

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine protease 1 (Bos taurus (bovine)) | BDBM16127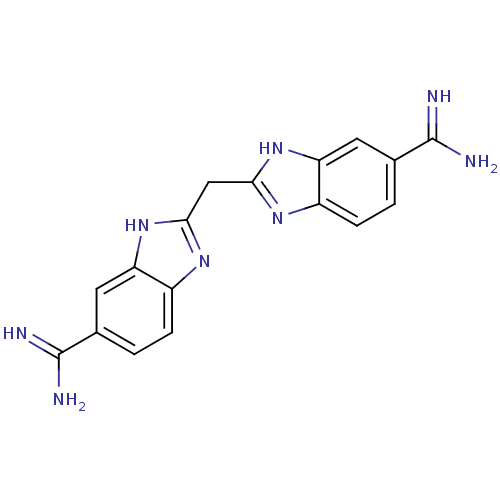 (2,2 -methanediylbis(1H-benzimidazole-6-carboximida...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant against bovine trypsin | J Med Chem 26: 294-8 (1983) BindingDB Entry DOI: 10.7270/Q2NV9H8B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glandular kallikrein (Sus scrofa) | BDBM50010135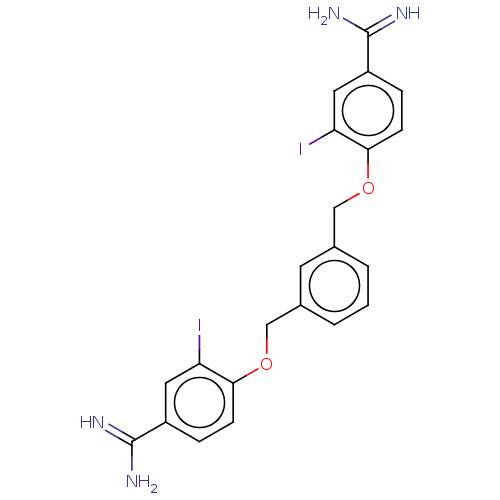 (CHEMBL548400) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic kallikrein using BPPANA as substrate after 15 to 180 mins | J Med Chem 19: 634-9 (1976) BindingDB Entry DOI: 10.7270/Q2KD20FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glandular kallikrein (Sus scrofa) | BDBM50010130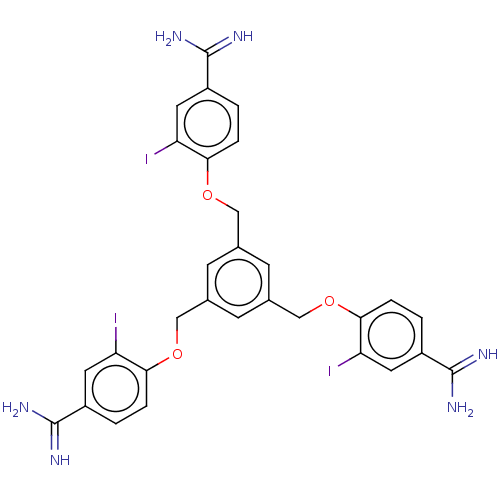 (CHEMBL3245418) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic kallikrein | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010103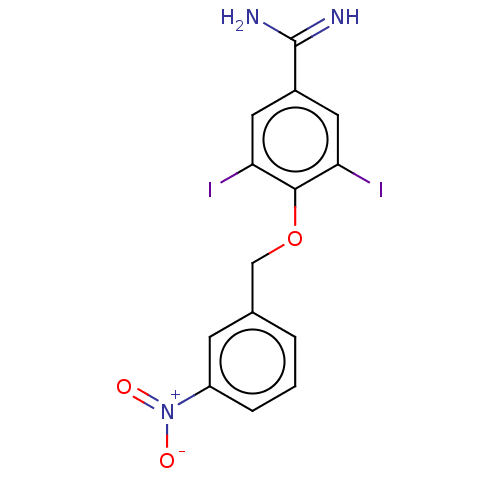 (CHEMBL2418042) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50379838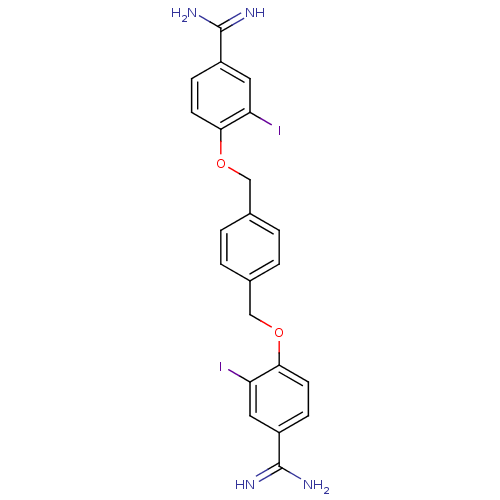 (CHEMBL2011750) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amidase activity of bovine thrombin using BPVANA as substrate after 15 to 40 mins | J Med Chem 19: 634-9 (1976) BindingDB Entry DOI: 10.7270/Q2KD20FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010128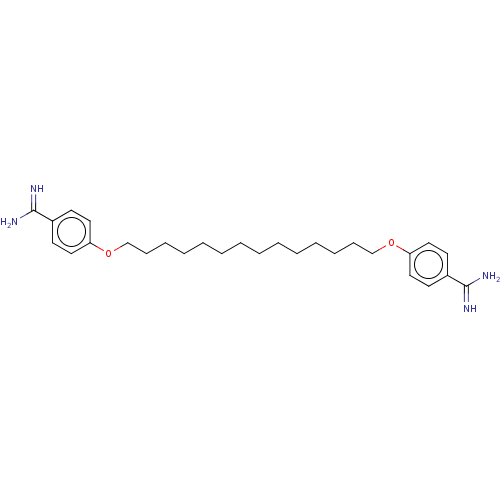 (CHEMBL3245416) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50016540 (CHEMBL3277922) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amidase activity of bovine thrombin using BPVANA as substrate after 15 to 40 mins | J Med Chem 19: 634-9 (1976) BindingDB Entry DOI: 10.7270/Q2KD20FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010110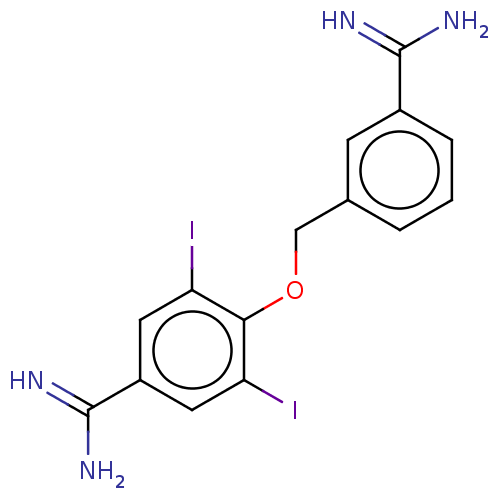 (CHEMBL2417998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010136 (CHEMBL3245422) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010124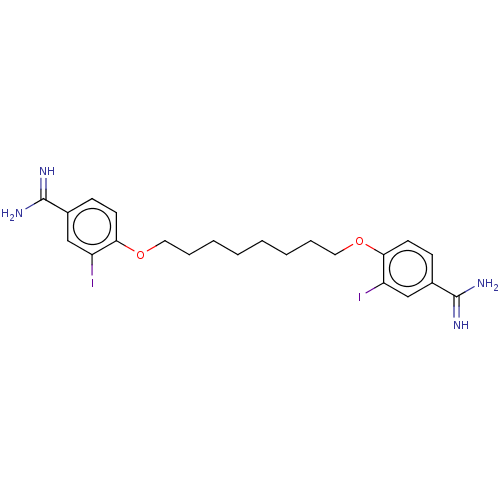 (CHEMBL3245412) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010126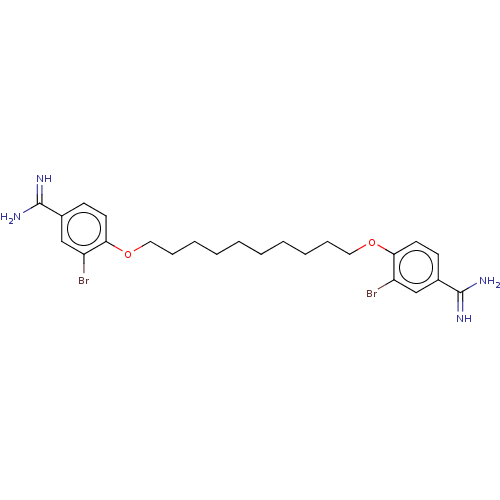 (CHEMBL3245414) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010118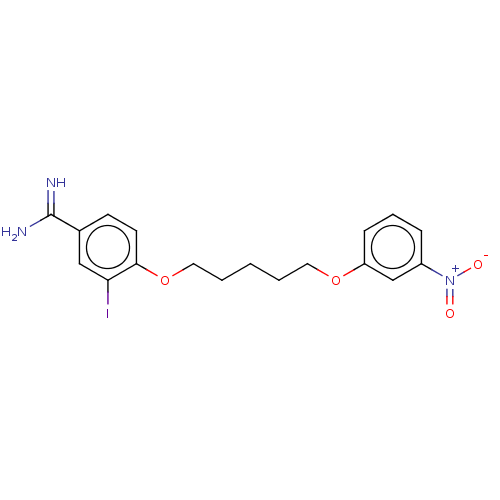 (CHEMBL3245406) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50010136 (CHEMBL3245422) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amidase activity of bovine thrombin using BPVANA as substrate after 15 to 40 mins | J Med Chem 19: 634-9 (1976) BindingDB Entry DOI: 10.7270/Q2KD20FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010113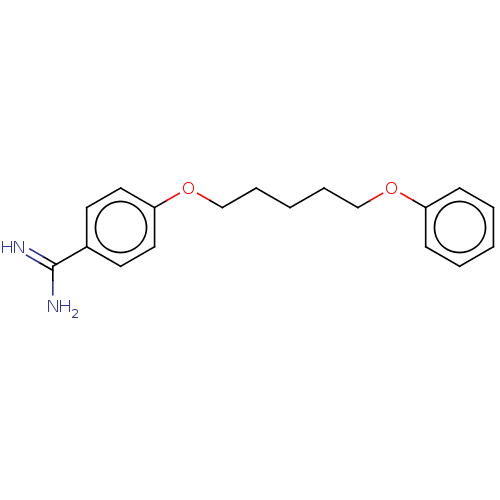 (CHEMBL3245402) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50379838 (CHEMBL2011750) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010104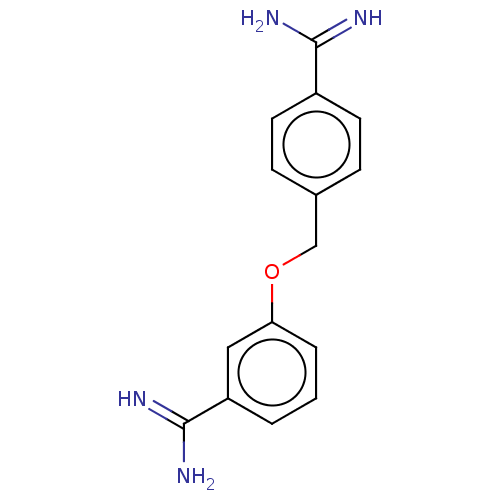 (CHEMBL2418010) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010092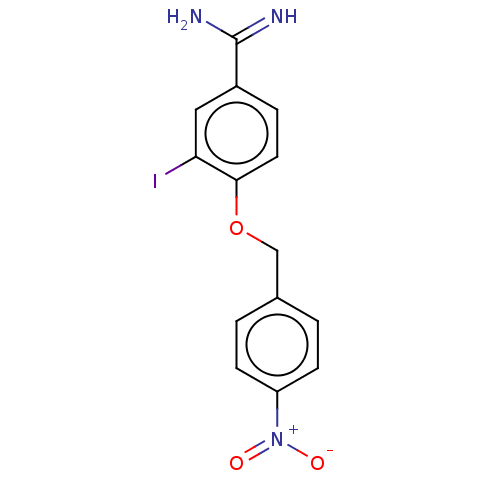 (CHEMBL2418041) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010127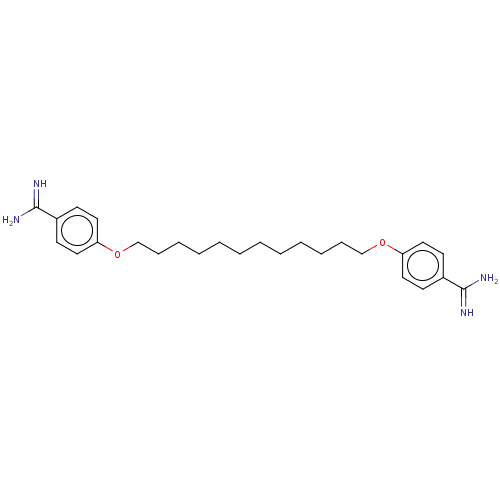 (CHEMBL3245415) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010109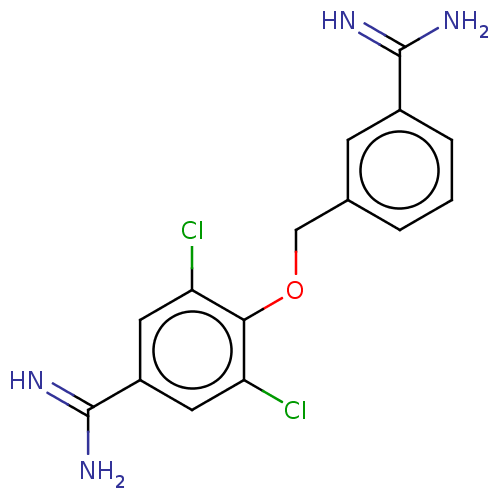 (CHEMBL2418035) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010107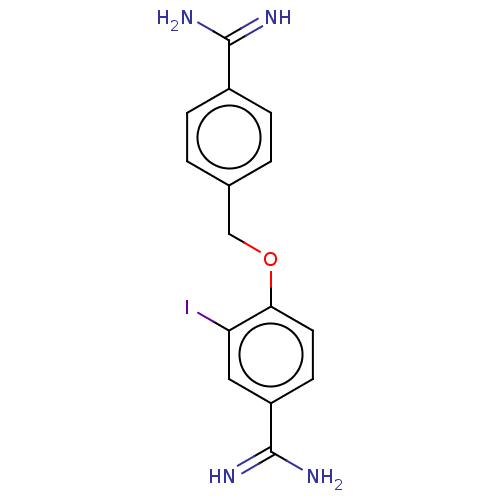 (CHEMBL2418015) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010105 (CHEMBL2418051) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010134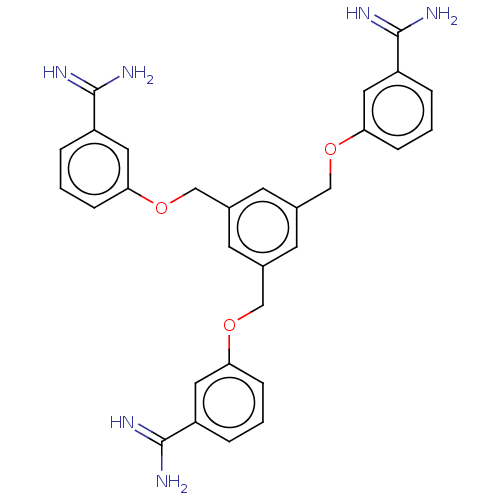 (CHEMBL3245421) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010120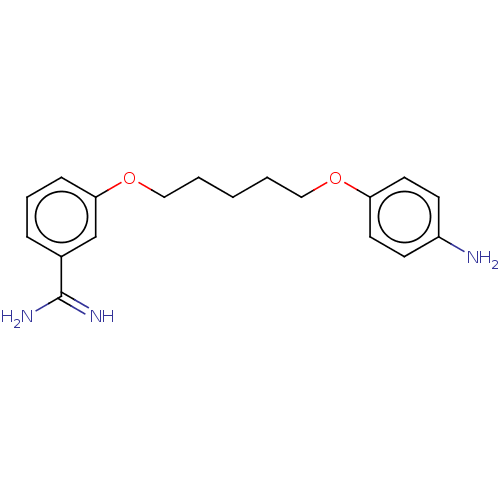 (CHEMBL3245408) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010125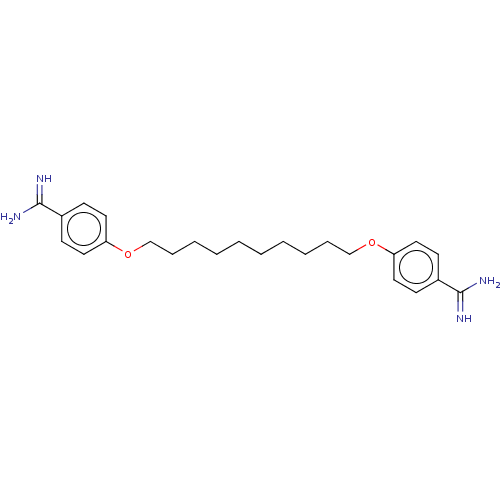 (CHEMBL3245413) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010112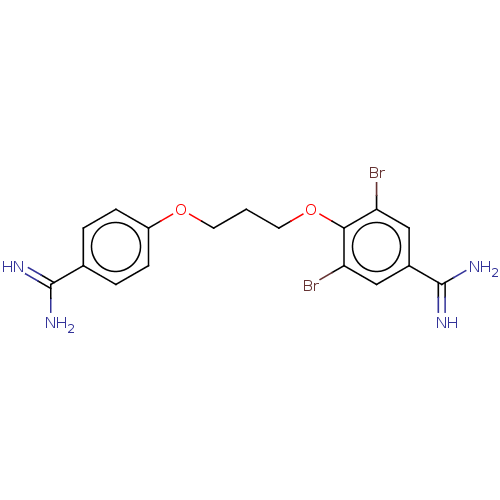 (CHEMBL3245401) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50016544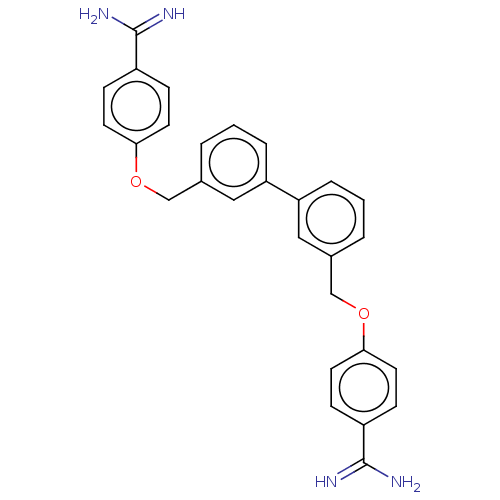 (CHEMBL3277934) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amidase activity of bovine thrombin using