 Found 67 hits with Last Name = 'gil' and Initial = 'mj'
Found 67 hits with Last Name = 'gil' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120056
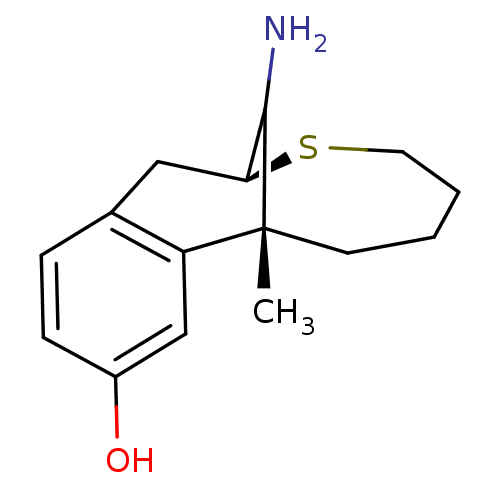
(15-Amino-1-methyl-10-thia-tricyclo[7.5.1.0*2,7*]pe...)Show InChI InChI=1S/C15H21NOS/c1-15-6-2-3-7-18-13(14(15)16)8-10-4-5-11(17)9-12(10)15/h4-5,9,13-14,17H,2-3,6-8,16H2,1H3/t13-,14?,15+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120057
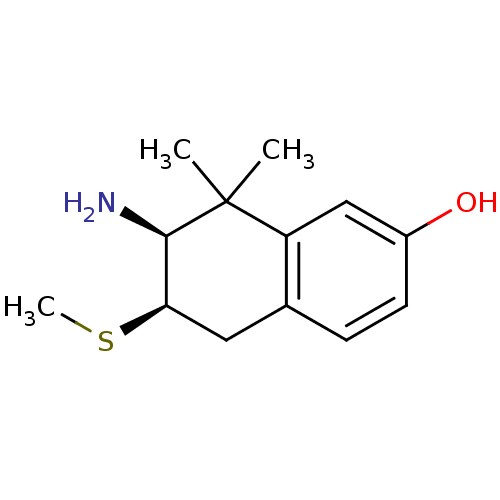
(7-Amino-8,8-dimethyl-6-methylsulfanyl-5,6,7,8-tetr...)Show InChI InChI=1S/C13H19NOS/c1-13(2)10-7-9(15)5-4-8(10)6-11(16-3)12(13)14/h4-5,7,11-12,15H,6,14H2,1-3H3/t11-,12-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120058
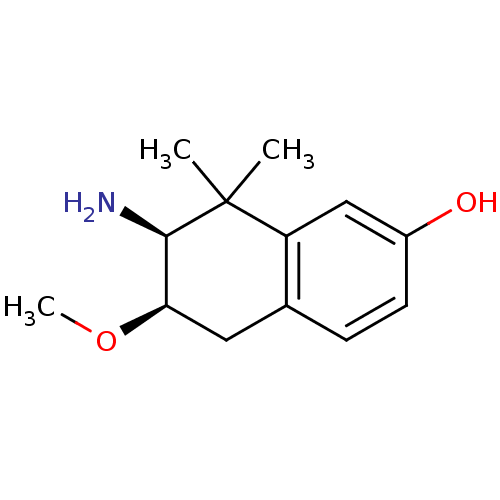
(7-Amino-6-methoxy-8,8-dimethyl-5,6,7,8-tetrahydro-...)Show InChI InChI=1S/C13H19NO2/c1-13(2)10-7-9(15)5-4-8(10)6-11(16-3)12(13)14/h4-5,7,11-12,15H,6,14H2,1-3H3/t11-,12-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50173870

