 Found 324 hits with Last Name = 'gilbertsen' and Initial = 'rb'
Found 324 hits with Last Name = 'gilbertsen' and Initial = 'rb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50005795
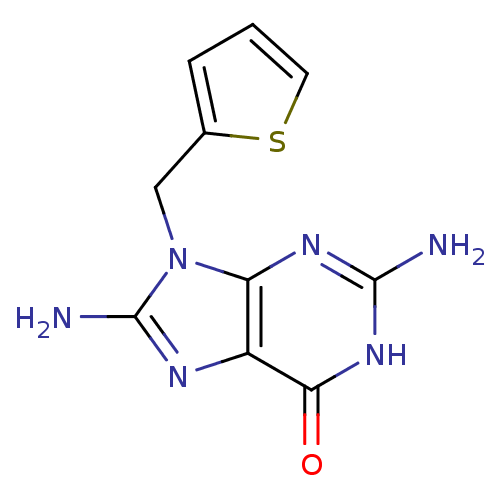
(2,8-Diamino-9-thiophen-2-ylmethyl-1,9-dihydro-puri...)Show InChI InChI=1S/C10H10N6OS/c11-9-14-7-6(8(17)15-9)13-10(12)16(7)4-5-2-1-3-18-5/h1-3H,4H2,(H2,12,13)(H3,11,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit purine nucleoside phosphorylase (PNP) |
J Med Chem 35: 1605-9 (1992)
BindingDB Entry DOI: 10.7270/Q2KW5GPH |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50368609

(CHEMBL604660)Show SMILES O[C@@H]1[C@@H](CI)OC([C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H12IN3O4/c12-1-5-8(16)9(17)10(19-5)4-2-13-7-6(4)14-3-15-11(7)18/h2-3,5,8-10,13,16-17H,1H2,(H,14,15,18)/t5-,8-,9-,10?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit purine nucleoside phosphorylase (PNP) |
J Med Chem 35: 1605-9 (1992)
BindingDB Entry DOI: 10.7270/Q2KW5GPH |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50368611
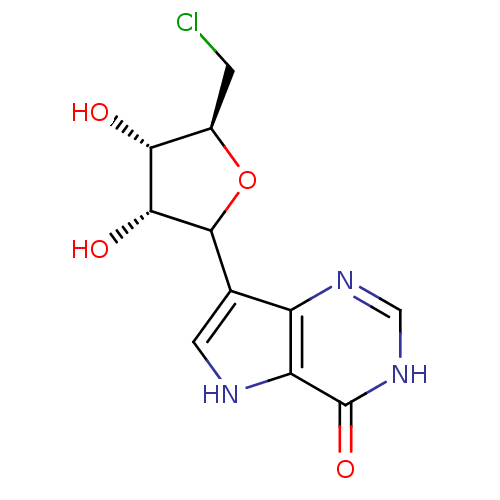
(CHEMBL604864)Show SMILES O[C@@H]1[C@@H](CCl)OC([C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H12ClN3O4/c12-1-5-8(16)9(17)10(19-5)4-2-13-7-6(4)14-3-15-11(7)18/h2-3,5,8-10,13,16-17H,1H2,(H,14,15,18)/t5-,8-,9-,10?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit purine nucleoside phosphorylase (PNP) |
J Med Chem 35: 1605-9 (1992)
BindingDB Entry DOI: 10.7270/Q2KW5GPH |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50005916

(2,6-diamino-7-(thien-3-ylmethyl)-3,5-dihydro-4H-py...)Show InChI InChI=1S/C11H11N5OS/c12-9-6(3-5-1-2-18-4-5)7-8(14-9)10(17)16-11(13)15-7/h1-2,4,14H,3,12H2,(H3,13,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit purine nucleoside phosphorylase (PNP) |
J Med Chem 35: 1605-9 (1992)
BindingDB Entry DOI: 10.7270/Q2KW5GPH |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50230691

