

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxytocin receptor (RAT) | BDBM50406692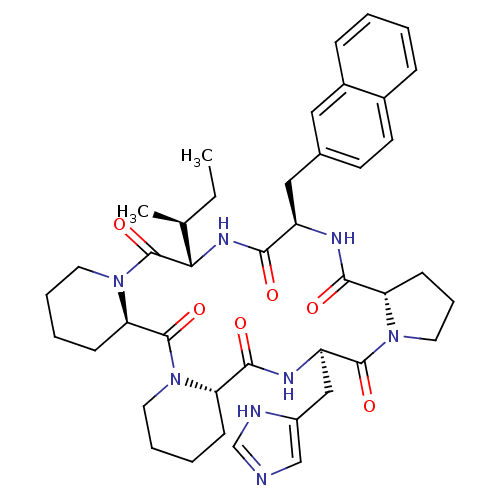 (CHEMBL2112249) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81894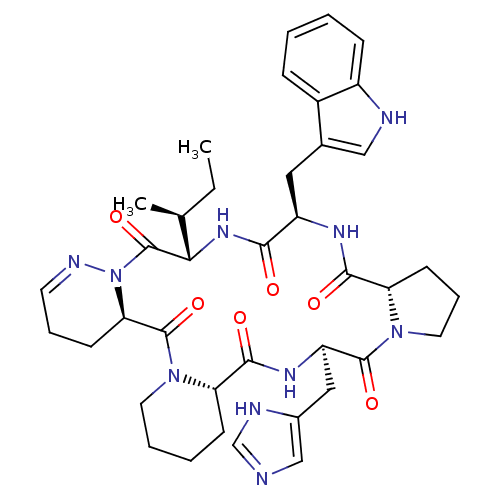 (Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to inhibit AVP stimulation of adenylate cyclase activity in the rat kidney medulla (AVP-V2) receptor | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM81894 (Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM81894 (Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from near-term pregnant rhesus m... | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50001311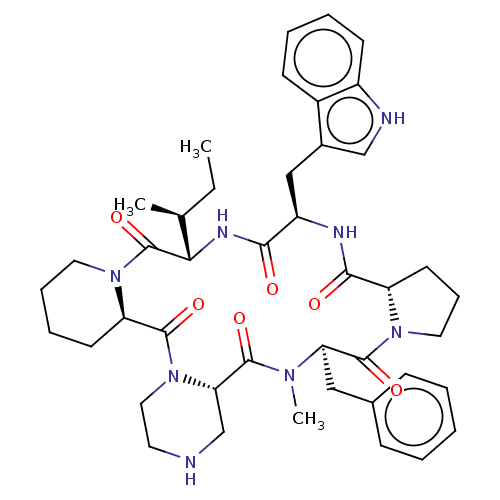 (24-benzyl-16-(1H-3-indolylmethyl)-25-methyl-13-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50013636 (22-benzyl-13-(1H-3-indolylmethyl)-23-methyl-10-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50001311 (24-benzyl-16-(1H-3-indolylmethyl)-25-methyl-13-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50001311 (24-benzyl-16-(1H-3-indolylmethyl)-25-methyl-13-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from near-term pregnant rhesus m... | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50406692 (CHEMBL2112249) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50406692 (CHEMBL2112249) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50013628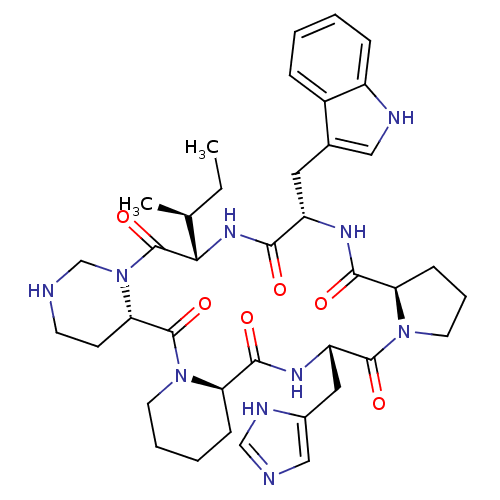 (24-(1H-5-imidazolylmethyl)-16-(1H-3-indolylmethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50013630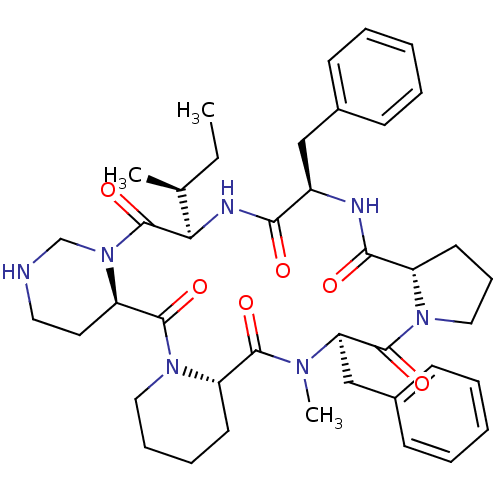 (16,24-dibenzyl-25-methyl-13-[1-methyl-(1S)-propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50001307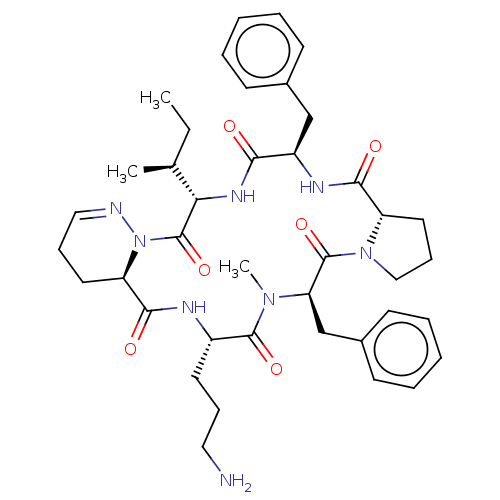 (18-(3-Amino-propyl)-6,15-dibenzyl-3-sec-butyl-16-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]arginine vasopressin binding to AVP-V2 site in rat kidney medulla. | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50368435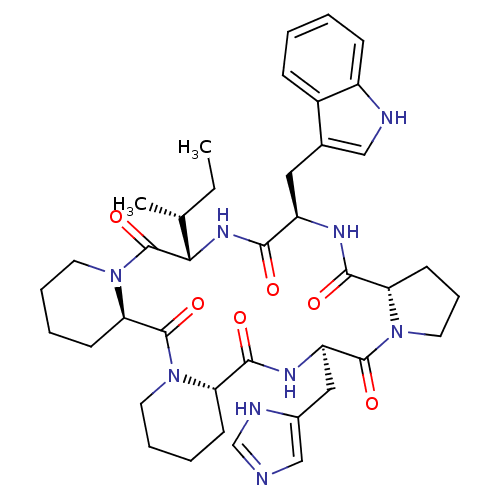 (CHEMBL1790937) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50001309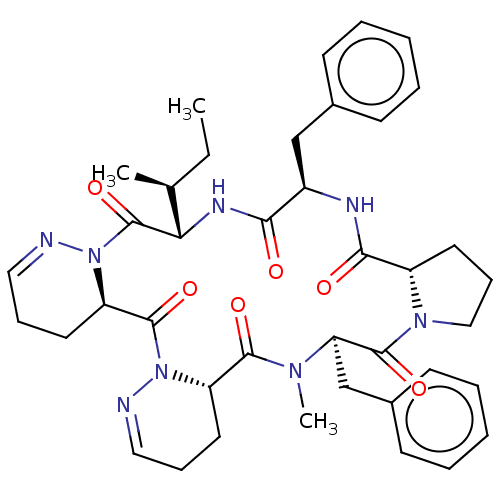 ((cyclo-[L-propyl-D-phenylalanyl-L-isoleucyl-D-dehy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards rat uterine receptor was determined using [3H]oxytocin as radioligand | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50001309 ((cyclo-[L-propyl-D-phenylalanyl-L-isoleucyl-D-dehy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50013634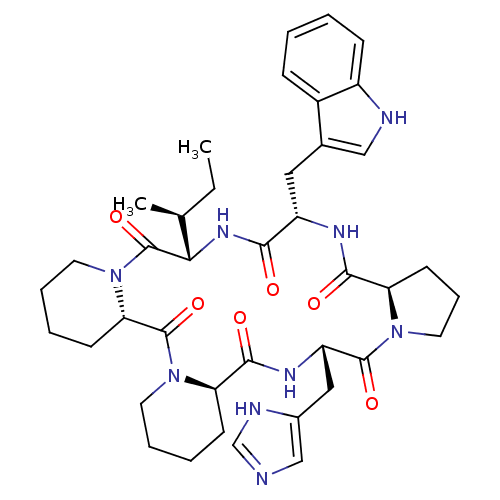 (24-(1H-5-imidazolylmethyl)-16-(1H-3-indolylmethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50013638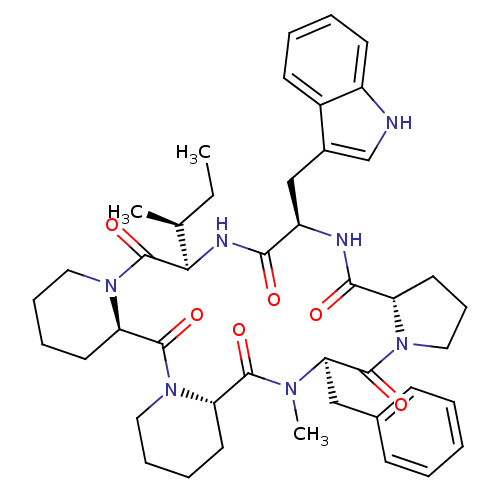 (24-benzyl-16-(1H-3-indolylmethyl)-25-methyl-13-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50368435 (CHEMBL1790937) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50368435 (CHEMBL1790937) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from near-term pregnant rhesus m... | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50013633 (18-(3-Amino-propyl)-6,15-dibenzyl-3-sec-butyl-16-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50001307 (18-(3-Amino-propyl)-6,15-dibenzyl-3-sec-butyl-16-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50001307 (18-(3-Amino-propyl)-6,15-dibenzyl-3-sec-butyl-16-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from near-term pregnant rhesus m... | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50013640 (18-(3-Amino-propyl)-15-benzyl-3-sec-butyl-6-(1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50013640 (18-(3-Amino-propyl)-15-benzyl-3-sec-butyl-6-(1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-arginine vasopressin binding to rat kidney medulla Vasopressin V2 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50013631 (24-benzyl-16-(4-ethoxybenzyl)-25-methyl-13-[1-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50013631 (24-benzyl-16-(4-ethoxybenzyl)-25-methyl-13-[1-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-arginine vasopressin binding to rat kidney medulla Vasopressin V2 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50013632 (16,24-dibenzyl-13-[1-methyl-(1S)-propyl]perhydrodi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50013633 (18-(3-Amino-propyl)-6,15-dibenzyl-3-sec-butyl-16-m...