(3-Benzyloxy-1,1-dioxo-2,3-dihydro-1H-1lambda*6*-be...)Show SMILES NS(=O)(=O)c1ccc2C(CS(=O)(=O)c2c1)OCc1ccccc1 Show InChI InChI=1S/C15H15NO5S2/c16-23(19,20)12-6-7-13-14(10-22(17,18)15(13)8-12)21-9-11-4-2-1-3-5-11/h1-8,14H,9-10H2,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50173865
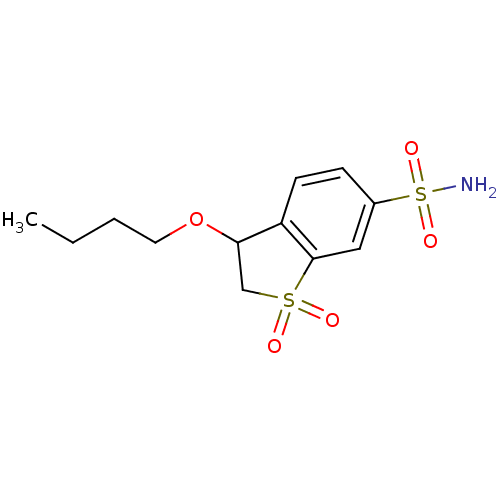
(3-Butoxy-1,1-dioxo-2,3-dihydro-1H-1lambda*6*-benzo...)Show InChI InChI=1S/C12H17NO5S2/c1-2-3-6-18-11-8-19(14,15)12-7-9(20(13,16)17)4-5-10(11)12/h4-5,7,11H,2-3,6,8H2,1H3,(H2,13,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50173865

(3-Butoxy-1,1-dioxo-2,3-dihydro-1H-1lambda*6*-benzo...)Show InChI InChI=1S/C12H17NO5S2/c1-2-3-6-18-11-8-19(14,15)12-7-9(20(13,16)17)4-5-10(11)12/h4-5,7,11H,2-3,6,8H2,1H3,(H2,13,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50173871

(1,1-Dioxo-1H-1lambda*6*-benzo[b]thiophene-5-sulfon...)Show InChI InChI=1S/C8H7NO4S2/c9-15(12,13)7-1-2-8-6(5-7)3-4-14(8,10)11/h1-5H,(H2,9,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50173862

(1,1-Dioxo-1H-1lambda*6*-benzo[b]thiophene-6-sulfon...)Show InChI InChI=1S/C8H7NO4S2/c9-15(12,13)7-2-1-6-3-4-14(10,11)8(6)5-7/h1-5H,(H2,9,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50173870

(3-Benzyloxy-1,1-dioxo-2,3-dihydro-1H-1lambda*6*-be...)Show SMILES NS(=O)(=O)c1ccc2C(CS(=O)(=O)c2c1)OCc1ccccc1 Show InChI InChI=1S/C15H15NO5S2/c16-23(19,20)12-6-7-13-14(10-22(17,18)15(13)8-12)21-9-11-4-2-1-3-5-11/h1-8,14H,9-10H2,(H2,16,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50173867
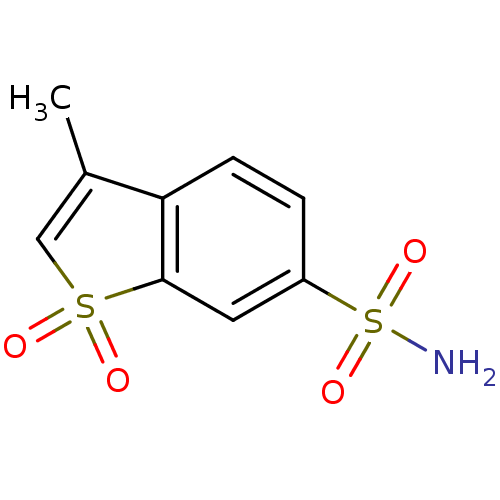
(3-Methyl-1,1-dioxo-1H-1lambda*6*-benzo[b]thiophene...)Show InChI InChI=1S/C9H9NO4S2/c1-6-5-15(11,12)9-4-7(16(10,13)14)2-3-8(6)9/h2-5H,1H3,(H2,10,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50173864

(1,1-Dioxo-2,3-dihydro-1H-1lambda*6*-benzo[b]thioph...)Show InChI InChI=1S/C8H9NO4S2/c9-15(12,13)7-2-1-6-3-4-14(10,11)8(6)5-7/h1-2,5H,3-4H2,(H2,9,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50173864

(1,1-Dioxo-2,3-dihydro-1H-1lambda*6*-benzo[b]thioph...)Show InChI InChI=1S/C8H9NO4S2/c9-15(12,13)7-2-1-6-3-4-14(10,11)8(6)5-7/h1-2,5H,3-4H2,(H2,9,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50173871

(1,1-Dioxo-1H-1lambda*6*-benzo[b]thiophene-5-sulfon...)Show InChI InChI=1S/C8H7NO4S2/c9-15(12,13)7-1-2-8-6(5-7)3-4-14(8,10)11/h1-5H,(H2,9,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50173867