(CHEMBL3143996)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)C1=CNC2C1=NC=NC2=O |r,c:17,t:10,15| Show InChI InChI=1S/C11H13N3O5/c15-2-5-8(16)9(17)10(19-5)4-1-12-7-6(4)13-3-14-11(7)18/h1,3,5,7-10,12,15-17H,2H2/t5-,7?,8-,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit purine nucleoside phosphorylase (PNP) |
J Med Chem 35: 1605-9 (1992)
BindingDB Entry DOI: 10.7270/Q2KW5GPH |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50230690
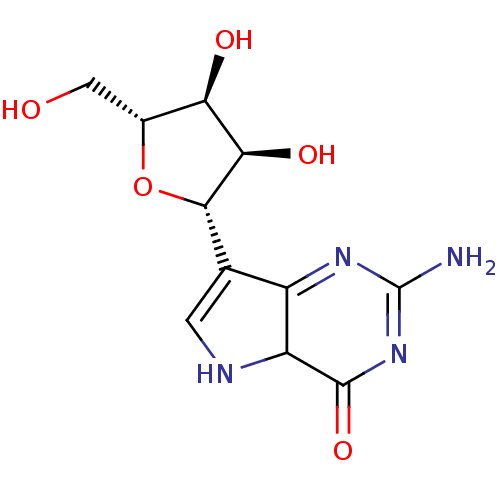
(CHEMBL3143997)Show SMILES NC1=NC(=O)C2NC=C([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)C2=N1 |r,c:20,t:1,7| Show InChI InChI=1S/C11H14N4O5/c12-11-14-5-3(1-13-6(5)10(19)15-11)9-8(18)7(17)4(2-16)20-9/h1,4,6-9,13,16-18H,2H2,(H2,12,15,19)/t4-,6?,7-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit purine nucleoside phosphorylase (PNP) |
J Med Chem 35: 1605-9 (1992)
BindingDB Entry DOI: 10.7270/Q2KW5GPH |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382321
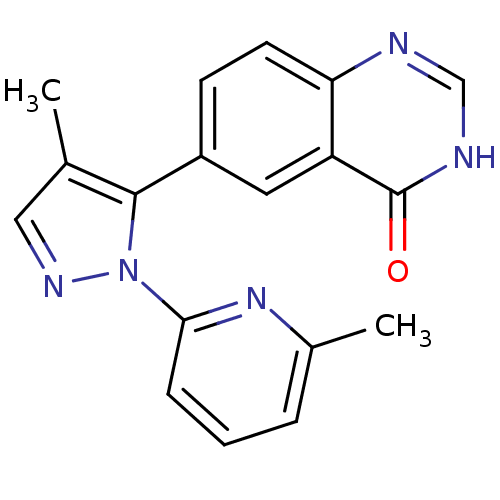
(CHEMBL2024688)Show SMILES Cc1cnn(c1-c1ccc2nc[nH]c(=O)c2c1)-c1cccc(C)n1 Show InChI InChI=1S/C18H15N5O/c1-11-9-21-23(16-5-3-4-12(2)22-16)17(11)13-6-7-15-14(8-13)18(24)20-10-19-15/h3-10H,1-2H3,(H,19,20,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382322
(CHEMBL2024689)Show SMILES CCc1cc(-c2ccc3ncn(C)c(=O)c3c2)n(n1)-c1cccc(C)n1 Show InChI InChI=1S/C20H19N5O/c1-4-15-11-18(25(23-15)19-7-5-6-13(2)22-19)14-8-9-17-16(10-14)20(26)24(3)12-21-17/h5-12H,4H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382324

(CHEMBL2024691)Show SMILES CCc1cnn(c1-c1ccc2nc[nH]c(=O)c2c1)-c1cccc(C)n1 Show InChI InChI=1S/C19H17N5O/c1-3-13-10-22-24(17-6-4-5-12(2)23-17)18(13)14-7-8-16-15(9-14)19(25)21-11-20-16/h4-11H,3H2,1-2H3,(H,20,21,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382320
(CHEMBL2024687)Show SMILES Cc1cc(-c2ccc3ncn(C)c(=O)c3c2)n(n1)-c1cccc(C)n1 Show InChI InChI=1S/C19H17N5O/c1-12-5-4-6-18(21-12)24-17(9-13(2)22-24)14-7-8-16-15(10-14)19(25)23(3)11-20-16/h4-11H,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382319

