

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159379 ((R)-3-[4-(1,1-Dioxo-1,2,3,6-tetrahydro-1lambda*6*-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159388 ((R)-3-[4-(1,1-Dioxo-1,2,3,6-tetrahydro-1lambda*6*-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159382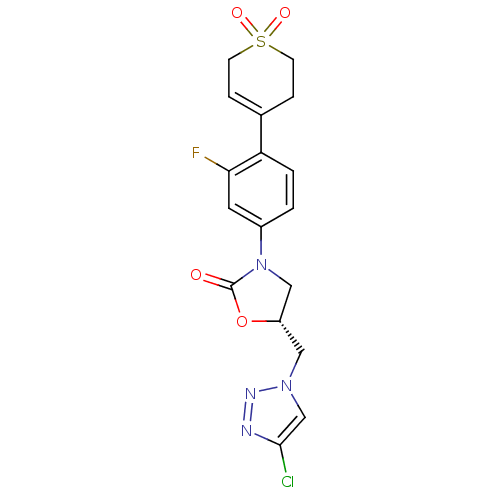 ((R)-5-(4-Chloro-[1,2,3]triazol-1-ylmethyl)-3-[4-(1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159372 (1-{(R)-3-[4-(1,1-Dioxo-1,2,3,6-tetrahydro-1lambda*...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159385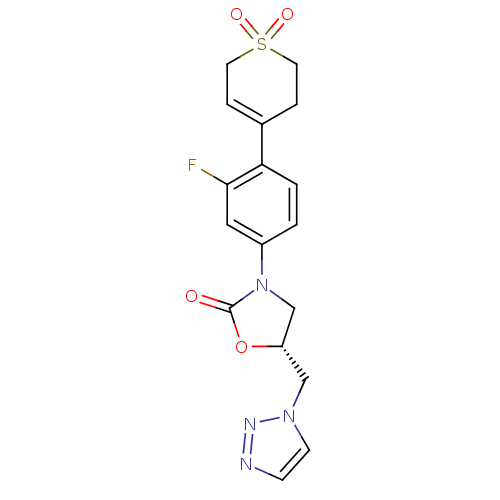 ((R)-3-[4-(1,1-Dioxo-1,2,3,6-tetrahydro-1lambda*6*-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159370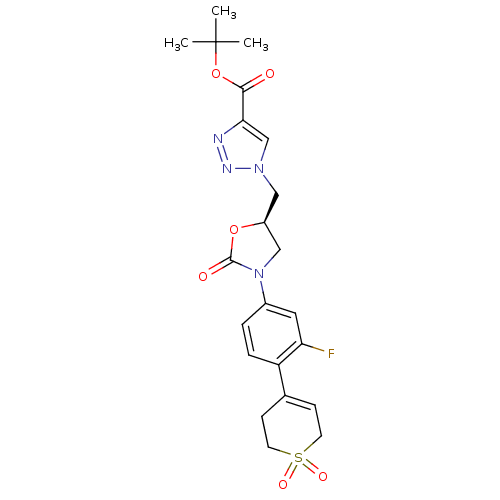 (1-{(R)-3-[4-(1,1-Dioxo-1,2,3,6-tetrahydro-1lambda*...