(3-Methyl-1,1-dioxo-1H-1lambda*6*-benzo[b]thiophene...)Show InChI InChI=1S/C9H9NO4S2/c1-6-5-15(11,12)9-4-7(16(10,13)14)2-3-8(6)9/h2-5H,1H3,(H2,10,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50173862

(1,1-Dioxo-1H-1lambda*6*-benzo[b]thiophene-6-sulfon...)Show InChI InChI=1S/C8H7NO4S2/c9-15(12,13)7-2-1-6-3-4-14(10,11)8(6)5-7/h1-5H,(H2,9,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120053
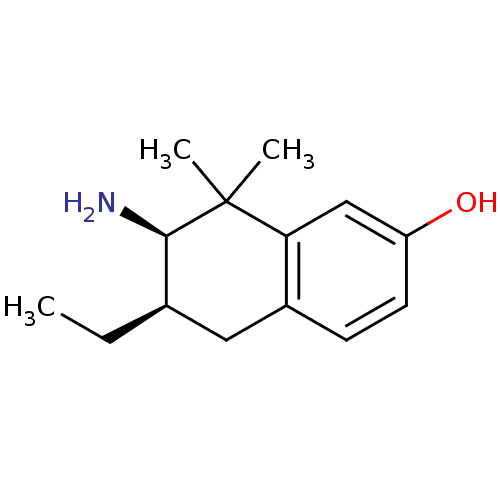
(7-Amino-6-ethyl-8,8-dimethyl-5,6,7,8-tetrahydro-na...)Show InChI InChI=1S/C14H21NO/c1-4-9-7-10-5-6-11(16)8-12(10)14(2,3)13(9)15/h5-6,8-9,13,16H,4,7,15H2,1-3H3/t9-,13-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase I by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase I by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
MMDB
PDB
Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50120056

(15-Amino-1-methyl-10-thia-tricyclo[7.5.1.0*2,7*]pe...)Show InChI InChI=1S/C15H21NOS/c1-15-6-2-3-7-18-13(14(15)16)8-10-4-5-11(17)9-12(10)15/h4-5,9,13-14,17H,2-3,6-8,16H2,1H3/t13-,14?,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor kappa 1 was determined. |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor kappa 1 was determined. |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50173862

(1,1-Dioxo-1H-1lambda*6*-benzo[b]thiophene-6-sulfon...)Show InChI InChI=1S/C8H7NO4S2/c9-15(12,13)7-2-1-6-3-4-14(10,11)8(6)5-7/h1-5H,(H2,9,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase I by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50120057

(7-Amino-8,8-dimethyl-6-methylsulfanyl-5,6,7,8-tetr...)Show InChI InChI=1S/C13H19NOS/c1-13(2)10-7-9(15)5-4-8(10)6-11(16-3)12(13)14/h4-5,7,11-12,15H,6,14H2,1-3H3/t11-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor kappa 1 was determined. |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50173864

(1,1-Dioxo-2,3-dihydro-1H-1lambda*6*-benzo[b]thioph...)Show InChI InChI=1S/C8H9NO4S2/c9-15(12,13)7-2-1-6-3-4-14(10,11)8(6)5-7/h1-2,5H,3-4H2,(H2,9,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase I by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50173871

(1,1-Dioxo-1H-1lambda*6*-benzo[b]thiophene-5-sulfon...)Show InChI InChI=1S/C8H7NO4S2/c9-15(12,13)7-1-2-8-6(5-7)3-4-14(8,10)11/h1-5H,(H2,9,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase I by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50173869
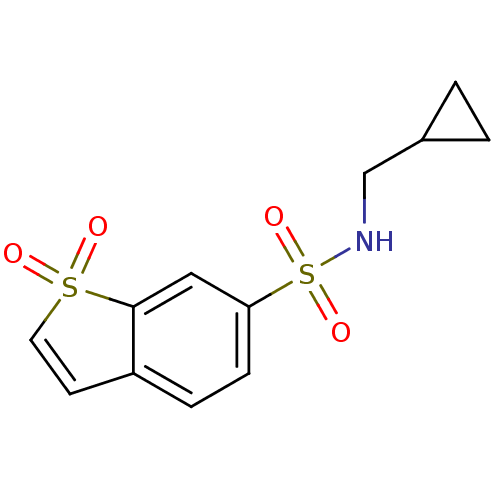
(1,1-Dioxo-1H-1lambda*6*-benzo[b]thiophene-6-sulfon...)Show SMILES O=S(=O)(NCC1CC1)c1ccc2C=CS(=O)(=O)c2c1 |c:13| Show InChI InChI=1S/C12H13NO4S2/c14-18(15)6-5-10-3-4-11(7-12(10)18)19(16,17)13-8-9-1-2-9/h3-7,9,13H,1-2,8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50173867