(CHEMBL2024686)Show SMILES Cc1cnn(c1-c1ccc2ncn(C)c(=O)c2c1)-c1cccc(C)n1 Show InChI InChI=1S/C19H17N5O/c1-12-10-21-24(17-6-4-5-13(2)22-17)18(12)14-7-8-16-15(9-14)19(25)23(3)11-20-16/h4-11H,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382318

(CHEMBL2024685)Show SMILES Cc1cccc(n1)-n1nccc1-c1ccc2ncn(C=C)c(=O)c2c1 Show InChI InChI=1S/C19H15N5O/c1-3-23-12-20-16-8-7-14(11-15(16)19(23)25)17-9-10-21-24(17)18-6-4-5-13(2)22-18/h3-12H,1H2,2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382325

(CHEMBL2024693)Show SMILES Cc1cccc(n1)-n1nc2CCCc2c1-c1ccc2nc[nH]c(=O)c2c1 Show InChI InChI=1S/C20H17N5O/c1-12-4-2-7-18(23-12)25-19(14-5-3-6-17(14)24-25)13-8-9-16-15(10-13)20(26)22-11-21-16/h2,4,7-11H,3,5-6H2,1H3,(H,21,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382323
(CHEMBL2024690)Show InChI InChI=1S/C17H13N5O/c1-11-3-2-4-16(21-11)22-15(7-8-20-22)12-5-6-14-13(9-12)17(23)19-10-18-14/h2-10H,1H3,(H,18,19,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50044072
(CHEMBL16434 | N-[5-(3,5-Di-tert-butyl-4-hydroxy-be...)Show SMILES CC(C)(C)c1cc(C=C2SC(NC(N)=N)=NC2=O)cc(c1O)C(C)(C)C |w:7.6,c:14| Show InChI InChI=1S/C19H26N4O2S/c1-18(2,3)11-7-10(8-12(14(11)24)19(4,5)6)9-13-15(25)22-17(26-13)23-16(20)21/h7-9,24H,1-6H3,(H4,20,21,22,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 against ovine Prostaglandin G/H synthase 1 |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 1 of human platelet rich plasma |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 against Prostaglandin G/H synthase 1 from human platelet rich plasma |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50046902

