Patent
| Assay Description
The ability of the compounds of the present invention to inhibit the activity of four PI3K isoforms, PI3Kα, PI3Kβ, PI3Kγ, and PI3K&d... |
US Patent US9216985 (2015)
BindingDB Entry DOI: 10.7270/Q22V2DZ4 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-8
(Homo sapiens (Human)) | BDBM606865

(US11685738, Compound A4)Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1cnc2ccc(F)cc2c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 21.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-8
(Homo sapiens (Human)) | BDBM606883

(US11685738, Compound A6)Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1cnc2cc(F)c(F)cc2c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-8
(Homo sapiens (Human)) | BDBM606919

(US11685738, Compound A28 | acid )Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1cc(ccc1F)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 34.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-8
(Homo sapiens (Human)) | BDBM606918

(US11685738, Compound A24 | acid )Show SMILES OC(=O)CC(CCCCCCc1ccc2CCCNc2n1)c1cc(Cl)cc(c1)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 35.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50554708
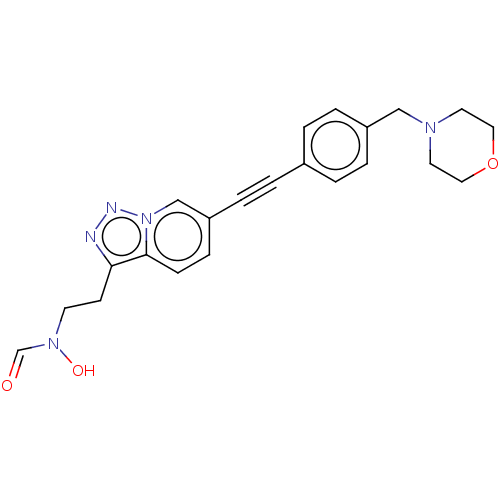
(CHEMBL4746493)Show SMILES ON(CCc1nnn2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115826
BindingDB Entry DOI: 10.7270/Q2C2513C |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50554707

(CHEMBL4751248)Show SMILES ON(CCn1nnc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115826
BindingDB Entry DOI: 10.7270/Q2C2513C |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50554702
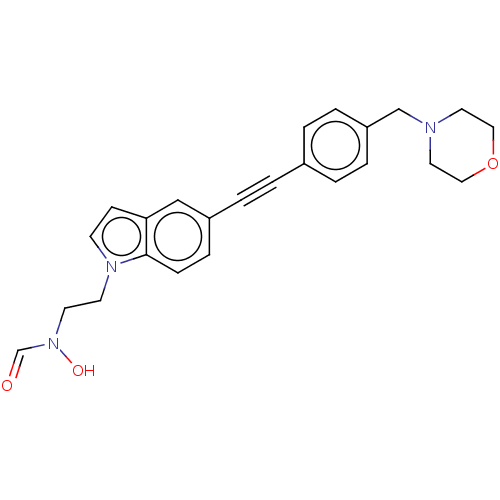
(CHEMBL4776055)Show SMILES ON(CCn1ccc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent ligand from Pseudomonas aeruginosa LpxC measured after 30 mins by fluorescence anisotrophy assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115826
BindingDB Entry DOI: 10.7270/Q2C2513C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM197721

(US9216985, 7A)Show SMILES CC[C@H](Nc1ncnc2[nH]ccc12)c1nc2cccc(F)c2c(=O)n1-c1cccc(CC(F)(F)F)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
SciFluor Life Sciences, Inc.
US Patent
| Assay Description
The ability of the compounds of the present invention to inhibit the activity of four PI3K isoforms, PI3Kα, PI3Kβ, PI3Kγ, and PI3K&d... |
US Patent US9216985 (2015)
BindingDB Entry DOI: 10.7270/Q22V2DZ4 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-8
(Homo sapiens (Human)) | BDBM606955

(US11685738, Compound A21-2)Show SMILES OC(=O)C[C@H](CCCCCCc1ccc2CCCNc2n1)c1cccc(c1)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z77CR |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-8
(Homo sapiens (Human)) | BDBM297773

(US10118929, Compound A1)Show SMILES OC(=O)CC(N1CCN(CCCCNc2nc3ccccc3[nH]2)C1=O)c1cccc(c1)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 50.4 | n/a | n/a | n/a | n/a | n/a | n/a |
SciFluor Life Sciences, Inc.
US Patent
| Assay Description
The optimized protocol was validated by employing reference compounds such as Cilengitide (+Vβ3/αVβ5−VN interaction) and CWHM12 ... |
US Patent US10118929 (2018)
BindingDB Entry DOI: 10.7270/Q2V126WM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data