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159380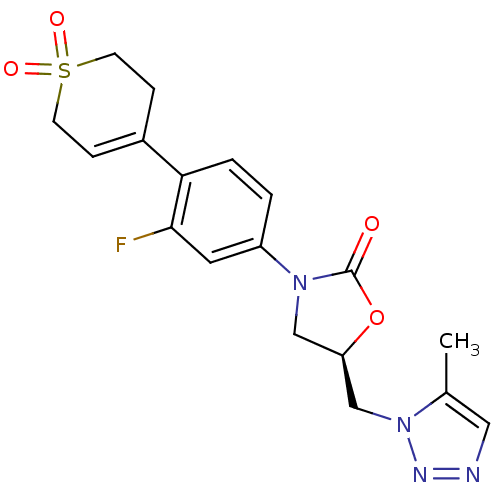 ((R)-3-[4-(1,1-Dioxo-1,2,3,6-tetrahydro-1lambda*6*-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159375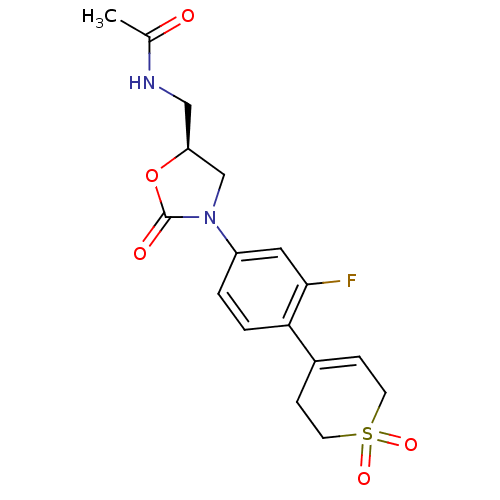 (CHEMBL179191 | N-{(S)-3-[4-(1,1-Dioxo-1,2,3,6-tetr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159378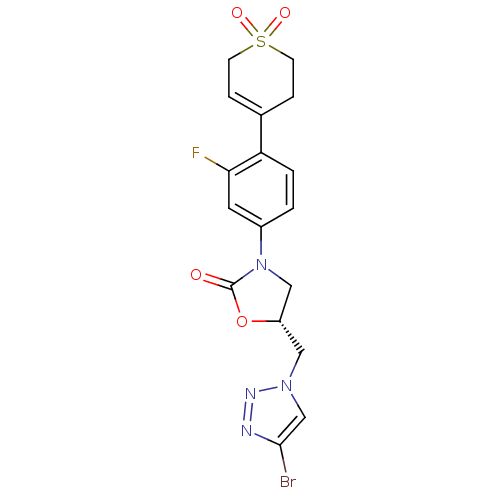 ((R)-5-(4-Bromo-[1,2,3]triazol-1-ylmethyl)-3-[4-(1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159386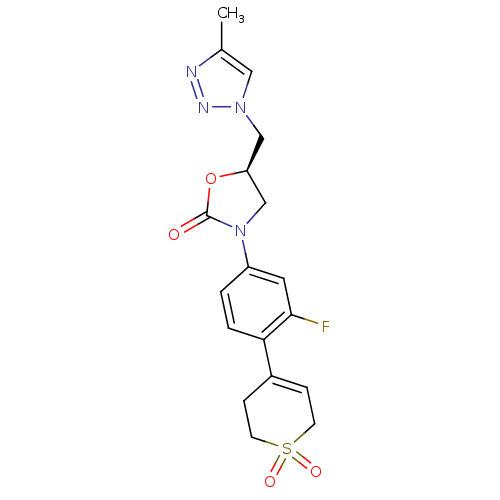 ((R)-3-[4-(1,1-Dioxo-1,2,3,6-tetrahydro-1lambda*6*-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159374 ((R)-3-[4-(1,1-Dioxo-1,2,3,6-tetrahydro-1lambda*6*-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159371 (CHEMBL360090 | Phosphoric acid 1-{(R)-3-[4-(1,1-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159373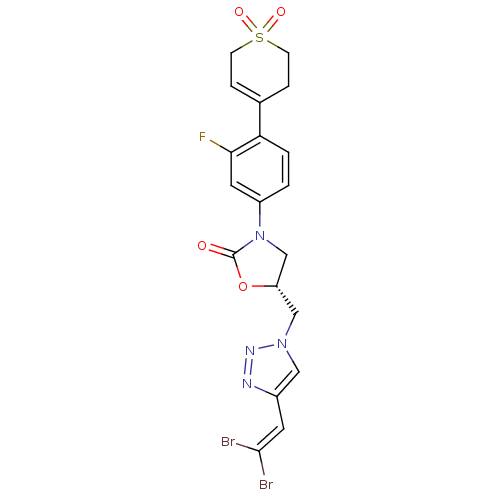 ((R)-5-[4-(2,2-Dibromo-vinyl)-[1,2,3]triazol-1-ylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159377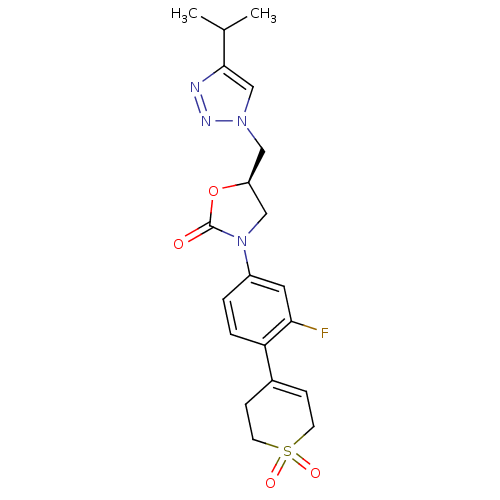 ((R)-3-[4-(1,1-Dioxo-1,2,3,6-tetrahydro-1lambda*6*-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159384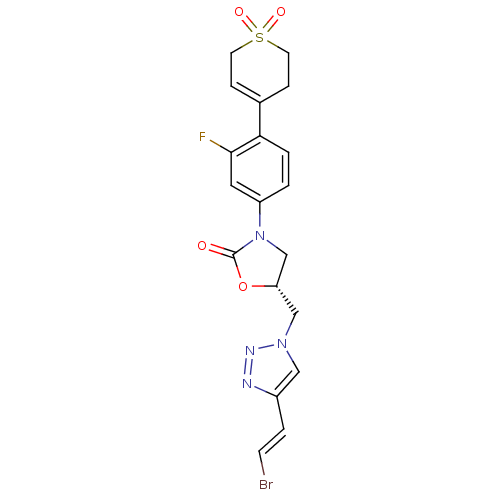 ((R)-5-[4-((E)-2-Bromo-vinyl)-[1,2,3]triazol-1-ylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159387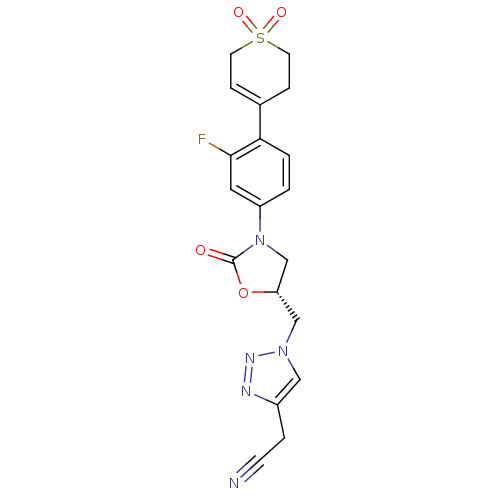 ((1-{(R)-3-[4-(1,1-Dioxo-1,2,3,6-tetrahydro-1lambda...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159383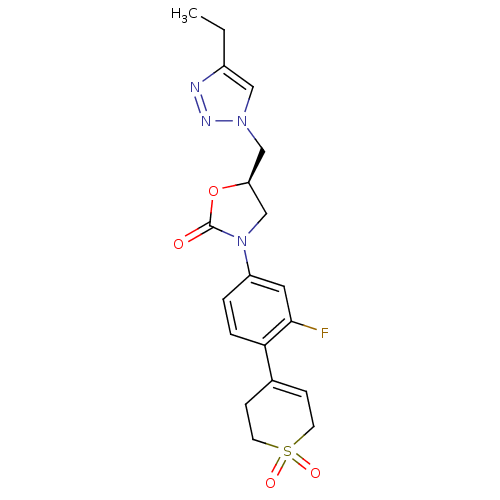 ((R)-3-[4-(1,1-Dioxo-1,2,3,6-tetrahydro-1lambda*6*-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159376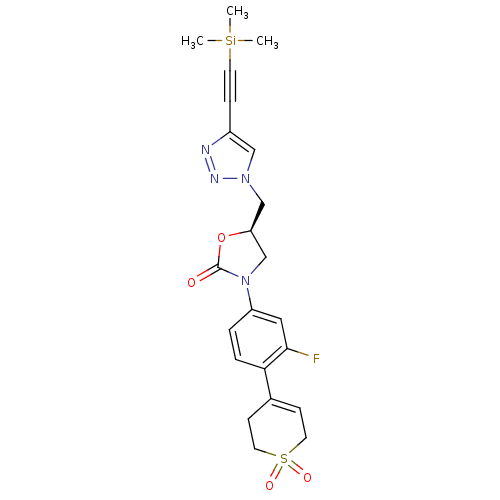 ((R)-3-[4-(1,1-Dioxo-1,2,3,6-tetrahydro-1lambda*6*-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50159381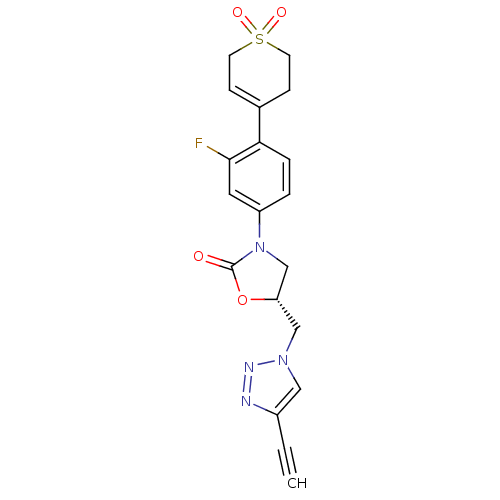 ((R)-3-[4-(1,1-Dioxo-1,2,3,6-tetrahydro-1lambda*6*-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Discovery Curated by ChEMBL | Assay