(2,6-Di-tert-butyl-4-(5-methylsulfanyl-[1,3,4]thiad...)Show SMILES CSc1nnc(s1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C17H24N2OS2/c1-16(2,3)11-8-10(14-18-19-15(21-7)22-14)9-12(13(11)20)17(4,5)6/h8-9,20H,1-7H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075529
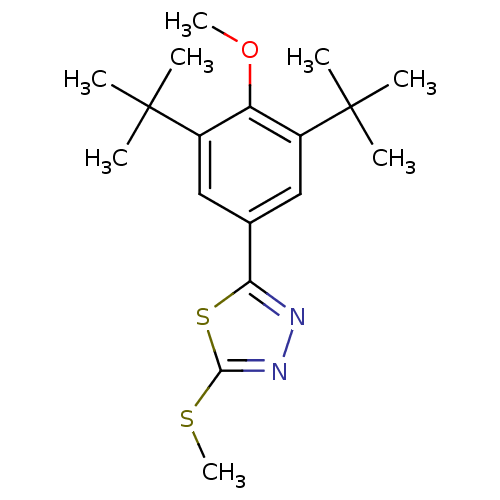
(2-(3,5-Di-tert-butyl-4-methoxy-phenyl)-5-methylsul...)Show InChI InChI=1S/C18H26N2OS2/c1-17(2,3)12-9-11(15-19-20-16(22-8)23-15)10-13(14(12)21-7)18(4,5)6/h9-10H,1-8H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 against ovine Prostaglandin G/H synthase 1 |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against ovine Prostaglandin G/H synthase 1 |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382317
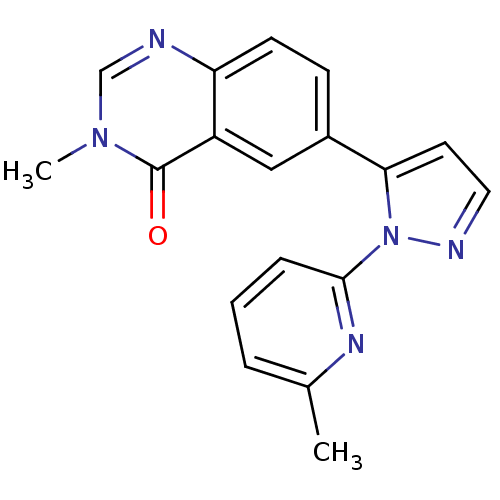
(CHEMBL2024684)Show InChI InChI=1S/C18H15N5O/c1-12-4-3-5-17(21-12)23-16(8-9-20-23)13-6-7-15-14(10-13)18(24)22(2)11-19-15/h3-11H,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075548
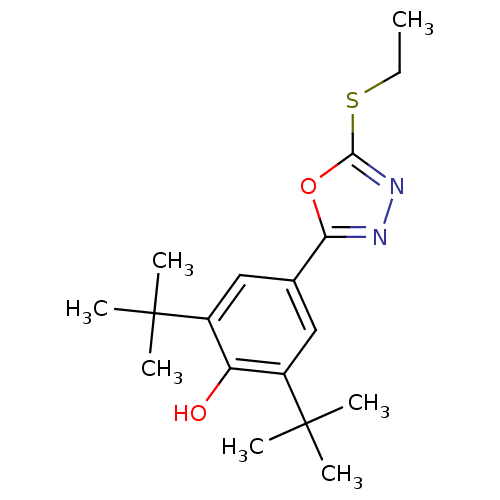
(2,6-Di-tert-butyl-4-(5-ethylsulfanyl-[1,3,4]oxadia...)Show SMILES CCSc1nnc(o1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C18H26N2O2S/c1-8-23-16-20-19-15(22-16)11-9-12(17(2,3)4)14(21)13(10-11)18(5,6)7/h9-10,21H,8H2,1-7H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075551
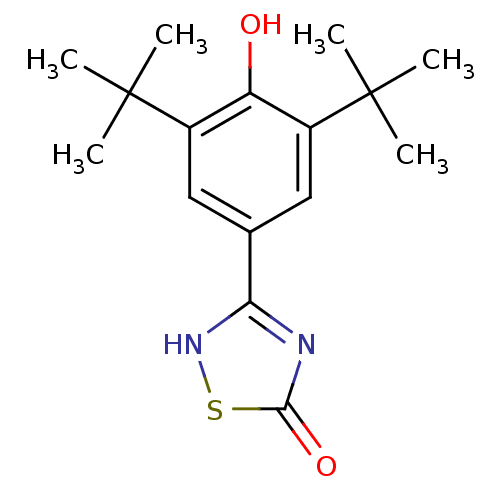
(3-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-[1,2,4]thia...)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1nc(=O)s[nH]1 Show InChI InChI=1S/C16H22N2O2S/c1-15(2,3)10-7-9(13-17-14(20)21-18-13)8-11(12(10)19)16(4,5)6/h7-8,19H,1-6H3,(H,17,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075541

(4,6-Di-tert-butyl-2-(5-ethylsulfanyl-[1,3,4]thiadi...)Show SMILES CCSc1nnc(s1)-c1nc(c(O)c(n1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C16H24N4OS2/c1-8-22-14-20-19-13(23-14)12-17-10(15(2,3)4)9(21)11(18-12)16(5,6)7/h21H,8H2,1-7H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50029593

(CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...)Show InChI InChI=1S/C13H18N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h7-9,11,14H,2-6H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50029593

(CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...)Show InChI InChI=1S/C13H18N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h7-9,11,14H,2-6H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075539

