

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM13065 (5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX1 | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50561580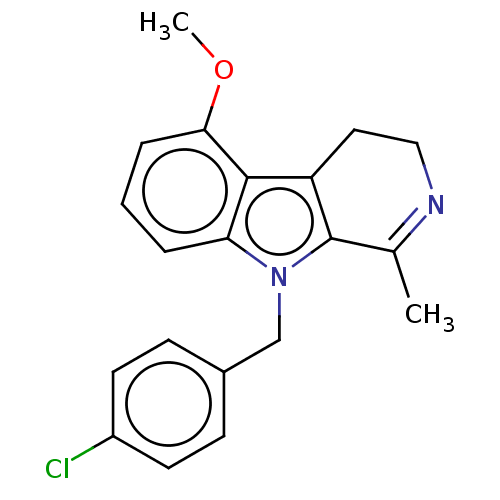 (CHEMBL4777544) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse COX2 using 2-arachidonylglycerol as substrate preincubated for 3 mins followed by substrate addition and measured for 30 sec by L... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00555 BindingDB Entry DOI: 10.7270/Q2N58R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50336971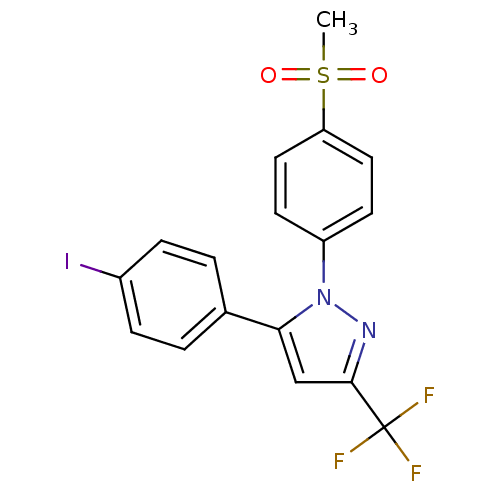 (5-(4-iodophenyl)-1-(4-(methylsulfonyl)phenyl)-3-(t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of COX2 in mouse LPS-stimulated RAW264.7 cells after 30 mins | ACS Med Chem Lett 2: 160-164 (2011) Article DOI: 10.1021/ml100232q BindingDB Entry DOI: 10.7270/Q25T3MG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of mouse purified COX2 after 20 mins | ACS Med Chem Lett 2: 160-164 (2011) Article DOI: 10.1021/ml100232q BindingDB Entry DOI: 10.7270/Q25T3MG9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description We evaluated the ability of test compounds to inhibit purified ovine COX-1 or murine COX-2 utilizing previously published protocols. [Uddin et al, Ca... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX2 | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50336964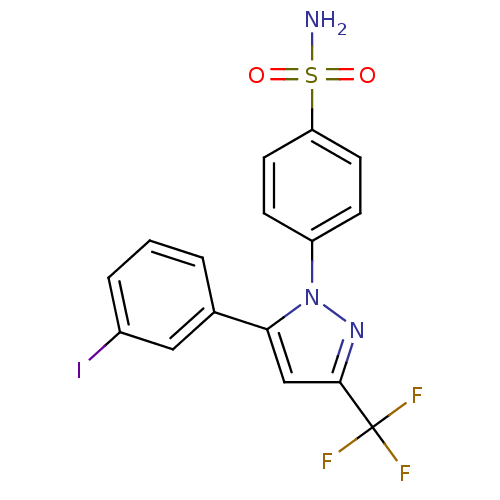 (4-[5-(3-Iodophenyl)-3-(trifluoromethyl)-1H-pyrazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of COX2 in mouse LPS-stimulated RAW264.7 cells after 30 mins | ACS Med Chem Lett 2: 160-164 (2011) Article DOI: 10.1021/ml100232q BindingDB Entry DOI: 10.7270/Q25T3MG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50561529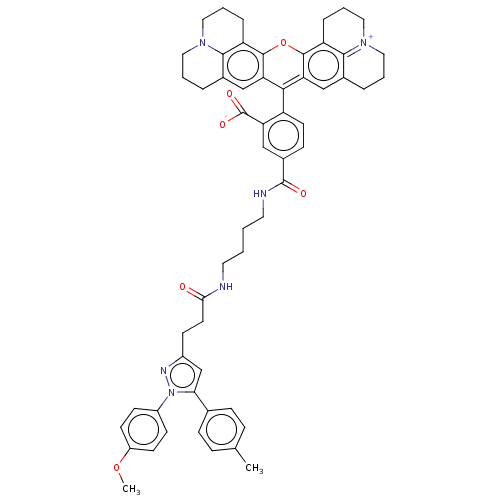 (CHEMBL4792985) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of COX-1 in human OVCAR3 cells assessed as [14C] arachidonic acid remaining using [14C] arachidonic acid as substrate preincubated for 30 ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00280 BindingDB Entry DOI: 10.7270/Q21C21J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50336971 (5-(4-iodophenyl)-1-(4-(methylsulfonyl)phenyl)-3-(t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of mouse purified COX2 after 20 mins | ACS Med Chem Lett 2: 160-164 (2011) Article DOI: 10.1021/ml100232q BindingDB Entry DOI: 10.7270/Q25T3MG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX1 | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX-2 in human HNSCC 1483 cells using [14C] arachidonic acid as substrate preincubated for 30 mins before substrate addition measured a... | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse COX-2 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50336964 (4-[5-(3-Iodophenyl)-3-(trifluoromethyl)-1H-pyrazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of mouse purified COX2 after 20 mins | ACS Med Chem Lett 2: 160-164 (2011) Article DOI: 10.1021/ml100232q BindingDB Entry DOI: 10.7270/Q25T3MG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50033168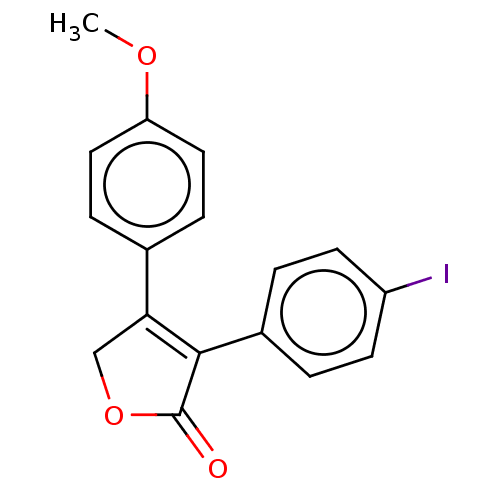 (CHEMBL3357106) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 [18-604,R106Q] (Mus musculus (Mouse)) | BDBM50312668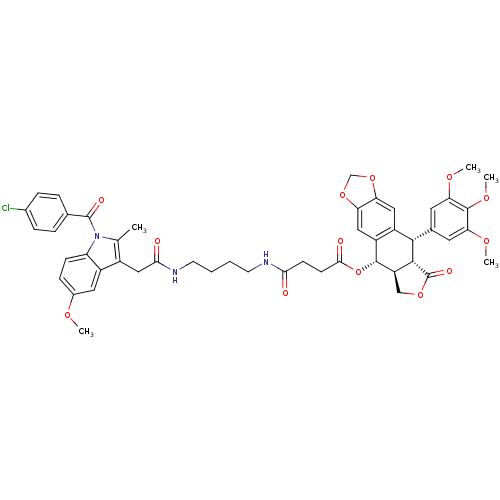 (CHEMBL1076638 | Chemocoxib A (12) | N-{(Succinylpo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description We evaluated the ability of test compounds to inhibit purified ovine COX-1 or murine COX-2 utilizing previously published protocols. [Uddin et al, Ca... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50561529 (CHEMBL4792985) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ovine COX-1 assessed as [14C] arachidonic acid remaining using [14C] arachidonic acid as substrate preincubated for 20 mins followed by... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00280 BindingDB Entry DOI: 10.7270/Q21C21J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205489 (Cytotoxic conjugates, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50110164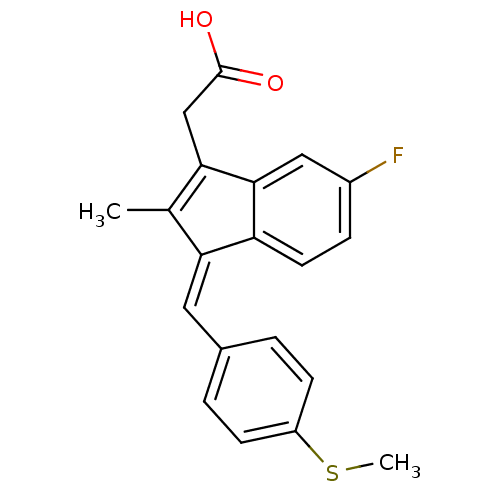 ((Z)-2-(3-(4-(methylthio)benzylidene)-6-fluoro-2-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX1 | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50272862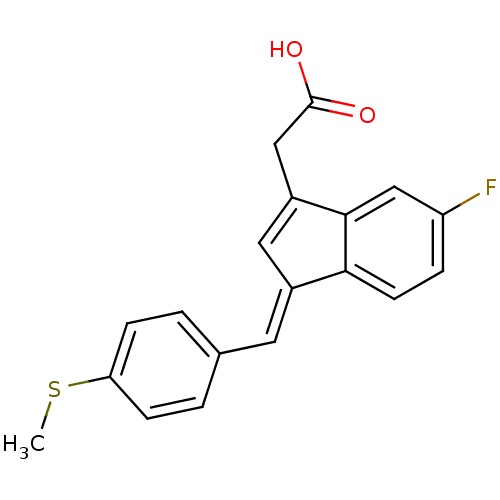 ((E)-2'-des-methyl sulindac sulfide | 2-desmethylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX-1 in human OVCAR3 cells using [14C] arachidonic acid as substrate preincubated for 30 mins before substrate addition measured after... | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50033167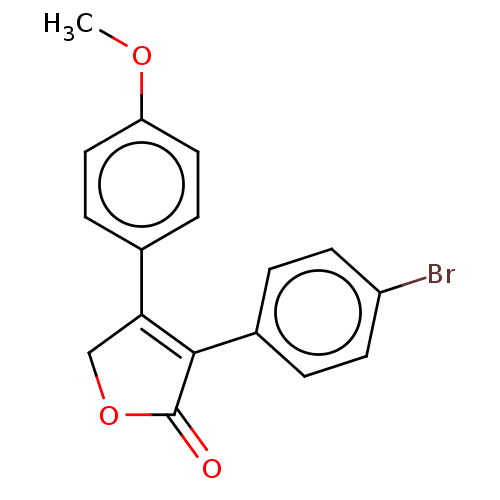 (CHEMBL3357105) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110164 ((Z)-2-(3-(4-(methylthio)benzylidene)-6-fluoro-2-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX2 | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205496 (Cytotoxic conjugates, 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50561580 (CHEMBL4777544) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse COX2 using arachidonic acid and 2-arachidonylglycerol as substrate preincubated for 5 mins followed by substrate addition and mea... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00555 BindingDB Entry DOI: 10.7270/Q2N58R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM13065 (5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX-1 in human OVCAR3 cells using [14C] arachidonic acid as substrate preincubated for 30 mins before substrate addition measured after... | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205497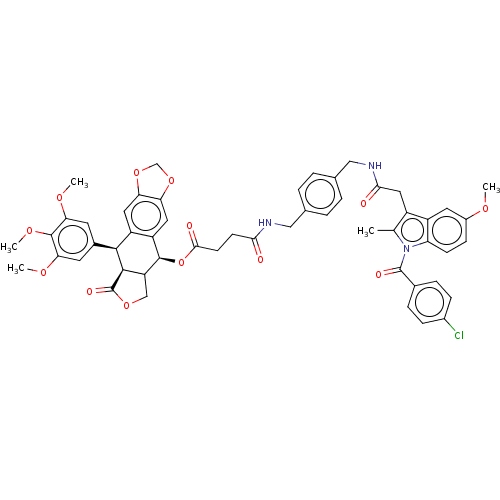 (Cytotoxic conjugates, 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50033277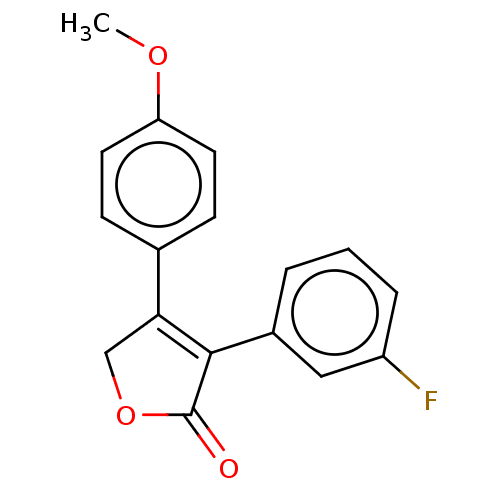 (CHEMBL3357103) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of COX-1 in human OVCAR3 cells by cell-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50561580 (CHEMBL4777544) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse COX2 using [1-14C]AA as substrate preincubated for 20 mins followed by substrate addition and measured for 30 sec by thin layer c... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00555 BindingDB Entry DOI: 10.7270/Q2N58R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 [18-604,S516A] (Mus musculus (Mouse)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description We evaluated the ability of test compounds to inhibit purified ovine COX-1 or murine COX-2 utilizing previously published protocols. [Uddin et al, Ca... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205487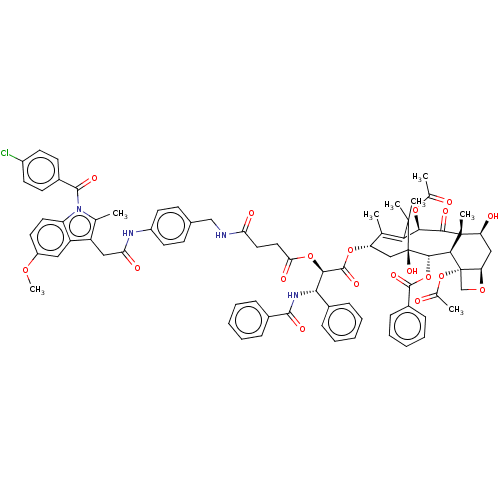 (Cytotoxic conjugates, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205495 (Cytotoxic conjugates, 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description We evaluated the ability of test compounds to inhibit purified ovine COX-1 or murine COX-2 utilizing previously published protocols. [Uddin et al, Ca... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50336967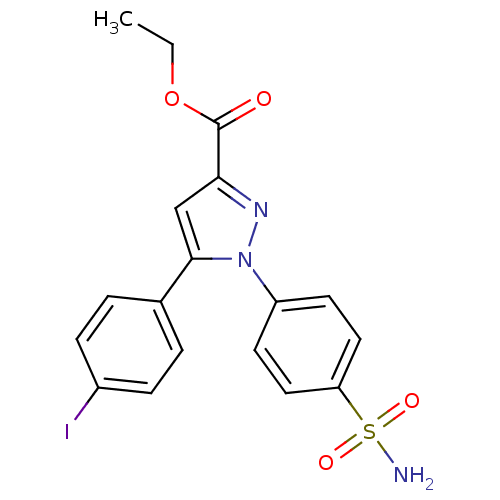 (CHEMBL1672579 | ethyl 5-(4-iodophenyl)-1-(4-sulfam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of mouse purified COX2 after 20 mins | ACS Med Chem Lett 2: 160-164 (2011) Article DOI: 10.1021/ml100232q BindingDB Entry DOI: 10.7270/Q25T3MG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50312668 (CHEMBL1076638 | Chemocoxib A (12) | N-{(Succinylpo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description We evaluated the ability of test compounds to inhibit purified ovine COX-1 or murine COX-2 utilizing previously published protocols. [Uddin et al, Ca... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50312668 (CHEMBL1076638 | Chemocoxib A (12) | N-{(Succinylpo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 [18-604,R106Q] (Mus musculus (Mouse)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description We evaluated the ability of test compounds to inhibit purified ovine COX-1 or murine COX-2 utilizing previously published protocols. [Uddin et al, Ca... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50033170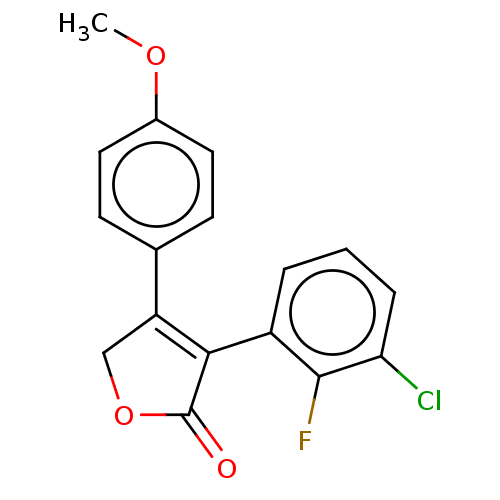 (CHEMBL3357108) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50273065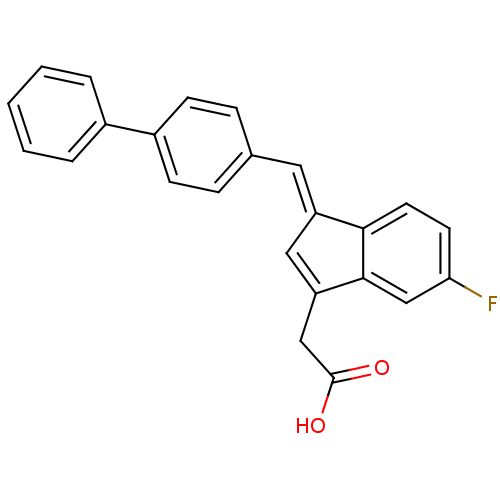 ((E)-2-(1-(Biphenyl-4-ylmethylene)-5-fluoro-1H-inde...