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]arginine vasopressin binding to rat liver Vasopressin V1 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50013636 (22-benzyl-13-(1H-3-indolylmethyl)-23-methyl-10-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-arginine vasopressin binding to rat kidney medulla Vasopressin V2 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50013638 (24-benzyl-16-(1H-3-indolylmethyl)-25-methyl-13-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-arginine vasopressin binding to rat kidney medulla Vasopressin V2 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50013635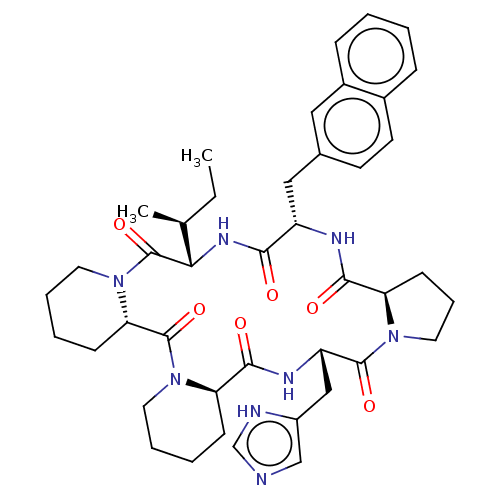 (24-(1H-5-imidazolylmethyl)-13-[1-methyl-(1S)-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50013635 (24-(1H-5-imidazolylmethyl)-13-[1-methyl-(1S)-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-arginine vasopressin binding to rat kidney medulla Vasopressin V2 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50013628 (24-(1H-5-imidazolylmethyl)-16-(1H-3-indolylmethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-arginine vasopressin binding to rat kidney medulla Vasopressin V2 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50001309 ((cyclo-[L-propyl-D-phenylalanyl-L-isoleucyl-D-dehy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50013630 (16,24-dibenzyl-25-methyl-13-[1-methyl-(1S)-propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-arginine vasopressin binding to rat kidney medulla Vasopressin V2 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50013641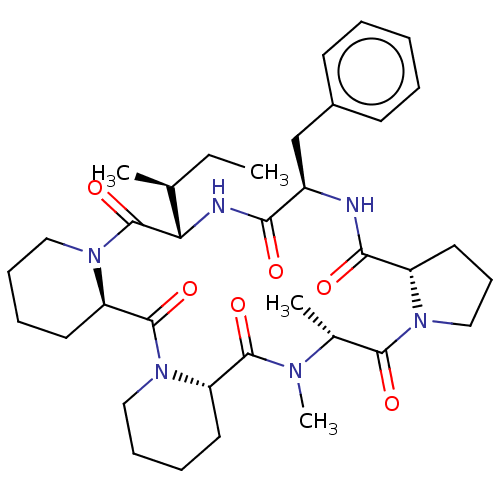 (16-benzyl-24,25-dimethyl-13-[1-methyl-(1S)-propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50001309 ((cyclo-[L-propyl-D-phenylalanyl-L-isoleucyl-D-dehy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]arginine vasopressin binding to rat kidney medulla Vasopressin V2 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50013633 (18-(3-Amino-propyl)-6,15-dibenzyl-3-sec-butyl-16-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-arginine vasopressin binding to rat kidney medulla Vasopressin V2 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50001309 ((cyclo-[L-propyl-D-phenylalanyl-L-isoleucyl-D-dehy...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]arginine vasopressin binding to rat liver Vasopressin V1 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50013635 (24-(1H-5-imidazolylmethyl)-13-[1-methyl-(1S)-propy...