(3-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-[1,2,4]thia...)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1nsc(NC#N)n1 Show InChI InChI=1S/C17H22N4OS/c1-16(2,3)11-7-10(8-12(13(11)22)17(4,5)6)14-20-15(19-9-18)23-21-14/h7-8,22H,1-6H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075519
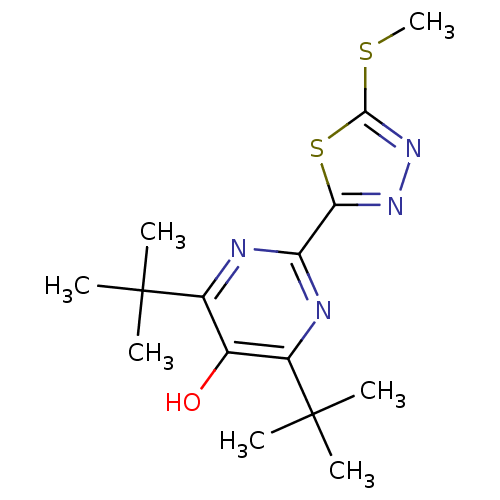
(4,6-Di-tert-butyl-2-(5-methylsulfanyl-[1,3,4]thiad...)Show SMILES CSc1nnc(s1)-c1nc(c(O)c(n1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C15H22N4OS2/c1-14(2,3)9-8(20)10(15(4,5)6)17-11(16-9)12-18-19-13(21-7)22-12/h20H,1-7H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50044068

(CHEMBL15904 | N-[5-(3,5-Di-tert-butyl-4-hydroxy-be...)Show SMILES CN1C(NC(N)=N)=NC(=O)C1=Cc1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C |w:11.12,c:6| Show InChI InChI=1S/C20H29N5O2/c1-19(2,3)12-8-11(9-13(15(12)26)20(4,5)6)10-14-16(27)23-18(25(14)7)24-17(21)22/h8-10,26H,1-7H3,(H4,21,22,23,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 against ovine Prostaglandin G/H synthase 1 |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Mus musculus) | BDBM50382318

(CHEMBL2024685)Show SMILES Cc1cccc(n1)-n1nccc1-c1ccc2ncn(C=C)c(=O)c2c1 Show InChI InChI=1S/C19H15N5O/c1-3-23-12-20-16-8-7-14(11-15(16)19(23)25)17-9-10-21-24(17)18-6-4-5-13(2)22-18/h3-12H,1H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 in mouse NIH/3T3 cells by smad binding element reporter based assay |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Mus musculus) | BDBM50382325

(CHEMBL2024693)Show SMILES Cc1cccc(n1)-n1nc2CCCc2c1-c1ccc2nc[nH]c(=O)c2c1 Show InChI InChI=1S/C20H17N5O/c1-12-4-2-7-18(23-12)25-19(14-5-3-6-17(14)24-25)13-8-9-16-15(10-13)20(26)22-11-21-16/h2,4,7-11H,3,5-6H2,1H3,(H,21,22,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 in mouse NIH/3T3 cells by smad binding element reporter based assay |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50075539

(3-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-[1,2,4]thia...)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1nsc(NC#N)n1 Show InChI InChI=1S/C17H22N4OS/c1-16(2,3)11-7-10(8-12(13(11)22)17(4,5)6)14-20-15(19-9-18)23-21-14/h7-8,22H,1-6H3,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Mus musculus) | BDBM50382324

(CHEMBL2024691)Show SMILES CCc1cnn(c1-c1ccc2nc[nH]c(=O)c2c1)-c1cccc(C)n1 Show InChI InChI=1S/C19H17N5O/c1-3-13-10-22-24(17-6-4-5-12(2)23-17)18(13)14-7-8-16-15(9-14)19(25)21-11-20-16/h4-11H,3H2,1-2H3,(H,20,21,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 in mouse NIH/3T3 cells by smad binding element reporter based assay |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM50382317