(3-Methyl-1,1-dioxo-1H-1lambda*6*-benzo[b]thiophene...)Show InChI InChI=1S/C9H9NO4S2/c1-6-5-15(11,12)9-4-7(16(10,13)14)2-3-8(6)9/h2-5H,1H3,(H2,10,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase I by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50173865

(3-Butoxy-1,1-dioxo-2,3-dihydro-1H-1lambda*6*-benzo...)Show InChI InChI=1S/C12H17NO5S2/c1-2-3-6-18-11-8-19(14,15)12-7-9(20(13,16)17)4-5-10(11)12/h4-5,7,11H,2-3,6,8H2,1H3,(H2,13,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase I by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor delta 1 was determined |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120061
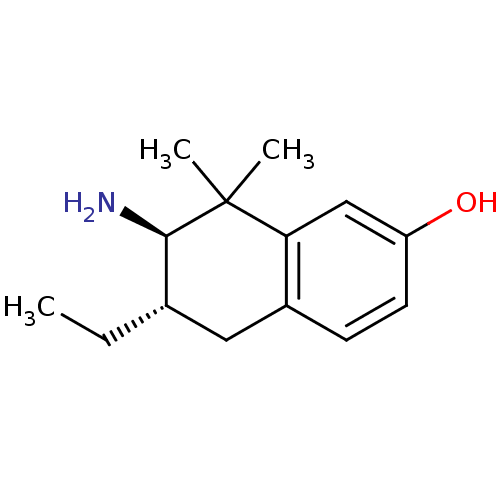
(7-Amino-6-ethyl-8,8-dimethyl-5,6,7,8-tetrahydro-na...)Show InChI InChI=1S/C14H21NO/c1-4-9-7-10-5-6-11(16)8-12(10)14(2,3)13(9)15/h5-6,8-9,13,16H,4,7,15H2,1-3H3/t9-,13+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50173870

(3-Benzyloxy-1,1-dioxo-2,3-dihydro-1H-1lambda*6*-be...)Show SMILES NS(=O)(=O)c1ccc2C(CS(=O)(=O)c2c1)OCc1ccccc1 Show InChI InChI=1S/C15H15NO5S2/c16-23(19,20)12-6-7-13-14(10-22(17,18)15(13)8-12)21-9-11-4-2-1-3-5-11/h1-8,14H,9-10H2,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase I by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120062

(7-Amino-6-mercapto-8,8-dimethyl-5,6,7,8-tetrahydro...)Show InChI InChI=1S/C12H17NOS/c1-12(2)9-6-8(14)4-3-7(9)5-10(15)11(12)13/h3-4,6,10-11,14-15H,5,13H2,1-2H3/t10-,11-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50120058

