

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111939 (US8618107, 105) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length His-tagged BTK expressed in baculovirus expression system by Z-LYTE assay | ACS Med Chem Lett 8: 608-613 (2017) Article DOI: 10.1021/acsmedchemlett.7b00103 BindingDB Entry DOI: 10.7270/Q24M96ZH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065147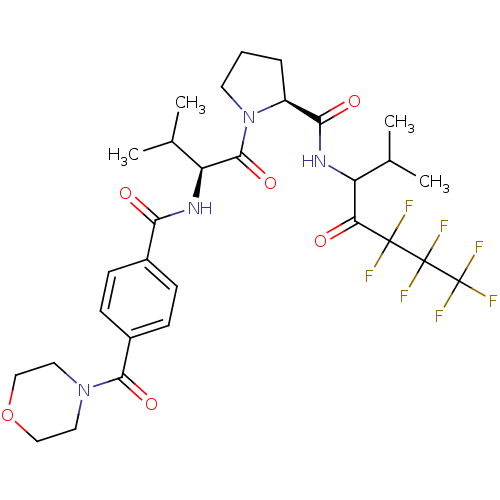 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035495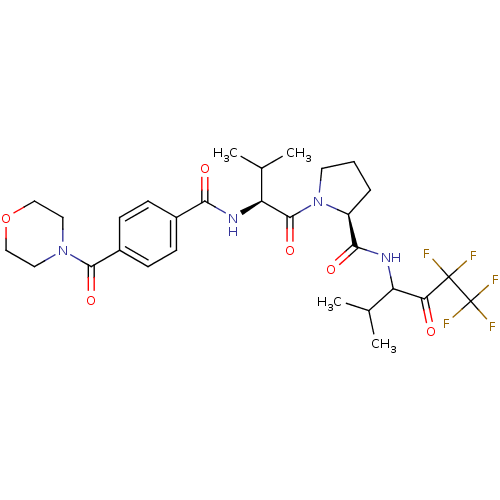 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065158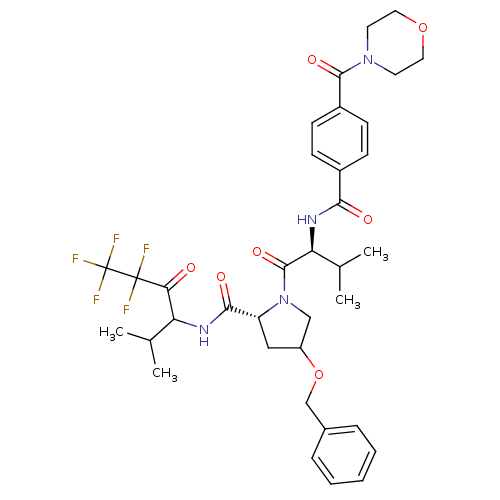 ((R)-4-Benzyloxy-1-{(S)-3-methyl-2-[4-(morpholine-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065157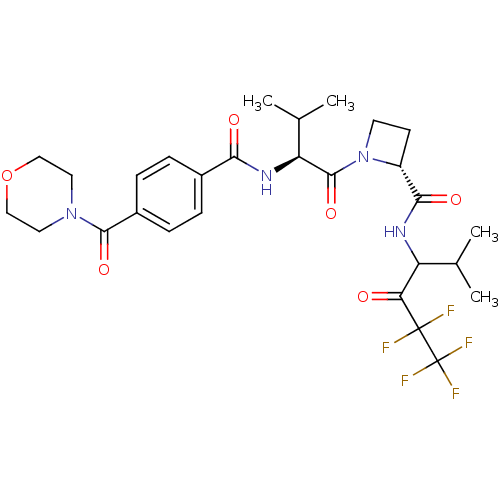 ((R)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065161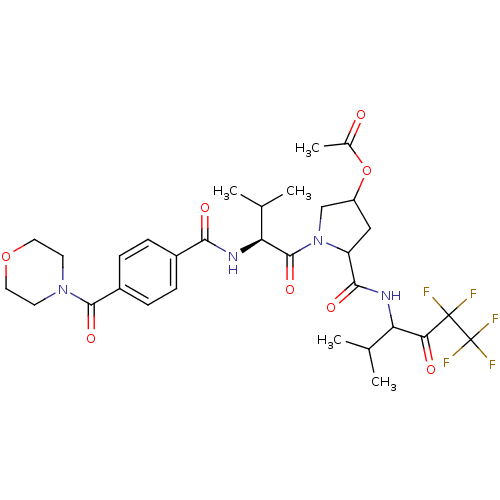 (Acetic acid 1-{(S)-3-methyl-2-[4-(morpholine-4-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065163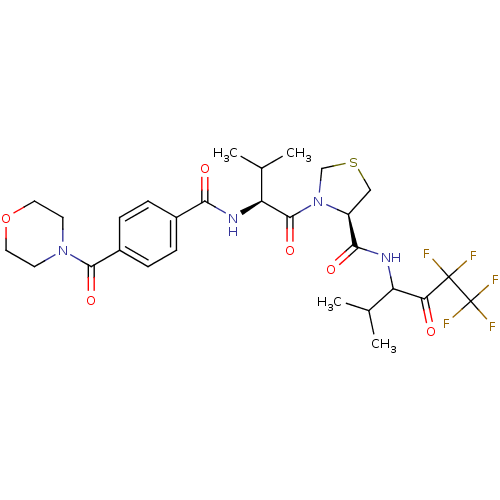 ((R)-3-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065155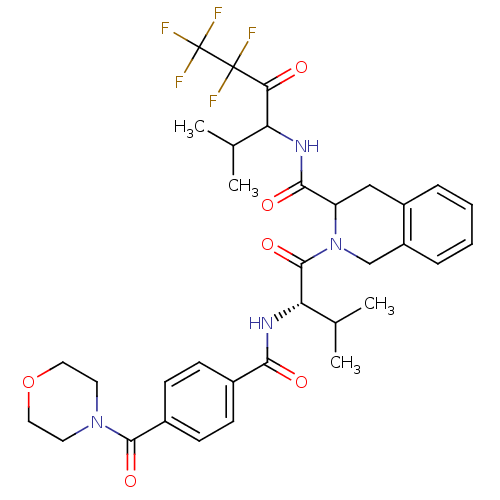 (2-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50282635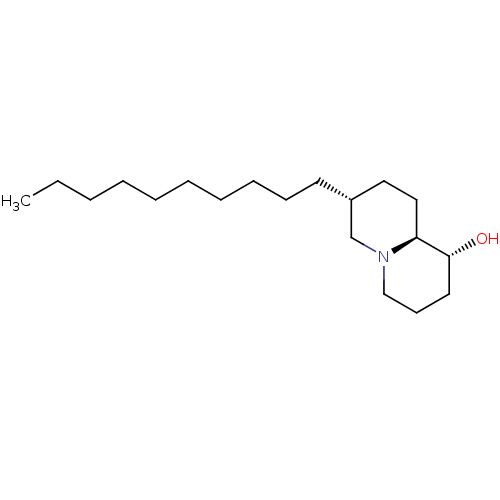 ((1R,7R,9aS)-7-Decyl-octahydro-quinolizin-1-ol | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for its activity to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase, activity expressed as Ki | Bioorg Med Chem Lett 4: 1317-1318 (1994) Article DOI: 10.1016/S0960-894X(01)80352-8 BindingDB Entry DOI: 10.7270/Q23T9H5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065154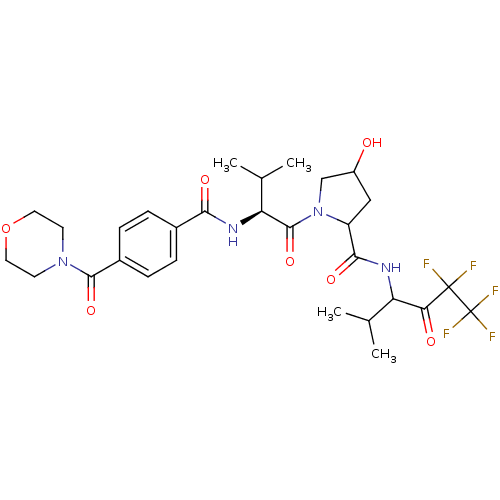 (4-Hydroxy-1-{(S)-3-methyl-2-[4-(morpholine-4-carbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065146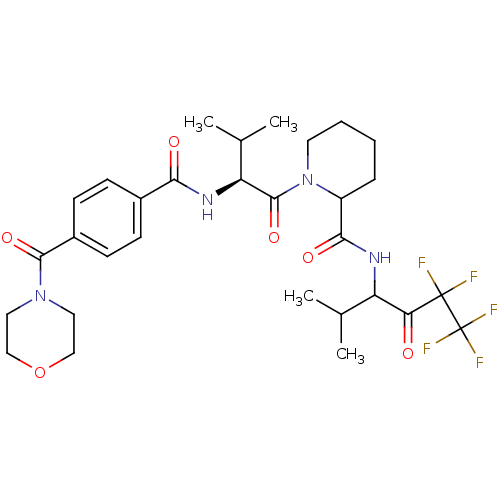 (1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065164 ((S)-1-{(R)-3,3-Dimethyl-2-[4-(morpholine-4-carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065156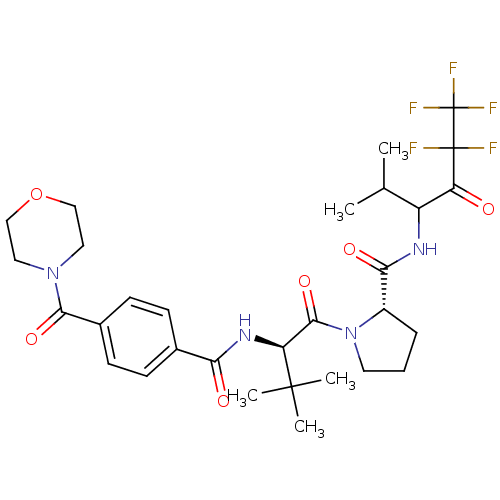 ((S)-1-{(R)-3,3-Dimethyl-2-[4-(morpholine-4-carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50282634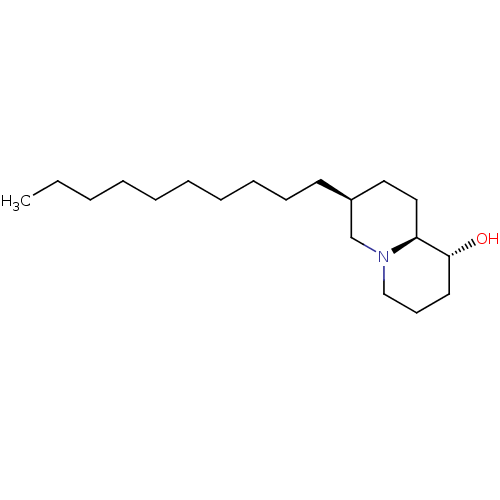 ((1R,7S,9aS)-7-Decyl-octahydro-quinolizin-1-ol | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for its activity to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase, activity expressed as Ki | Bioorg Med Chem Lett 4: 1317-1318 (1994) Article DOI: 10.1016/S0960-894X(01)80352-8 BindingDB Entry DOI: 10.7270/Q23T9H5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065162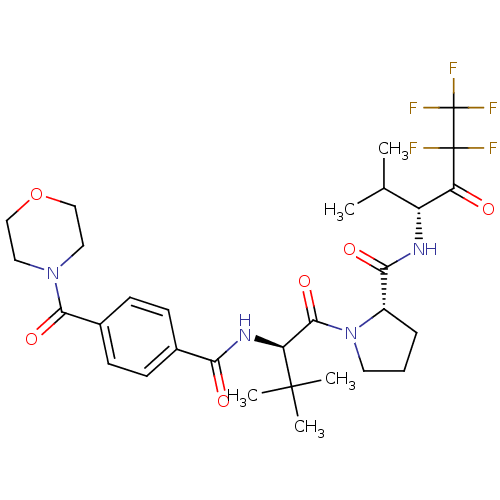 ((S)-1-{(R)-3,3-Dimethyl-2-[4-(morpholine-4-carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065153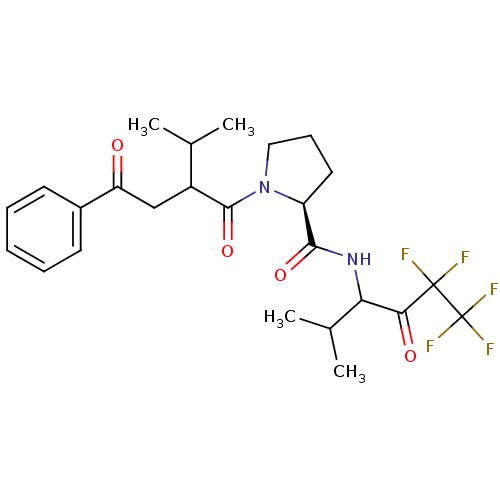 ((S)-1-[3-Methyl-2-(2-oxo-2-phenyl-ethyl)-butyryl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065151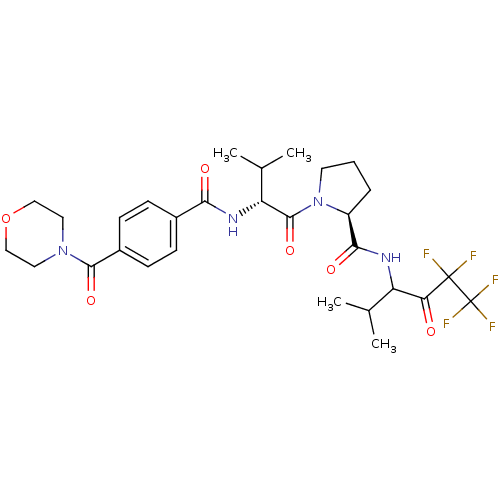 ((S)-1-{(R)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065150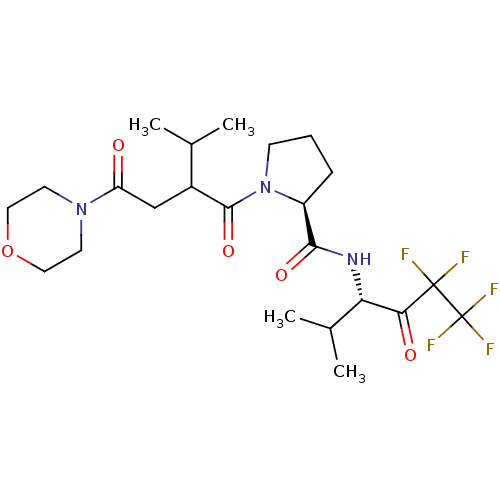 ((S)-1-[3-Methyl-2-(2-morpholin-4-yl-2-oxo-ethyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065148 (4-(Morpholine-4-carbonyl)-N-{(S)-2-oxo-1-[(S)-1-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065159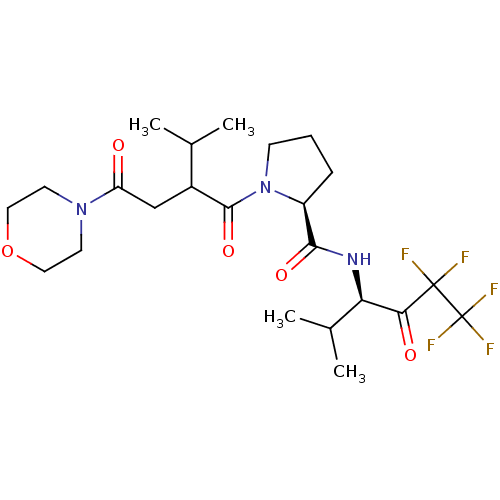 ((S)-1-[3-Methyl-2-(2-morpholin-4-yl-2-oxo-ethyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065160 (4-(Morpholine-4-carbonyl)-N-{(R)-2-oxo-1-[(3,3,4,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065149 (4-(Morpholine-4-carbonyl)-N-{(R)-2-oxo-1-[(3,3,4,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065152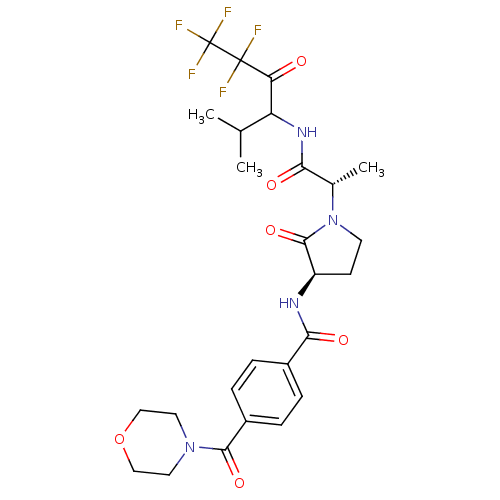 (4-(Morpholine-4-carbonyl)-N-{(R)-2-oxo-1-[(S)-1-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015453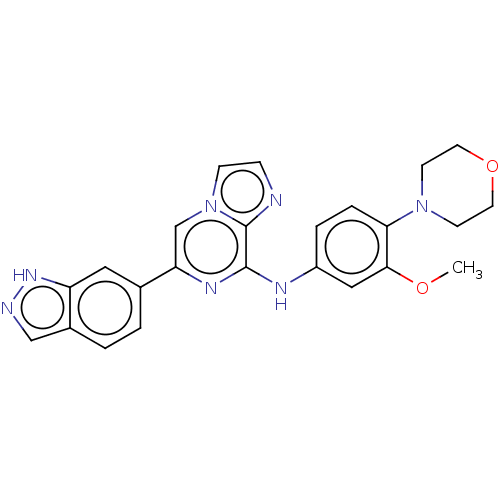 (CHEMBL3265037) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015454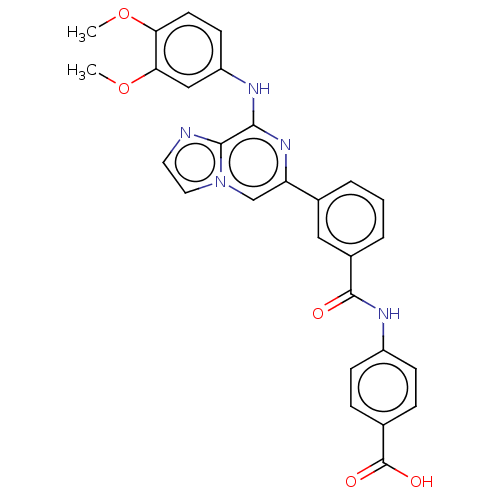 (CHEMBL3264995) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50134365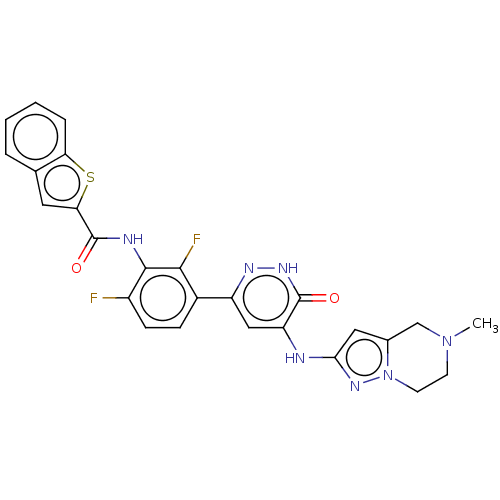 (CHEMBL3745935) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of BTK (unknown origin) by Lanthascreen assay | Bioorg Med Chem Lett 26: 575-9 (2016) Article DOI: 10.1016/j.bmcl.2015.11.076 BindingDB Entry DOI: 10.7270/Q2222WMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111938 (US8618107, 104) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... | ACS Med Chem Lett 8: 608-613 (2017) Article DOI: 10.1021/acsmedchemlett.7b00103 BindingDB Entry DOI: 10.7270/Q24M96ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015447 (CHEMBL3265031) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015460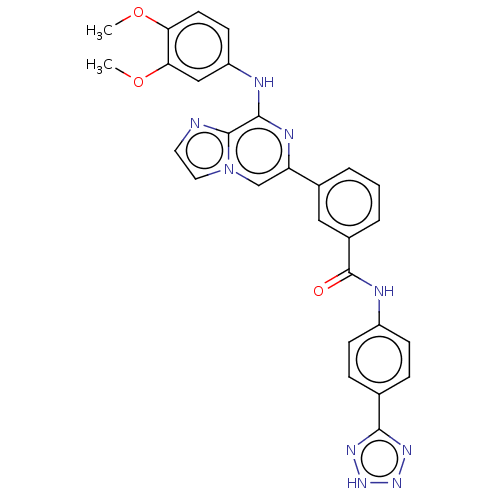 (CHEMBL3265001) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM36516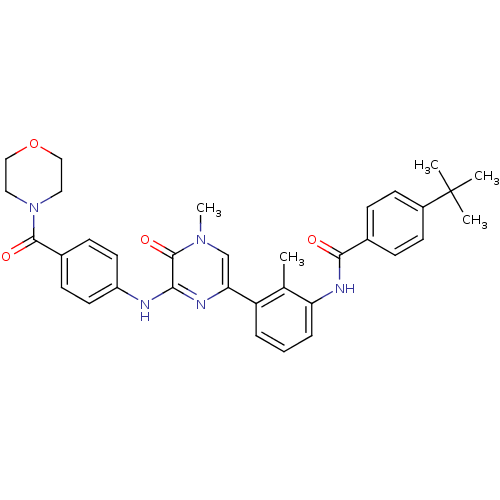 (4-(tert-Butyl)-N-(2-methyl-3-(4-methyl-6-((4-(morp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.90 | 1.5 | n/a | n/a | n/a | 7.5 | 23 |