(CHEMBL2024684)Show InChI InChI=1S/C18H15N5O/c1-12-4-3-5-17(21-12)23-16(8-9-20-23)13-6-7-15-14(10-13)18(24)22(2)11-19-15/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK4 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50044059
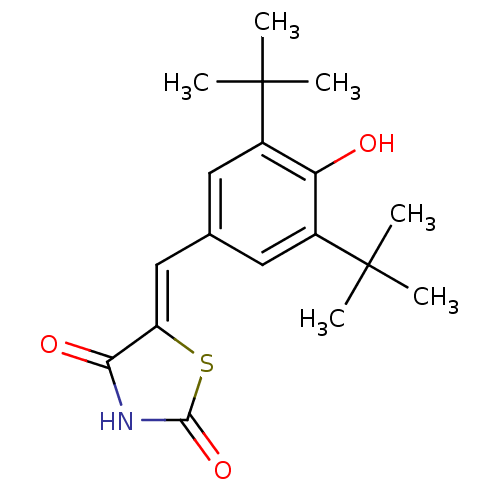
(5-(3,5-Di-tert-butyl-4-hydroxy-benzylidene)-thiazo...)Show SMILES CC(C)(C)c1cc(\C=C2/SC(=O)NC2=O)cc(c1O)C(C)(C)C Show InChI InChI=1S/C18H23NO3S/c1-17(2,3)11-7-10(8-12(14(11)20)18(4,5)6)9-13-15(21)19-16(22)23-13/h7-9,20H,1-6H3,(H,19,21,22)/b13-9- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Mus musculus) | BDBM50382321

(CHEMBL2024688)Show SMILES Cc1cnn(c1-c1ccc2nc[nH]c(=O)c2c1)-c1cccc(C)n1 Show InChI InChI=1S/C18H15N5O/c1-11-9-21-23(16-5-3-4-12(2)22-16)17(11)13-6-7-15-14(8-13)18(24)20-10-19-15/h3-10H,1-2H3,(H,19,20,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 in mouse NIH/3T3 cells by smad binding element reporter based assay |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50075526
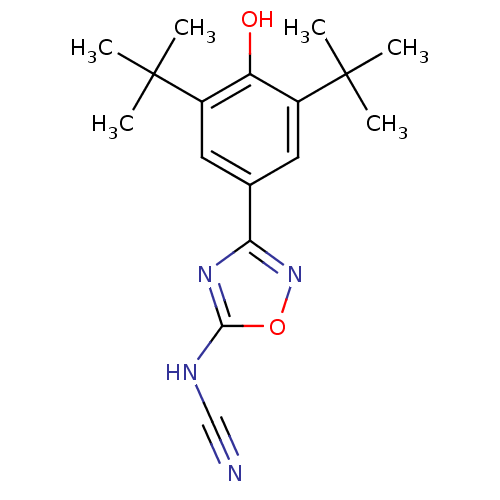
(3-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-[1,2,4]oxad...)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1noc(NC#N)n1 Show InChI InChI=1S/C17H22N4O2/c1-16(2,3)11-7-10(8-12(13(11)22)17(4,5)6)14-20-15(19-9-18)23-21-14/h7-8,22H,1-6H3,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50044080
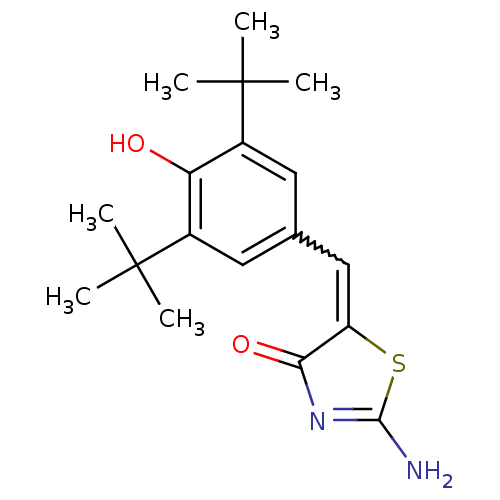
(2-Amino-5-[1-(3,5-di-tert-butyl-4-hydroxy-phenyl)-...)Show SMILES CC(C)(C)c1cc(C=C2SC(N)=NC2=O)cc(c1O)C(C)(C)C |w:7.6,c:11| Show InChI InChI=1S/C18H24N2O2S/c1-17(2,3)11-7-10(8-12(14(11)21)18(4,5)6)9-13-15(22)20-16(19)23-13/h7-9,21H,1-6H3,(H2,19,20,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382326

