

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50136106 (4-(6-Benzyl-benzo[1,3]dioxol-5-yl)-benzenesulfonam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc Curated by ChEMBL | Assay Description Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole blood | J Med Chem 46: 5484-504 (2003) Article DOI: 10.1021/jm030268b BindingDB Entry DOI: 10.7270/Q2FX78WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc Curated by ChEMBL | Assay Description Compound was tested for the inhibition of recombinant human Prostaglandin G/H synthase 2 (COX-2) | J Med Chem 46: 5484-504 (2003) Article DOI: 10.1021/jm030268b BindingDB Entry DOI: 10.7270/Q2FX78WK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50136109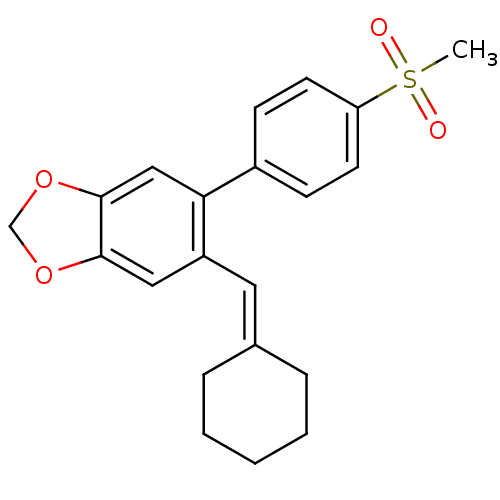 (5-Cyclohexylidenemethyl-6-(4-methanesulfonyl-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc Curated by ChEMBL | Assay Description Compound was tested for the inhibition of recombinant human Prostaglandin G/H synthase 2 (COX-2) by enzyme Assay | J Med Chem 46: 5484-504 (2003) Article DOI: 10.1021/jm030268b BindingDB Entry DOI: 10.7270/Q2FX78WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50136108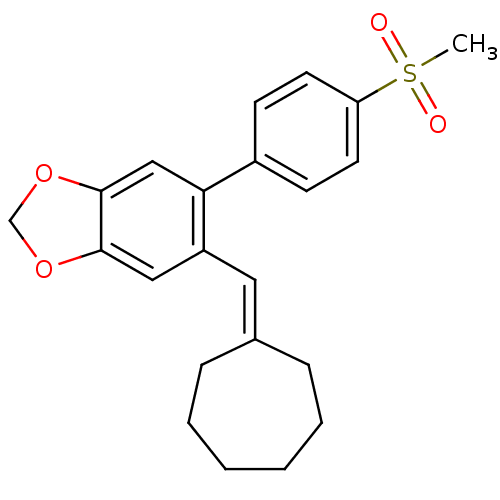 (5-Cycloheptylidenemethyl-6-(4-methanesulfonyl-phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc Curated by ChEMBL | Assay Description Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole blood | J Med Chem 46: 5484-504 (2003) Article DOI: 10.1021/jm030268b BindingDB Entry DOI: 10.7270/Q2FX78WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc Curated by ChEMBL | Assay Description Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole blood | J Med Chem 46: 5484-504 (2003) Article DOI: 10.1021/jm030268b BindingDB Entry DOI: 10.7270/Q2FX78WK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM443117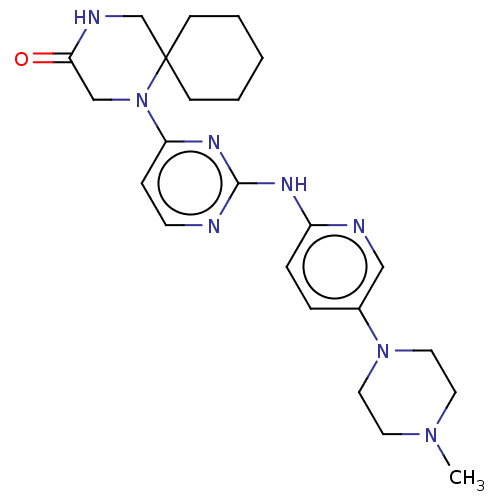 (US10654831, Compound 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM443117 (US10654831, Compound 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM443121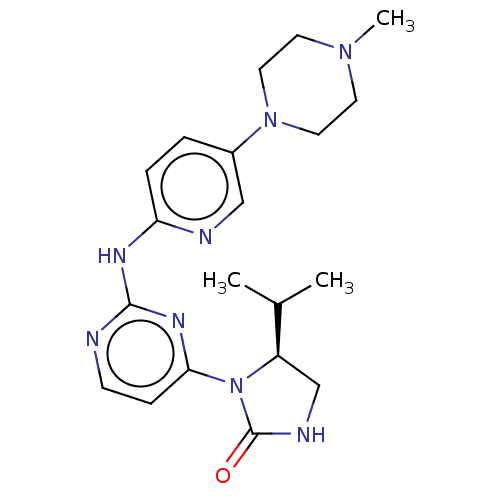 (US10654831, Compound 46) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 