CGI Pharmaceuticals | Assay Description Biochemical assay using Lanthascreen (human, full-lenght, C-terminal v5-His6 expressed in Sf9 cell) assay from Invitrogen. | Nat Chem Biol 7: 41-50 (2011) Article DOI: 10.1038/nchembio.481 BindingDB Entry DOI: 10.7270/Q2B56H2T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111939 (US8618107, 105) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... | ACS Med Chem Lett 8: 608-613 (2017) Article DOI: 10.1021/acsmedchemlett.7b00103 BindingDB Entry DOI: 10.7270/Q24M96ZH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50254236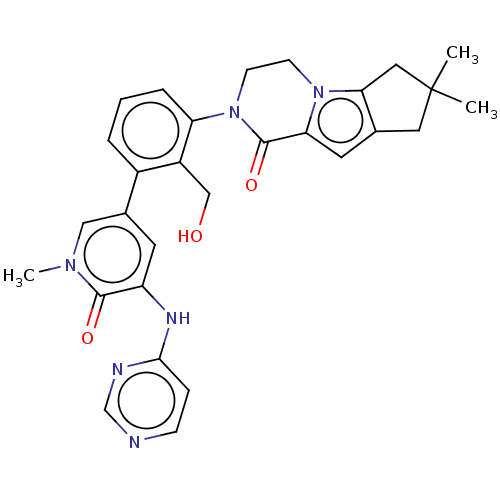 (CHEMBL4090189) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... | ACS Med Chem Lett 8: 608-613 (2017) Article DOI: 10.1021/acsmedchemlett.7b00103 BindingDB Entry DOI: 10.7270/Q24M96ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111951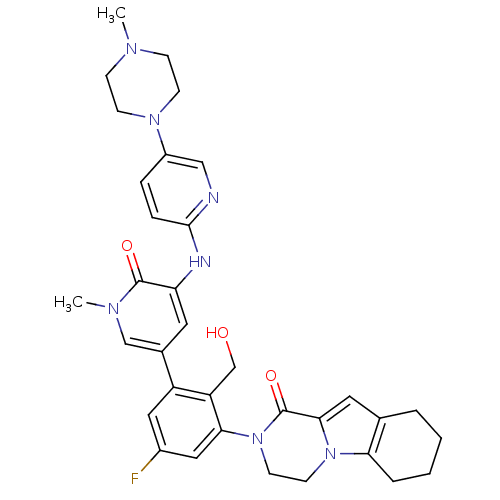 (US8618107, 197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... | ACS Med Chem Lett 8: 608-613 (2017) Article DOI: 10.1021/acsmedchemlett.7b00103 BindingDB Entry DOI: 10.7270/Q24M96ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015449 (CHEMBL3265033) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015459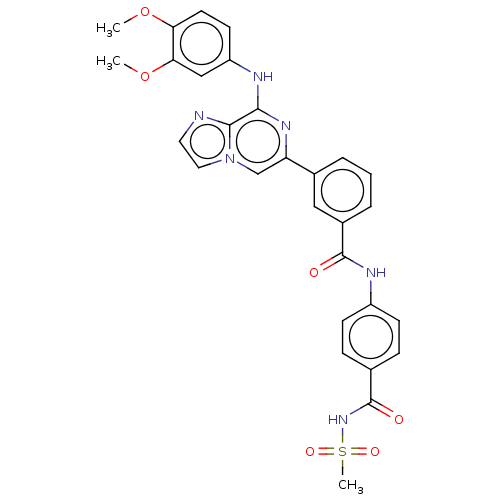 (CHEMBL3265000) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015439 (CHEMBL3265024) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015457 (CHEMBL3264998) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015425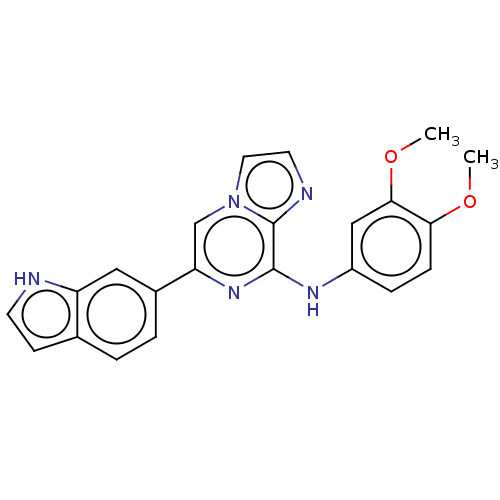 (CHEMBL3265016) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50134377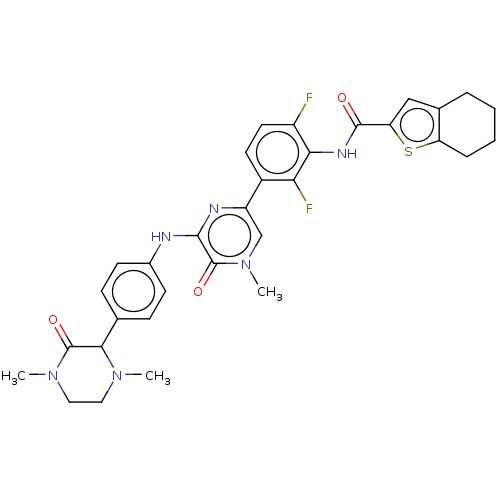 (CHEMBL3745934) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of BTK (unknown origin) by Lanthascreen assay | Bioorg Med Chem Lett 26: 575-9 (2016) Article DOI: 10.1016/j.bmcl.2015.11.076 BindingDB Entry DOI: 10.7270/Q2222WMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50254270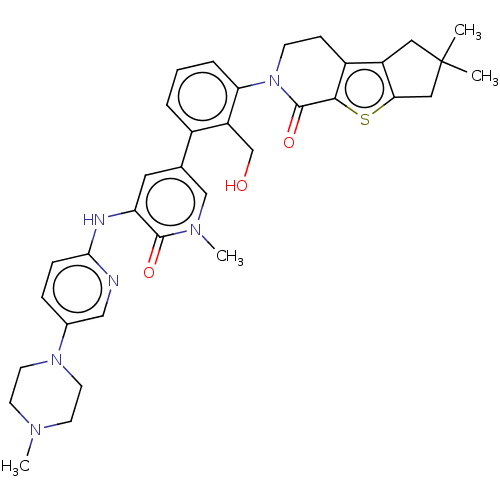 (CHEMBL4080943) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... | ACS Med Chem Lett 8: 608-613 (2017) Article DOI: 10.1021/acsmedchemlett.7b00103 BindingDB Entry DOI: 10.7270/Q24M96ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50254277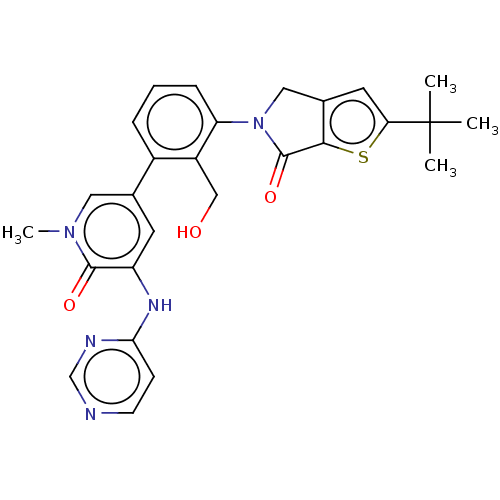 (CHEMBL4067714) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... | ACS