(CHEMBL2024692)Show InChI InChI=1S/C19H17N5O/c1-3-23-12-20-16-8-7-14(11-15(16)19(23)25)17-9-10-21-24(17)18-6-4-5-13(2)22-18/h4-12H,3H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50029616
(5-(4-Fluoro-phenyl)-1-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C17H12F4N2O2S/c1-26(24,25)14-8-6-13(7-9-14)23-15(10-16(22-23)17(19,20)21)11-2-4-12(18)5-3-11/h2-10H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075527

(2,6-Di-tert-butyl-4-(5-ethoxy-[1,3,4]thiadiazol-2-...)Show SMILES CCOc1nnc(s1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C18H26N2O2S/c1-8-22-16-20-19-15(23-16)11-9-12(17(2,3)4)14(21)13(10-11)18(5,6)7/h9-10,21H,8H2,1-7H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against Prostaglandin G/H synthase 2 of murine J774A.1 cell line |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50029616

(5-(4-Fluoro-phenyl)-1-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C17H12F4N2O2S/c1-26(24,25)14-8-6-13(7-9-14)23-15(10-16(22-23)17(19,20)21)11-2-4-12(18)5-3-11/h2-10H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50044081

(5-(3,5-Di-tert-butyl-4-hydroxy-benzylidene)-oxazol...)Show SMILES CC(C)(C)c1cc(\C=C2/OC(=O)NC2=O)cc(c1O)C(C)(C)C Show InChI InChI=1S/C18H23NO4/c1-17(2,3)11-7-10(8-12(14(11)20)18(4,5)6)9-13-15(21)19-16(22)23-13/h7-9,20H,1-6H3,(H,19,21,22)/b13-9- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Mus musculus) | BDBM50382323

(CHEMBL2024690)Show InChI InChI=1S/C17H13N5O/c1-11-3-2-4-16(21-11)22-15(7-8-20-22)12-5-6-14-13(9-12)17(23)19-10-18-14/h2-10H,1H3,(H,18,19,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 in mouse NIH/3T3 cells by smad binding element reporter based assay |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50075514

(5-[1-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-meth-(Z)...)Show SMILES CC(C)(C)c1cc(\C=C2/NC(=S)NC2=O)cc(c1O)C(C)(C)C Show InChI InChI=1S/C18H24N2O2S/c1-17(2,3)11-7-10(8-12(14(11)21)18(4,5)6)9-13-15(22)20-16(23)19-13/h7-9,21H,1-6H3,(H2,19,20,22,23)/b13-9- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50044081

(5-(3,5-Di-tert-butyl-4-hydroxy-benzylidene)-oxazol...)Show SMILES CC(C)(C)c1cc(\C=C2/OC(=O)NC2=O)cc(c1O)C(C)(C)C Show InChI InChI=1S/C18H23NO4/c1-17(2,3)11-7-10(8-12(14(11)20)18(4,5)6)9-13-15(21)19-16(22)23-13/h7-9,20H,1-6H3,(H,19,21,22)/b13-9- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 against ovine Prostaglandin G/H synthase 1 |
J Med Chem 42: 1151-60 (1999)
Article DOI: 10.1021/jm9805081
BindingDB Entry DOI: 10.7270/Q24X56Z3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50075519

(4,6-Di-tert-butyl-2-(5-methylsulfanyl-[1,3,4]thiad...)Show SMILES CSc1nnc(s1)-c1nc(c(O)c(n1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C15H22N4OS2/c1-14(2,3)9-8(20)10(15(4,5)6)17-11(16-9)12-18-19-13(21-7)22-12/h20H,1-7H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
IC50 value against ovine Prostaglandin G/H synthase 1 |
J Med Chem 42: 1161-9 (1999)
Article DOI: 10.1021/jm980570y
BindingDB Entry DOI: 10.7270/Q2154G6N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data