Description Binding affinity for human liver monoamine oxidase A | J Med Chem 48: 499-506 (2005) Article DOI: 10.1021/jm0400810 BindingDB Entry DOI: 10.7270/Q2VH5NBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121542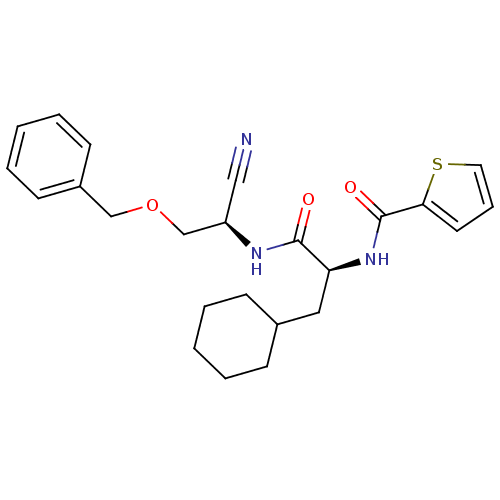 (CHEMBL155560 | Thiophene-2-carboxylic acid {1-[(be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121562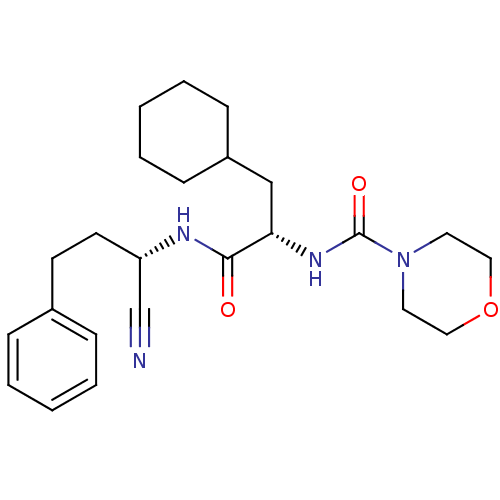 (CHEMBL153239 | Morpholine-4-carboxylic acid [1-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121554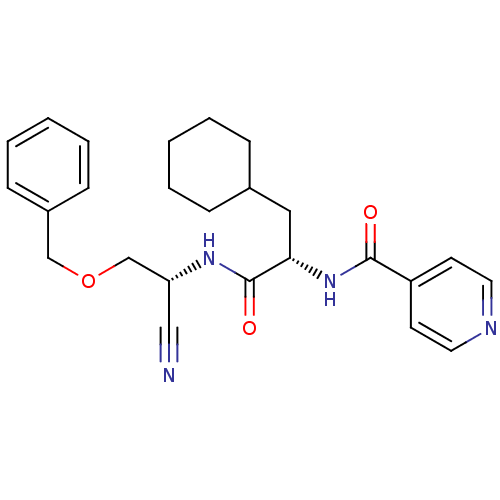 (CHEMBL356155 | N-{1-[(Benzyloxymethyl-cyano-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121549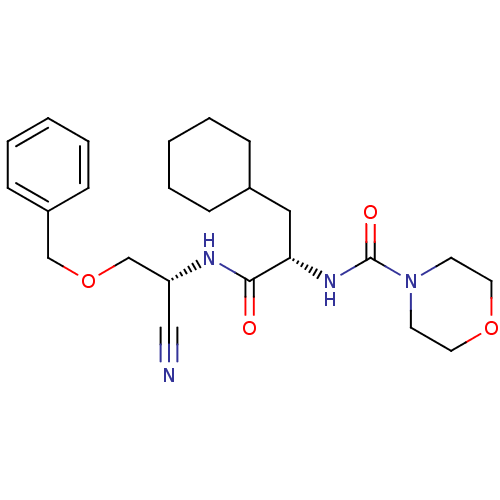 (CHEMBL347111 | Morpholine-4-carboxylic acid {1-[(b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121575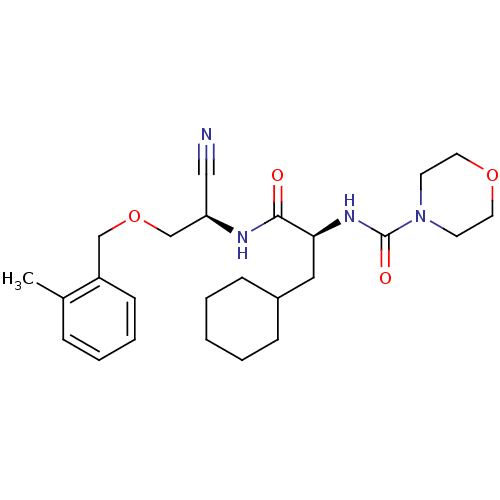 (CHEMBL356442 | Morpholine-4-carboxylic acid (1-{[c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121581 (CHEMBL150253 | Morpholine-4-carboxylic acid (1-{[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121569 (CHEMBL153783 | Morpholine-4-carboxylic acid {1-[(b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121543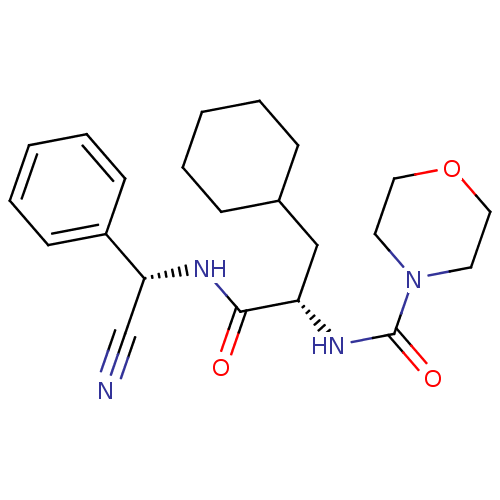 (CHEMBL435440 | Morpholine-4-carboxylic acid {1-[(c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121561 (CHEMBL356167 | Morpholine-4-carboxylic acid (1-{[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121545 (CHEMBL149523 | Morpholine-4-carboxylic acid (1-{[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121544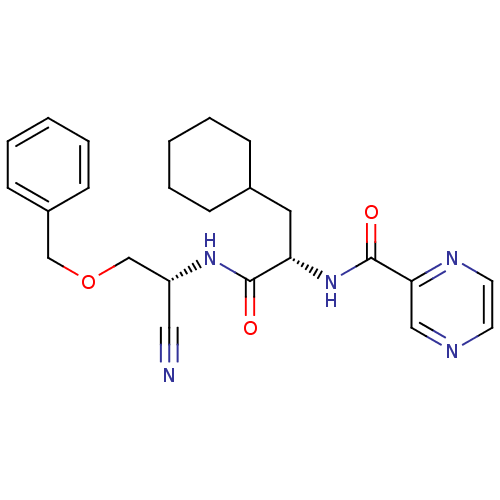 (CHEMBL153248 | Pyrazine-2-carboxylic acid {1-[(ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121558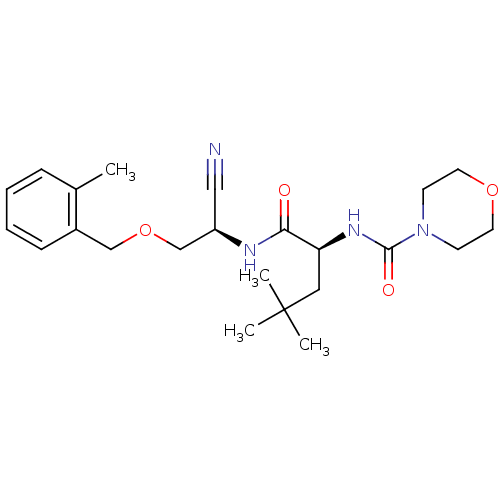 (CHEMBL152940 | Morpholine-4-carboxylic acid (1-{[c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121572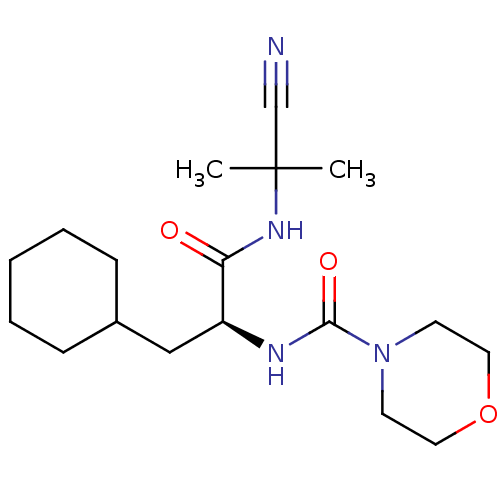 (CHEMBL150358 | Morpholine-4-carboxylic acid {1-[(c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121541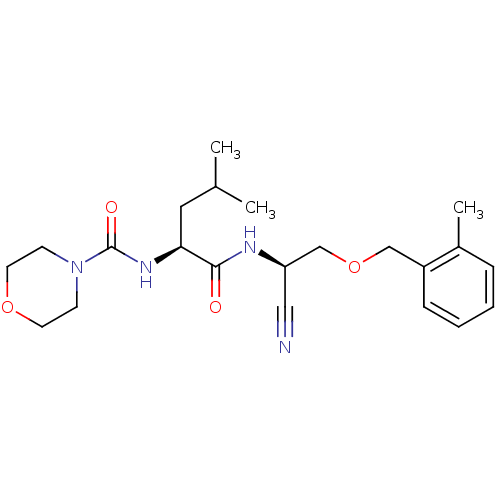 (CHEMBL346448 | Morpholine-4-carboxylic acid (1-{[c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121548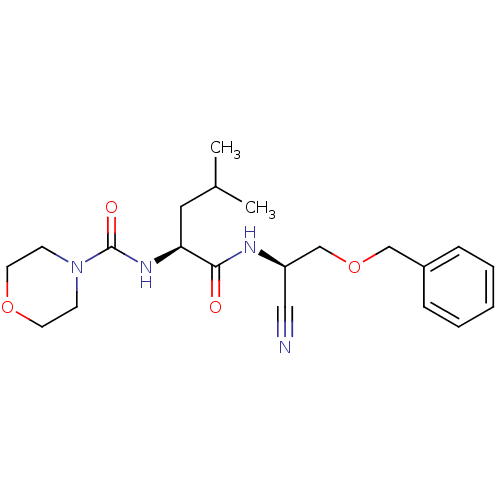 (CHEMBL153813 | MORPHOLINE-4-CARBOXYLIC ACID [1S-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121576 (CHEMBL153018 | Furan-2-carboxylic acid {1-[(benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121570 (CHEMBL348679 | Morpholine-4-carboxylic acid [1-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121557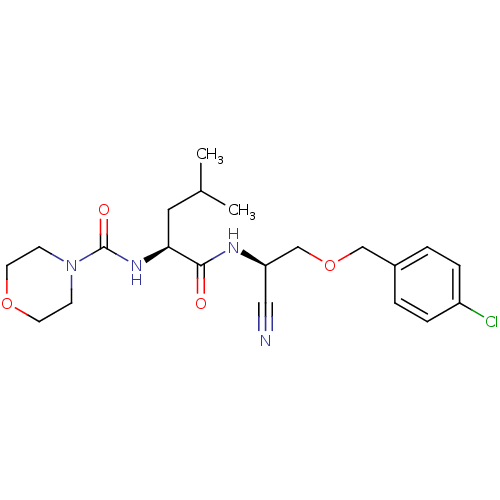 (CHEMBL151642 | Morpholine-4-carboxylic acid (1-{[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121551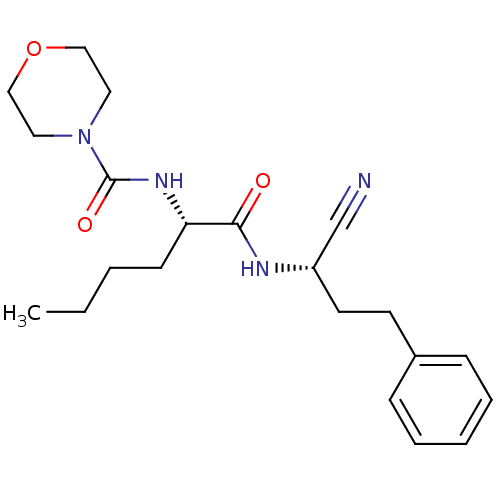 (CHEMBL150574 | Morpholine-4-carboxylic acid [1-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121564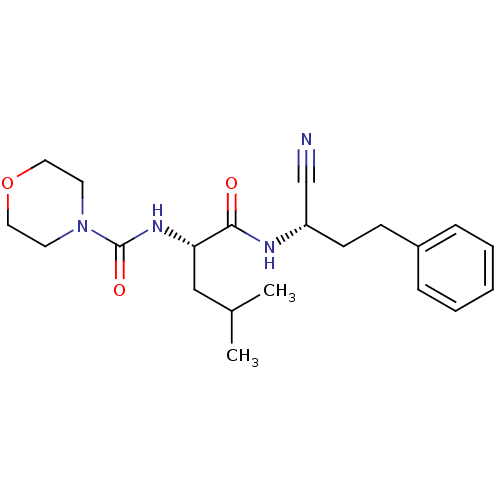 (CHEMBL153678 | Morpholine-4-carboxylic acid [1-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121546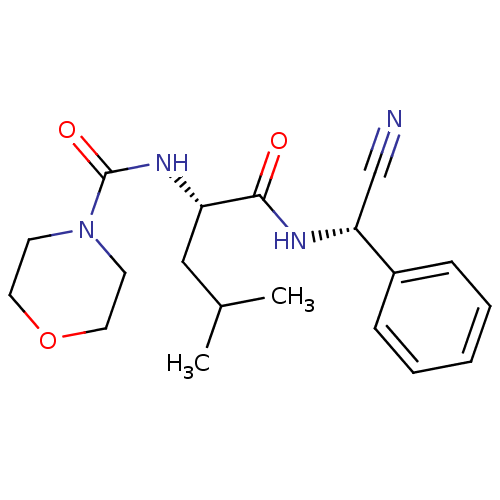 (CHEMBL347745 | Morpholine-4-carboxylic acid {1-[(c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121566 (CHEMBL153319 | Morpholine-4-carboxylic acid (1-{[c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121578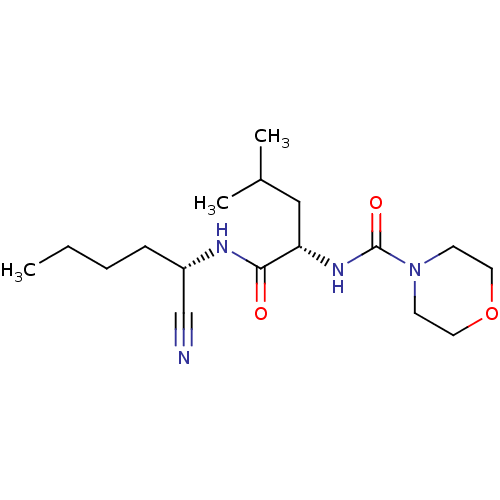 (CHEMBL435245 | Morpholine-4-carboxylic acid [1-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121550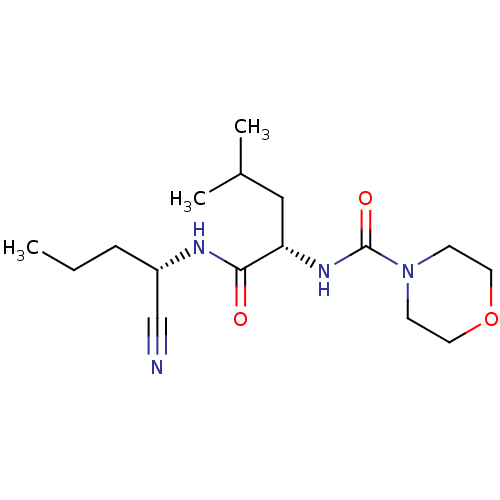 (CHEMBL153166 | Morpholine-4-carboxylic acid [1-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121560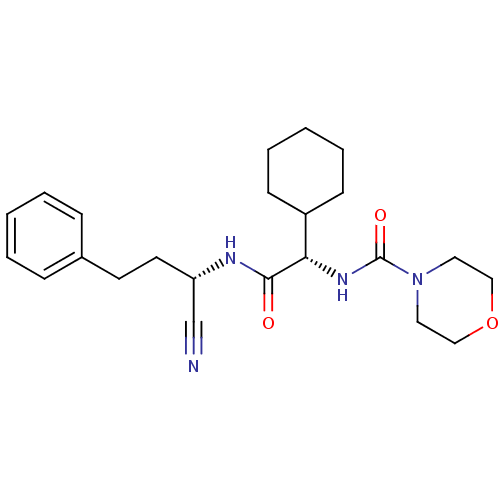 (CHEMBL345502 | Morpholine-4-carboxylic acid [(1-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121565 (CHEMBL345267 | Morpholine-4-carboxylic acid {1-[(c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121574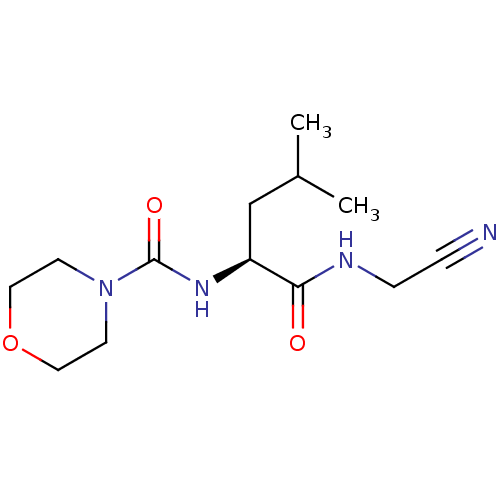 (CHEMBL153370 | Morpholine-4-carboxylic acid [1-(cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121547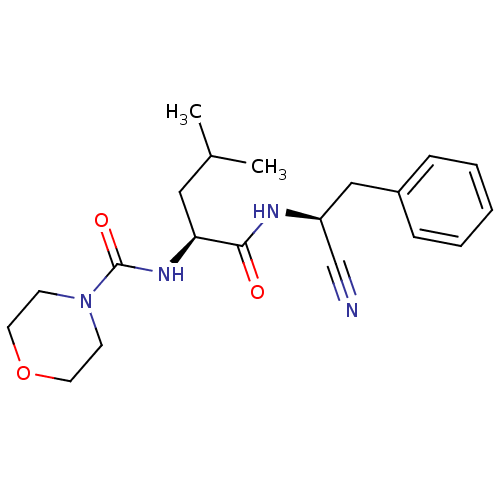 (CHEMBL154808 | Morpholine-4-carboxylic acid {1-[(b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121563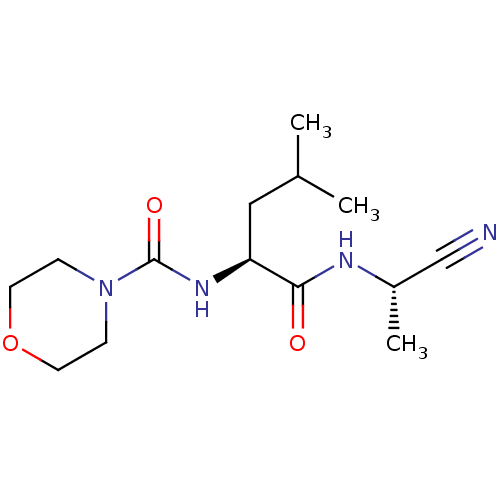 (CHEMBL149736 | Morpholine-4-carboxylic acid {1-[(c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121552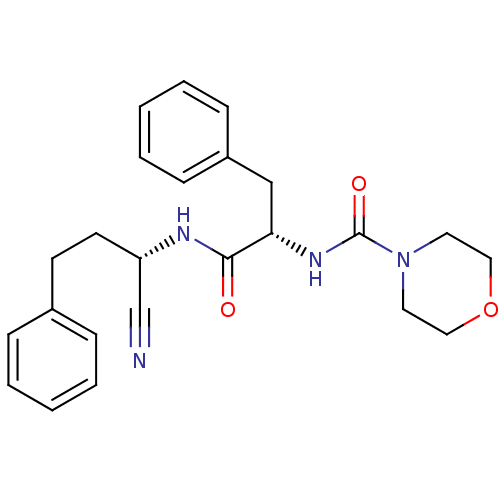 (CHEMBL356669 | Morpholine-4-carboxylic acid [1-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121573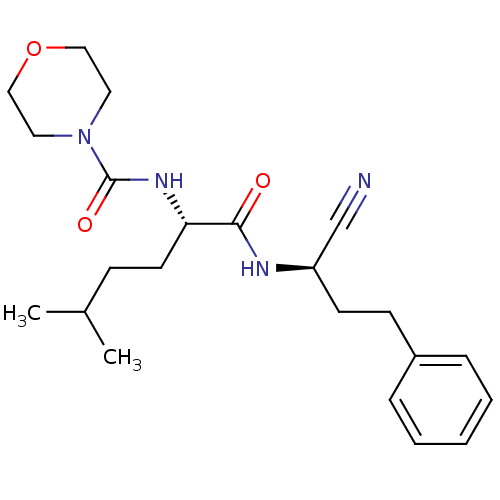 (CHEMBL358829 | Morpholine-4-carboxylic acid [1-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 387 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 87 total ) | Next | Last >> |