761 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM443113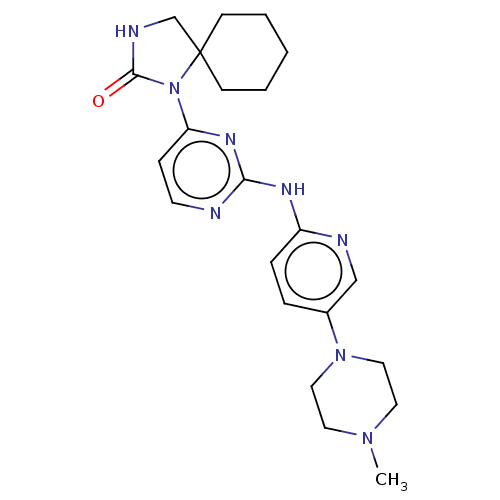 (US10654831, Compound 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM443117 (US10654831, Compound 20) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 (Homo sapiens (Human)) | BDBM50229787 ((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Induction of p53 in human HCT116 cells coexpressing pp53TA-luc assessed as inhibition of cell proliferation after 8 hrs by firefly/renilla luciferase... | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50136107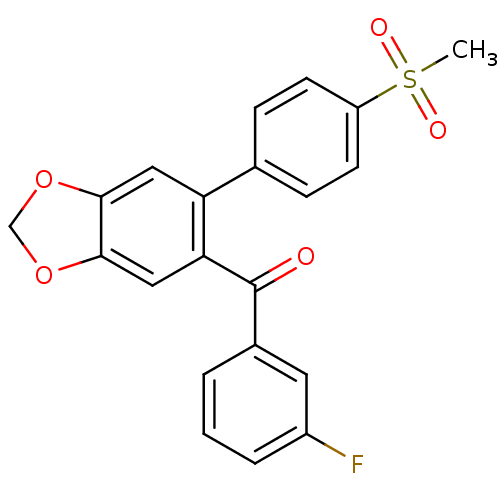 ((3-Fluoro-phenyl)-[6-(4-methanesulfonyl-phenyl)-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc Curated by ChEMBL | Assay Description Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole blood | J Med Chem 46: 5484-504 (2003) Article DOI: 10.1021/jm030268b BindingDB Entry DOI: 10.7270/Q2FX78WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc Curated by ChEMBL | Assay Description Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole blood | J Med Chem 46: 5484-504 (2003) Article DOI: 10.1021/jm030268b BindingDB Entry DOI: 10.7270/Q2FX78WK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cellular tumor antigen p53 (Homo sapiens (Human)) | BDBM50025671 (CHEMBL583413) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Induction of p53-Ser15 phosphorylation in human HCT116 cells after 24 hrs | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 (Homo sapiens (Human)) | BDBM50229787 ((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Induction of p53-Ser15 phosphorylation in human HCT116 cells after 24 hrs | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc Curated by ChEMBL | Assay Description Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole blood | J Med Chem 46: 5484-504 (2003) Article DOI: 10.1021/jm030268b BindingDB Entry DOI: 10.7270/Q2FX78WK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM443122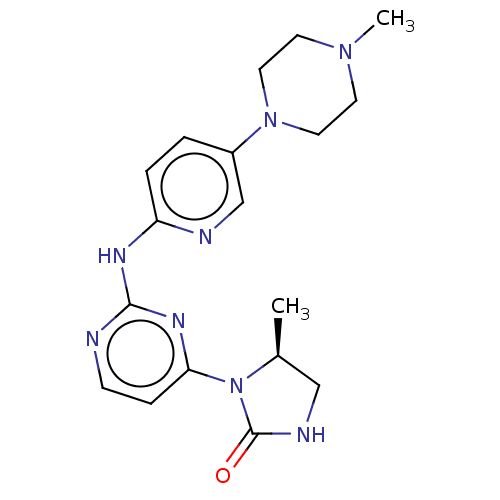 (US10654831, Compound 52) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM443120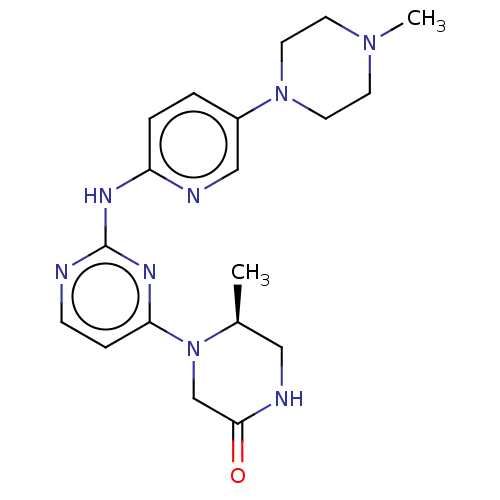 (US10654831, Compound 40) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM443115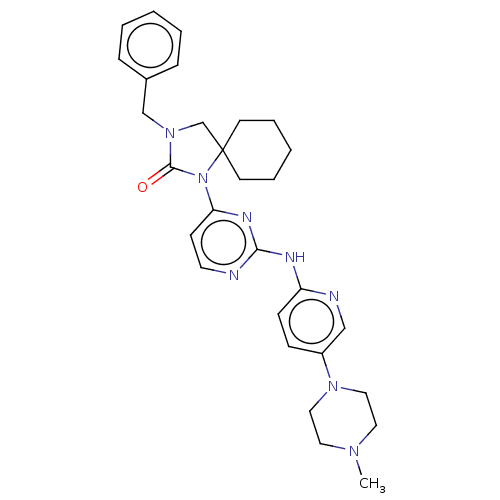 (US10654831, Compound 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 (Homo sapiens (Human)) | BDBM50025671 (CHEMBL583413) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Induction of p53 in human HCT116 cells coexpressing pp53TA-luc assessed as inhibition of cell proliferation after 8 hrs by firefly/renilla luciferase... | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM443114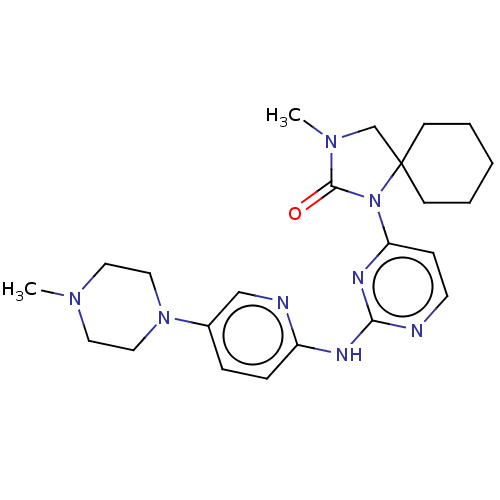 (US10654831, Compound 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM443121 (US10654831, Compound 46) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM443116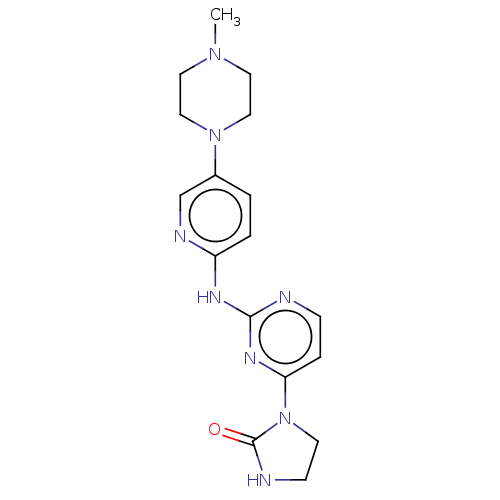 (US10654831, Compound 16) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM443126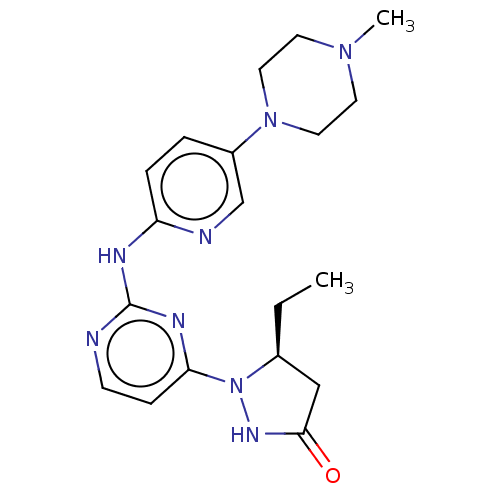 (US10654831, Compound 56) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM443122 (US10654831, Compound 52) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM443113 (US10654831, Compound 6) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM443120 (US10654831, Compound 40) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 (Homo sapiens (Human)) | BDBM50299820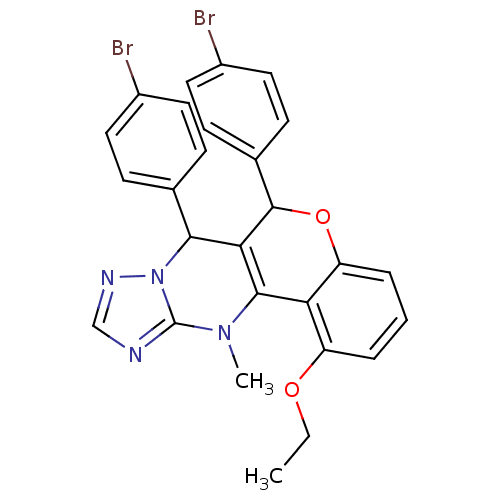 ((+/-)-syn-6,7-Bis(4-bromophenyl)-1-ethoxy-12-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Induction of p53 in human HCT116 cells coexpressing pp53TA-luc assessed as inhibition of cell proliferation after 8 hrs by firefly/renilla luciferase... | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM443116 (US10654831, Compound 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM443116 (US10654831, Compound 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 (Homo sapiens (Human)) | BDBM50299822 ((+/-)-syn-6,7-Bis(4-chlorophenyl)-12-methyl-7,12-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Induction of p53 in human HCT116 cells coexpressing pp53TA-luc assessed as inhibition of cell proliferation after 8 hrs by firefly/renilla luciferase... | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM443113 (US10654831, Compound 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM443121 (US10654831, Compound 46) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50136105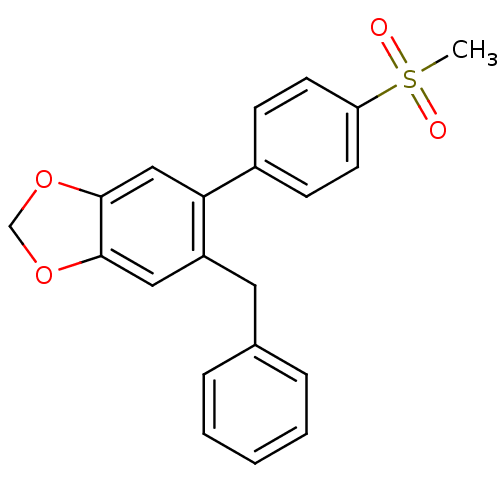 (5-Benzyl-6-(4-methanesulfonyl-phenyl)-benzo[1,3]di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc Curated by ChEMBL | Assay Description Compound was tested for the inhibition of human Prostaglandin G/H synthase 2 (COX-2) in human whole blood | J Med Chem 46: 5484-504 (2003) Article DOI: 10.1021/jm030268b BindingDB Entry DOI: 10.7270/Q2FX78WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 (Homo sapiens (Human)) | BDBM50299818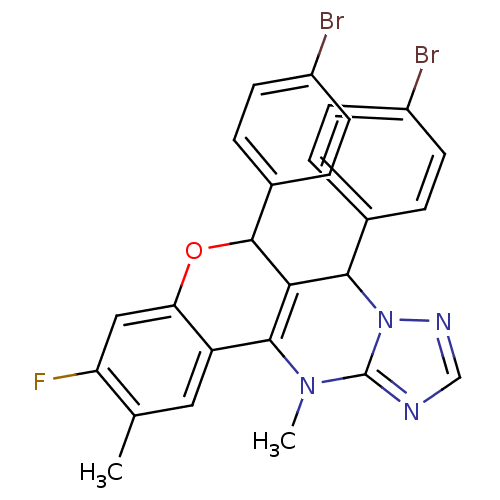 ((+/-)-syn-6,7-Bis(4-bromophenyl)-3-fluoro-2,12-dim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Induction of p53 in human HCT116 cells coexpressing pp53TA-luc assessed as inhibition of cell proliferation after 8 hrs by firefly/renilla luciferase... | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc Curated by ChEMBL | Assay Description Compound was tested for the inhibition of human Prostaglandin G/H synthase 1 (COX-1) in human whole blood | J Med Chem 46: 5484-504 (2003) Article DOI: 10.1021/jm030268b BindingDB Entry DOI: 10.7270/Q2FX78WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM443119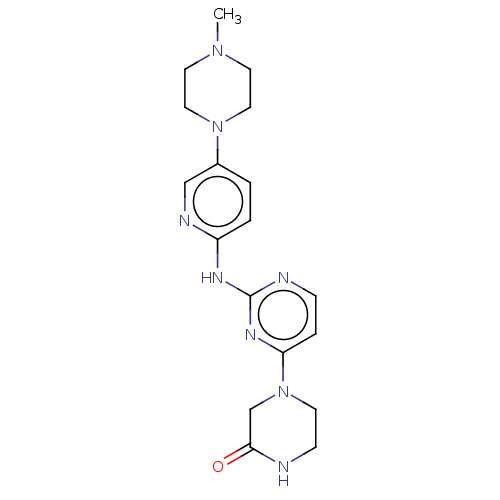 (US10654831, Compound 32) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 (Homo sapiens (Human)) | BDBM50299821 ((+/-)-syn-7-(4-Bromophenyl)-6-(4-chlorophenyl)-12-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Induction of p53 in human HCT116 cells coexpressing pp53TA-luc assessed as inhibition of cell proliferation after 8 hrs by firefly/renilla luciferase... | J Med Chem 52: 7044-53 (2009) Article DOI: 10.1021/jm900681h BindingDB Entry DOI: 10.7270/Q2ZK5GRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM443114 (US10654831, Compound 8) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50136107 ((3-Fluoro-phenyl)-[6-(4-methanesulfonyl-phenyl)-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc Curated by ChEMBL | Assay Description