BPVANA as substrate after 15 to 40 mins | J Med Chem 19: 634-9 (1976) BindingDB Entry DOI: 10.7270/Q2KD20FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010088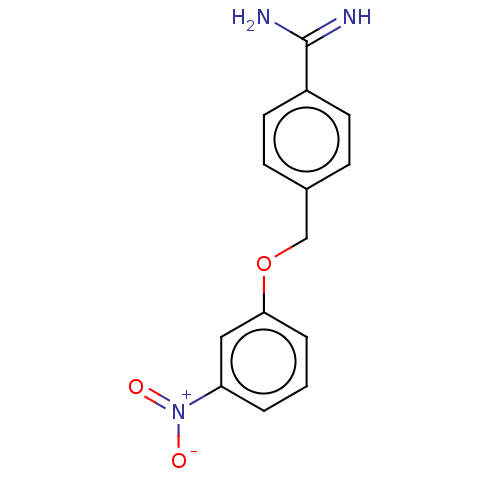 (CHEMBL2418049) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glandular kallikrein (Sus scrofa) | BDBM50379838 (CHEMBL2011750) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic kallikrein using BPPANA as substrate after 15 to 180 mins | J Med Chem 19: 634-9 (1976) BindingDB Entry DOI: 10.7270/Q2KD20FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010130 (CHEMBL3245418) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50010134 (CHEMBL3245421) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of thrombin (unknown origin) | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010111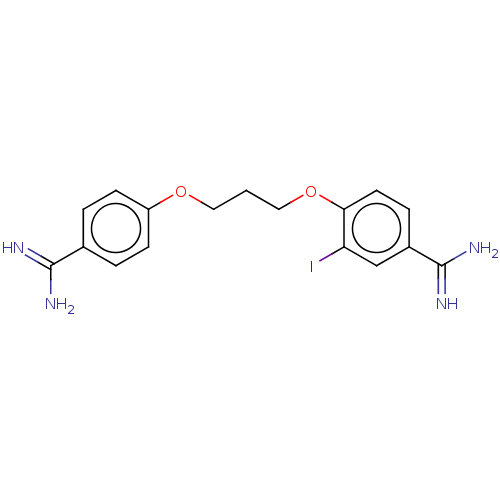 (CHEMBL3245400) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010119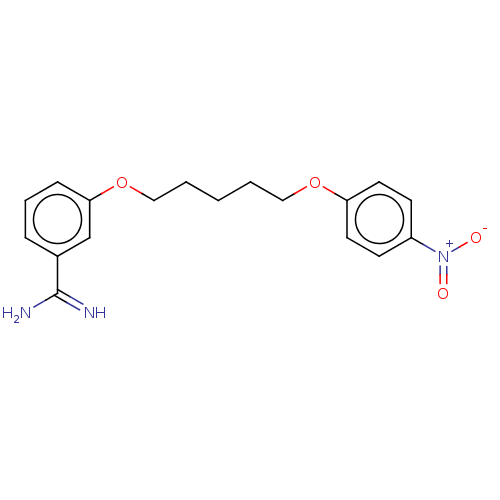 (CHEMBL3245407) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010106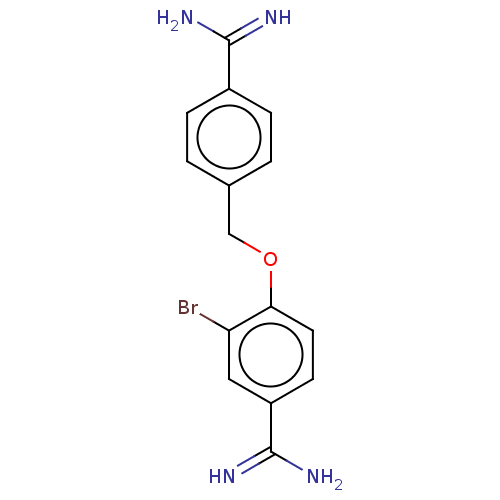 (CHEMBL2418012) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glandular kallikrein (Sus scrofa) | BDBM50016540 (CHEMBL3277922) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic kallikrein using BANA as substrate after 15 to 180 mins | J Med Chem 19: 634-9 (1976) BindingDB Entry DOI: 10.7270/Q2KD20FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glandular kallikrein (Sus scrofa) | BDBM50010025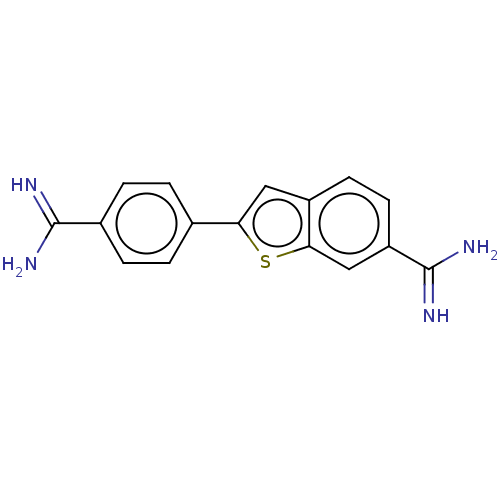 (CHEMBL352923) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive reversible inhibition of porcine pancreatic kallikrein using N-benzoyl-L-prolyl-L-phenylalanyl-L-arginine p-nitroanilide hydrochloride as... | J Med Chem 21: 613-23 (1978) BindingDB Entry DOI: 10.7270/Q23B61PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glandular kallikrein (Sus scrofa) | BDBM50010025 (CHEMBL352923) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive reversible inhibition of porcine pancreatic