(7-Amino-6-methoxy-8,8-dimethyl-5,6,7,8-tetrahydro-...)Show InChI InChI=1S/C13H19NO2/c1-13(2)10-7-9(15)5-4-8(10)6-11(16-3)12(13)14/h4-5,7,11-12,15H,6,14H2,1-3H3/t11-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor kappa 1 was determined. |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120054
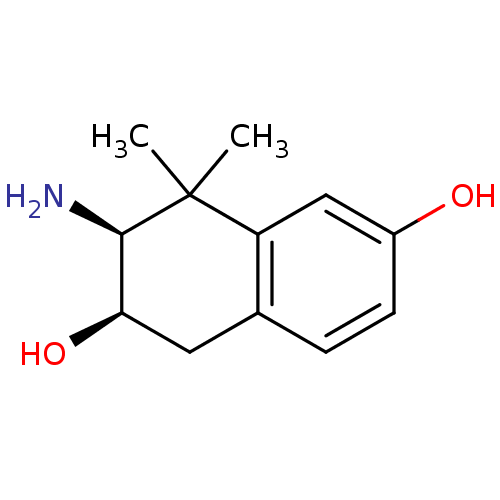
(3-Amino-4,4-dimethyl-1,2,3,4-tetrahydro-naphthalen...)Show InChI InChI=1S/C12H17NO2/c1-12(2)9-6-8(14)4-3-7(9)5-10(15)11(12)13/h3-4,6,10-11,14-15H,5,13H2,1-2H3/t10-,11-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50120059
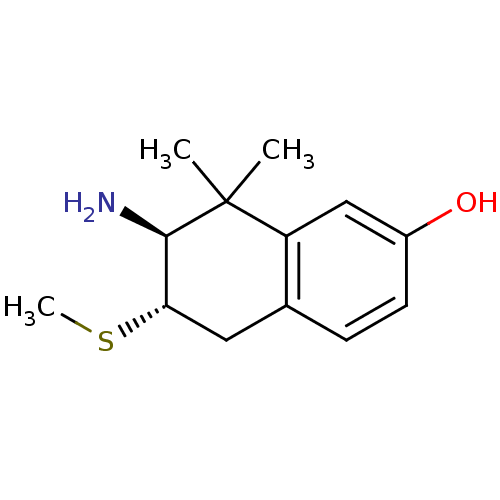
(7-Amino-8,8-dimethyl-6-methylsulfanyl-5,6,7,8-tetr...)Show InChI InChI=1S/C13H19NOS/c1-13(2)10-7-9(15)5-4-8(10)6-11(16-3)12(13)14/h4-5,7,11-12,15H,6,14H2,1-3H3/t11-,12+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 286 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity for Opioid receptor mu 1 |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50120053

(7-Amino-6-ethyl-8,8-dimethyl-5,6,7,8-tetrahydro-na...)Show InChI InChI=1S/C14H21NO/c1-4-9-7-10-5-6-11(16)8-12(10)14(2,3)13(9)15/h5-6,8-9,13,16H,4,7,15H2,1-3H3/t9-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 345 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor kappa 1 was determined. |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50120057

(7-Amino-8,8-dimethyl-6-methylsulfanyl-5,6,7,8-tetr...)Show InChI InChI=1S/C13H19NOS/c1-13(2)10-7-9(15)5-4-8(10)6-11(16-3)12(13)14/h4-5,7,11-12,15H,6,14H2,1-3H3/t11-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 349 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor delta 1 was determined |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50173868
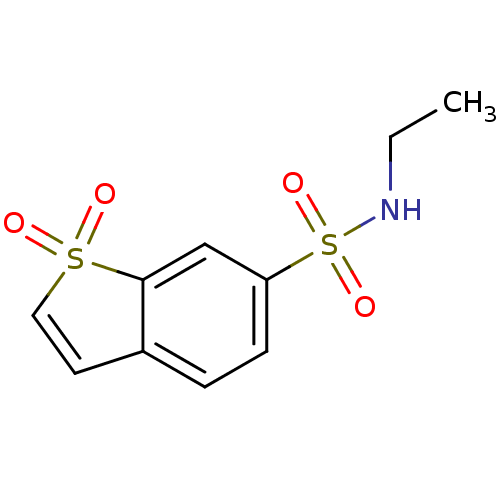
(1,1-Dioxo-1H-1lambda*6*-benzo[b]thiophene-6-sulfon...)Show InChI InChI=1S/C10H11NO4S2/c1-2-11-17(14,15)9-4-3-8-5-6-16(12,13)10(8)7-9/h3-7,11H,2H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 362 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against catalytic domain of human cloned Carbonic anhydrase IX by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50120056

(15-Amino-1-methyl-10-thia-tricyclo[7.5.1.0*2,7*]pe...)Show InChI InChI=1S/C15H21NOS/c1-15-6-2-3-7-18-13(14(15)16)8-10-4-5-11(17)9-12(10)15/h4-5,9,13-14,17H,2-3,6-8,16H2,1H3/t13-,14?,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire Biochem
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for Opioid receptor delta 1 was determined |
Bioorg Med Chem Lett 12: 3141-3 (2002)
BindingDB Entry DOI: 10.7270/Q21N80GS |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50173868

(1,1-Dioxo-1H-1lambda*6*-benzo[b]thiophene-6-sulfon...)Show InChI InChI=1S/C10H11NO4S2/c1-2-11-17(14,15)9-4-3-8-5-6-16(12,13)10(8)7-9/h3-7,11H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 436 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cloned Carbonic anhydrase II by the CO2 hydration method |
Bioorg Med Chem Lett 15: 4872-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.078
BindingDB Entry DOI: 10.7270/Q2F18Z86 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data