Compound was tested for the inhibition of human Prostaglandin G/H synthase 1 (COX-1) in human whole blood | J Med Chem 46: 5484-504 (2003) Article DOI: 10.1021/jm030268b BindingDB Entry DOI: 10.7270/Q2FX78WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM443119 (US10654831, Compound 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM443121 (US10654831, Compound 46) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50136106 (4-(6-Benzyl-benzo[1,3]dioxol-5-yl)-benzenesulfonam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc Curated by ChEMBL | Assay Description Compound was tested for the inhibition of recombinant human Prostaglandin G/H synthase 1 (COX-1) | J Med Chem 46: 5484-504 (2003) Article DOI: 10.1021/jm030268b BindingDB Entry DOI: 10.7270/Q2FX78WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CDK-activating kinase assembly factor MAT1/Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM443116 (US10654831, Compound 16) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM443126 (US10654831, Compound 56) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM443122 (US10654831, Compound 52) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM443115 (US10654831, Compound 10) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM443117 (US10654831, Compound 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 [99-307] (Homo sapiens (Human)) | BDBM443117 (US10654831, Compound 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G1 Therapeutics, Inc. US Patent | Assay Description Selected compounds disclosed herein were tested in CDK4/cyclinD1, CDK2/CycA and CDK2/cyclinE kinase assays to determine their inhibitory effect on th... | US Patent US10654831 (2020) BindingDB Entry DOI: 10.7270/Q2JS9TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NitroMed, Inc Curated by ChEMBL | Assay Description Compound was tested for the inhibition of human Prostaglandin G/H synthase 1 (COX-1) in human whole blood | J Med Chem 46: 5484-504 (2003) Article DOI: 10.1021/jm030268b BindingDB Entry DOI: 10.7270/Q2FX78WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 114 total ) | Next | Last >> |