kallikrein using alpha-N-benzoyl-DL-arginine-p-nitroanilide hydrochloride as substrate after ... | J Med Chem 21: 613-23 (1978) BindingDB Entry DOI: 10.7270/Q23B61PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010089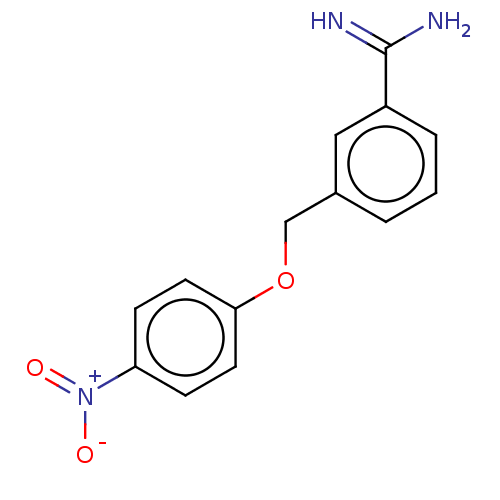 (CHEMBL3245397) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010115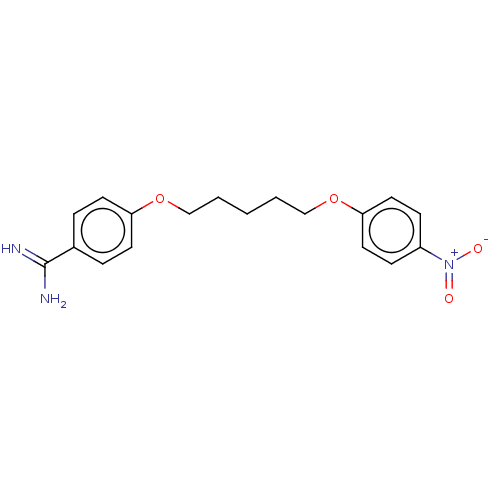 (CHEMBL3245403) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010090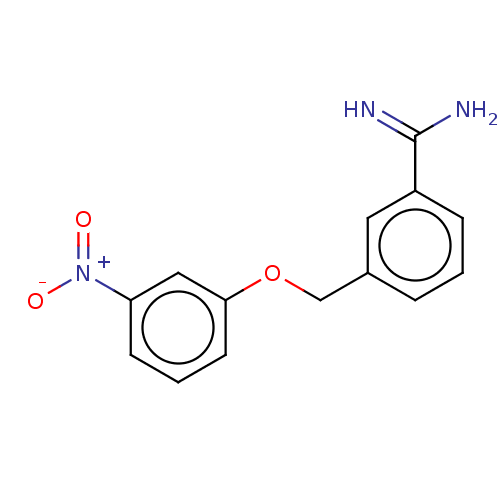 (CHEMBL2418050) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010082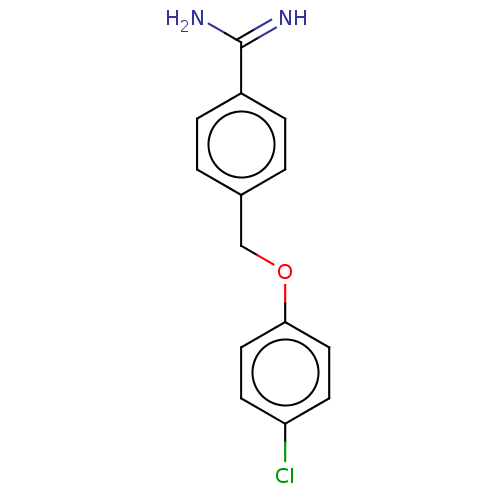 (CHEMBL2418045) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010116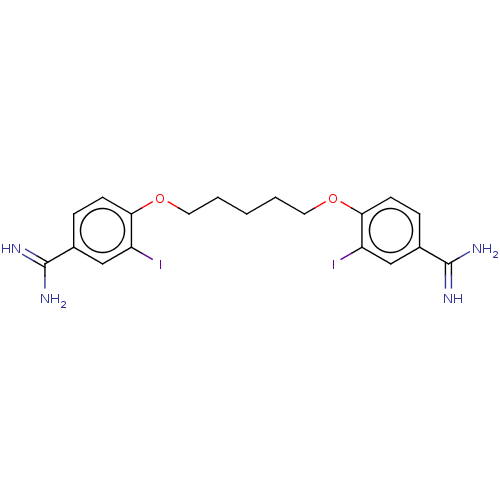 (CHEMBL3245404) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368077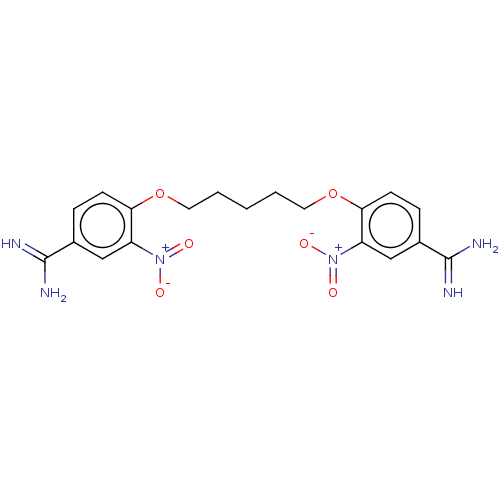 (CHEMBL3216901 | CHEMBL493336) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010122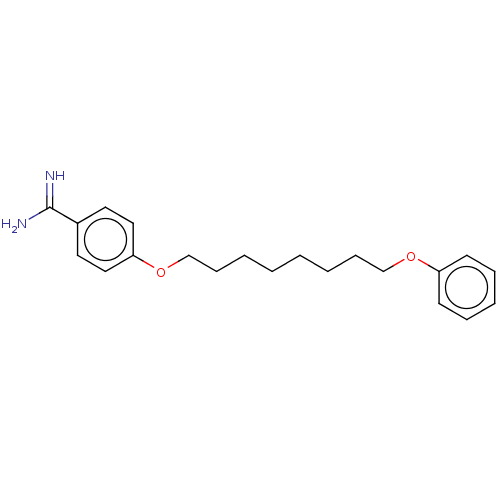 (CHEMBL3245410) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010121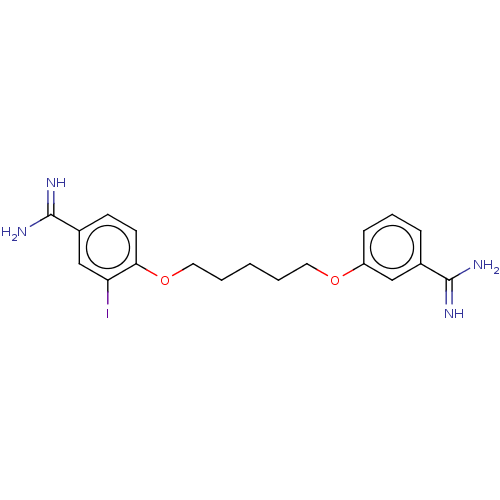 (CHEMBL3245409) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glandular kallikrein (Sus scrofa) | BDBM50016538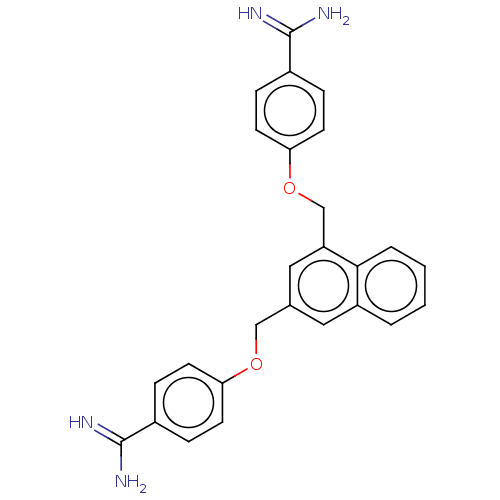 (CHEMBL3277933) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic kallikrein using BANA as substrate after 15 to 180 mins | J Med Chem 19: 634-9 (1976) BindingDB Entry DOI: 10.7270/Q2KD20FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368081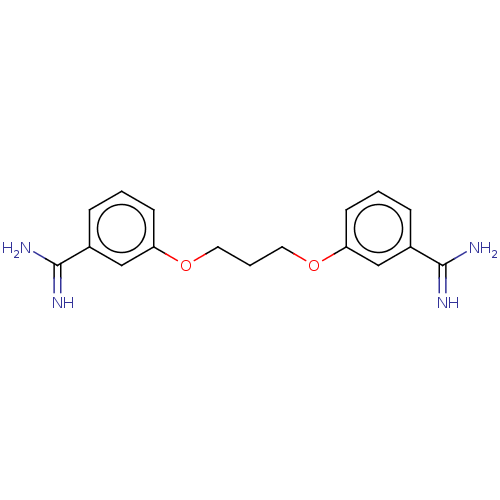 (CHEMBL3217116 | CHEMBL522538) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368096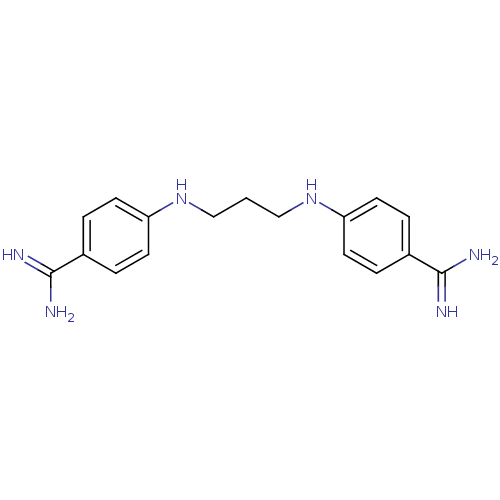 (CHEMBL1204157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010117 (CHEMBL3245405) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010108 (CHEMBL2418029) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010083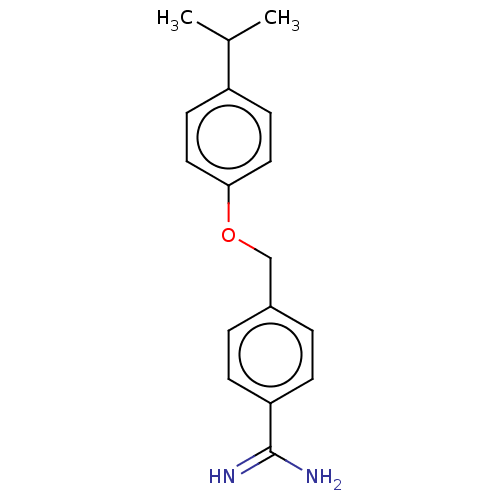 (CHEMBL2418046) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 356 total ) | Next | Last >> |