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX-1 in human OVCAR3 cells using [14C] arachidonic acid as substrate preincubated for 30 mins before substrate addition measured after... | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50336970 (5-(3-iodophenyl)-1-(4-(methylsulfonyl)phenyl)-3-(t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of mouse purified COX2 after 20 mins | ACS Med Chem Lett 2: 160-164 (2011) Article DOI: 10.1021/ml100232q BindingDB Entry DOI: 10.7270/Q25T3MG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX2 | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50033166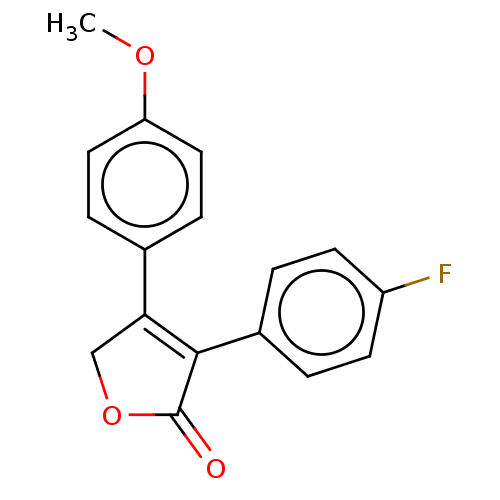 (CHEMBL3357104) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of COX-1 in human OVCAR3 cells by cell-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50033277 (CHEMBL3357103) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50033167 (CHEMBL3357105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse COX-2 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205488 (Cytotoxic conjugates, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 [18-604,V509I] (Mus musculus (Mouse)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description We evaluated the ability of test compounds to inhibit purified ovine COX-1 or murine COX-2 utilizing previously published protocols. [Uddin et al, Ca... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50388954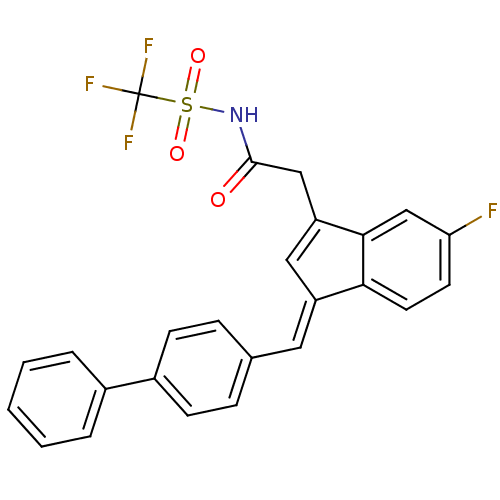 (CHEMBL2063559) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using [14C] arachidonic acid as substrate preincubated for 17 mins before substrate addition measured after 3 mins by thin-... | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205494 (Cytotoxic conjugates, 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50033173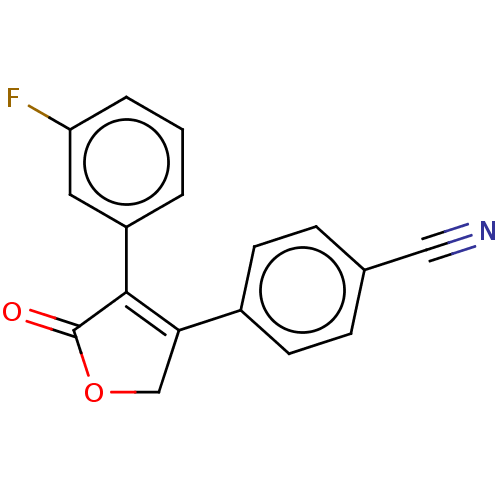 (CHEMBL3357111) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50033166 (CHEMBL3357104) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM205498 (Cytotoxic conjugates, 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine | Assay Description Each molecule was evaluated for its ability to inhibit purified mouse COX-2 or ovine COX-1 using a previously described assay. [Kalgutkar et al, J. M... | ACS Chem Biol 11: 3052-3060 (2016) Article DOI: 10.1021/acschembio.6b00560 BindingDB Entry DOI: 10.7270/Q23777JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 234 total ) | Next | Last >> |