Med Chem Lett 8: 608-613 (2017) Article DOI: 10.1021/acsmedchemlett.7b00103 BindingDB Entry DOI: 10.7270/Q24M96ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50254268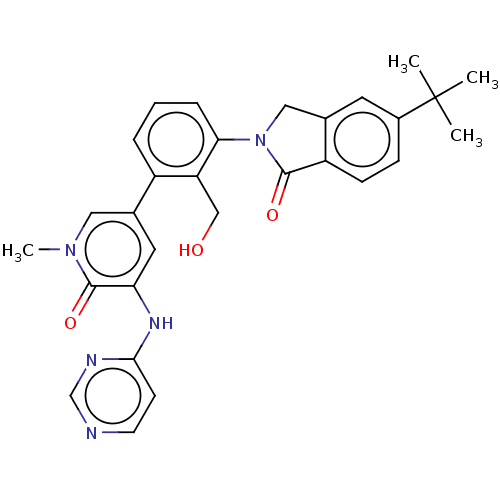 (CHEMBL4077625) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... | ACS Med Chem Lett 8: 608-613 (2017) Article DOI: 10.1021/acsmedchemlett.7b00103 BindingDB Entry DOI: 10.7270/Q24M96ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50134377 (CHEMBL3745934) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of BTK (unknown origin) by Lanthascreen assay | Bioorg Med Chem Lett 26: 575-9 (2016) Article DOI: 10.1016/j.bmcl.2015.11.076 BindingDB Entry DOI: 10.7270/Q2222WMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50134320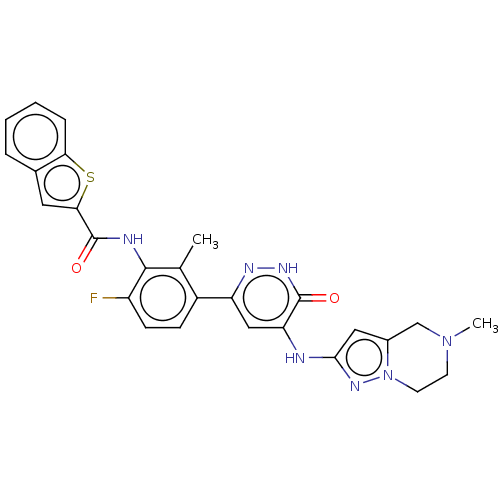 (CHEMBL3746293) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of BTK (unknown origin) by Lanthascreen assay | Bioorg Med Chem Lett 26: 575-9 (2016) Article DOI: 10.1016/j.bmcl.2015.11.076 BindingDB Entry DOI: 10.7270/Q2222WMH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015427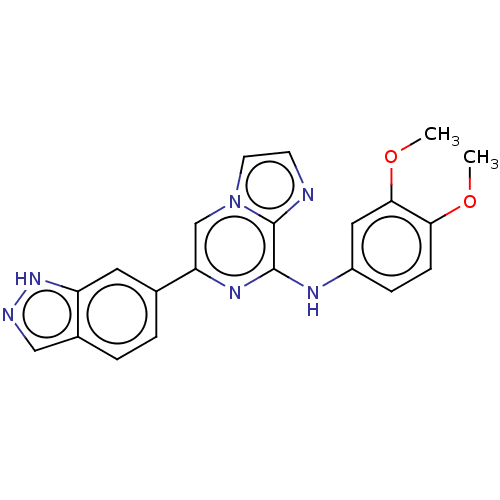 (CHEMBL3265017) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015450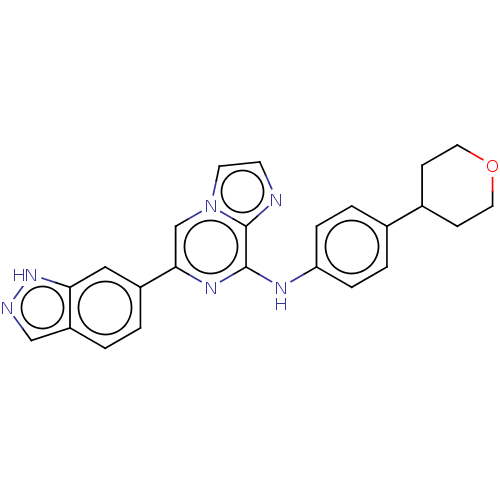 (CHEMBL3265034) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015446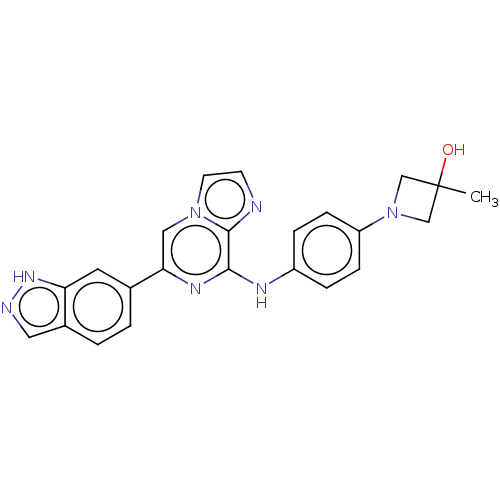 (CHEMBL3265030) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111952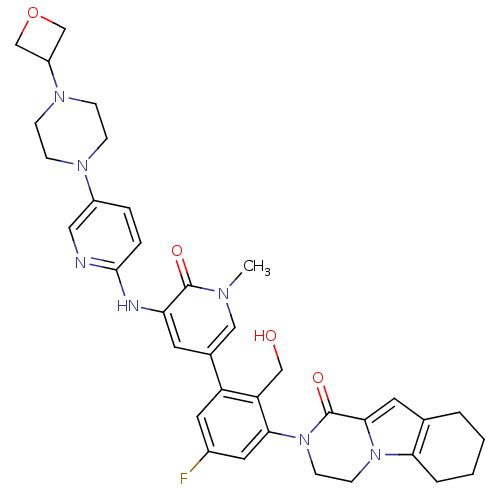 (US8618107, 210) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... | ACS Med Chem Lett 8: 608-613 (2017) Article DOI: 10.1021/acsmedchemlett.7b00103 BindingDB Entry DOI: 10.7270/Q24M96ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111945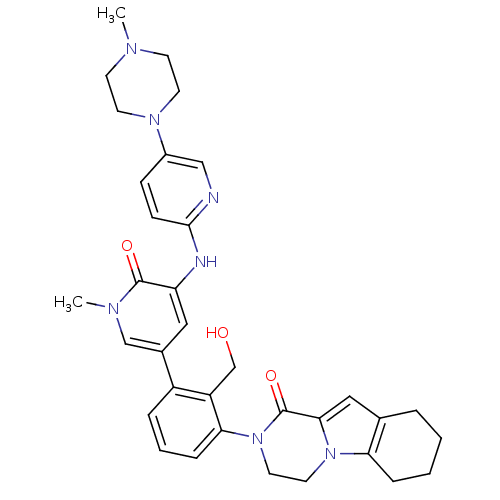 (US8618107, 113) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length His-tagged BTK expressed in baculovirus expression system by Z-LYTE assay | ACS Med Chem Lett 8: 608-613 (2017) Article DOI: 10.1021/acsmedchemlett.7b00103 BindingDB Entry DOI: 10.7270/Q24M96ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111998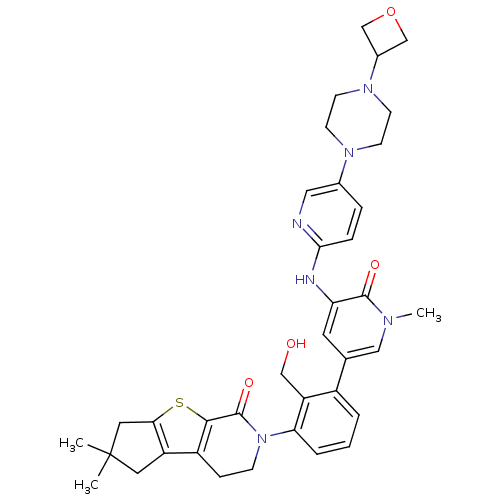 (US8618107, 312) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... | ACS Med Chem Lett 8: 608-613 (2017) Article DOI: 10.1021/acsmedchemlett.7b00103 BindingDB Entry DOI: 10.7270/Q24M96ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 400 total ) | Next | Last >> |