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]arginine vasopressin binding to rat liver Vasopressin V1 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50001309 ((cyclo-[L-propyl-D-phenylalanyl-L-isoleucyl-D-dehy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards rat kidney V2 receptor was determined using [3H]arginine vasopressin as radioligand | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50013637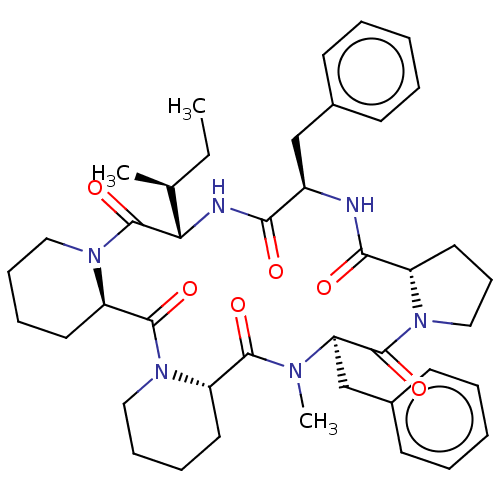 (16,24-dibenzyl-25-methyl-13-[1-methyl-(1S)-propyl]...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]arginine vasopressin binding to rat liver Vasopressin V1 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50013630 (16,24-dibenzyl-25-methyl-13-[1-methyl-(1S)-propyl]...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-arginine vasopressin binding to rat kidney medulla Vasopressin V2 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50013640 (18-(3-Amino-propyl)-15-benzyl-3-sec-butyl-6-(1H-in...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]arginine vasopressin binding to rat liver Vasopressin V1 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50013628 (24-(1H-5-imidazolylmethyl)-16-(1H-3-indolylmethyl)...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]arginine vasopressin binding to rat liver Vasopressin V1 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50001302 (14,22-dibenzyl-23-methyl-11-[1-methyl-(1S)-propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50013637 (16,24-dibenzyl-25-methyl-13-[1-methyl-(1S)-propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50013632 (16,24-dibenzyl-13-[1-methyl-(1S)-propyl]perhydrodi...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]- oxytocin binding to rat uterine Oxytocin receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50013637 (16,24-dibenzyl-25-methyl-13-[1-methyl-(1S)-propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-arginine vasopressin binding to rat kidney medulla Vasopressin V2 receptor | J Med Chem 33: 1843-5 (1990) BindingDB Entry DOI: 10.7270/Q2JS9PDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 770 total ) | Next | Last >> |