 Found 81 hits with Last Name = 'gregory' and Initial = 'gb'
Found 81 hits with Last Name = 'gregory' and Initial = 'gb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226528
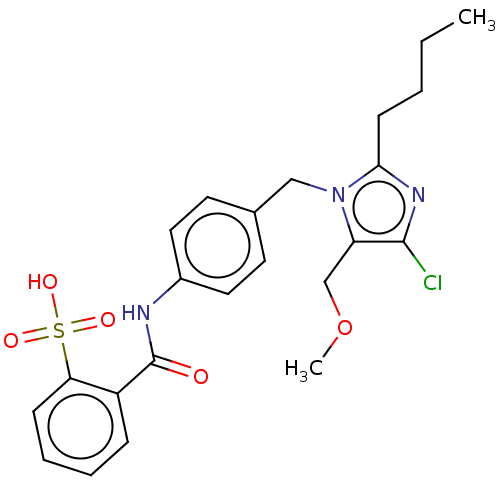
(CHEMBL284425)Show SMILES CCCCc1nc(Cl)c(COC)n1Cc1ccc(NC(=O)c2ccccc2S(O)(=O)=O)cc1 Show InChI InChI=1S/C23H26ClN3O5S/c1-3-4-9-21-26-22(24)19(15-32-2)27(21)14-16-10-12-17(13-11-16)25-23(28)18-7-5-6-8-20(18)33(29,30)31/h5-8,10-13H,3-4,9,14-15H2,1-2H3,(H,25,28)(H,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226522
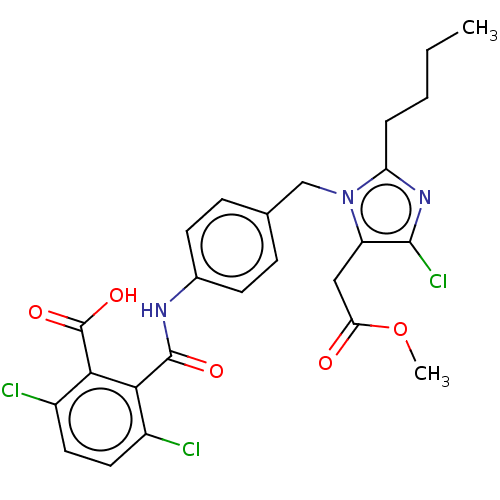
(CHEMBL1744348)Show SMILES C1CCC(CC1)NC1CCCCC1.CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2c(Cl)ccc(Cl)c2C(O)=O)cc1 Show InChI InChI=1S/C25H24Cl3N3O5/c1-3-4-5-19-30-23(28)18(12-20(32)36-2)31(19)13-14-6-8-15(9-7-14)29-24(33)21-16(26)10-11-17(27)22(21)25(34)35/h6-11H,3-5,12-13H2,1-2H3,(H,29,33)(H,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226421
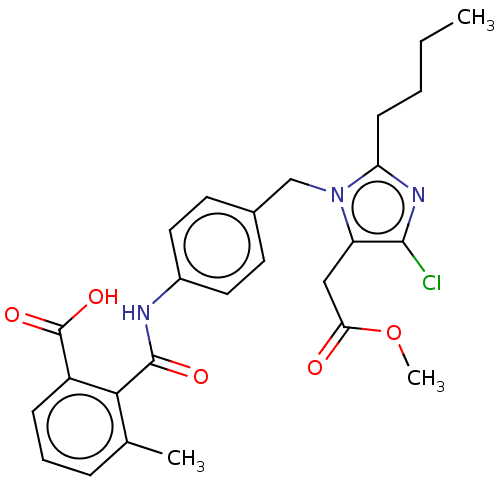
(CHEMBL281589)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2c(C)cccc2C(O)=O)cc1 Show InChI InChI=1S/C26H28ClN3O5/c1-4-5-9-21-29-24(27)20(14-22(31)35-3)30(21)15-17-10-12-18(13-11-17)28-25(32)23-16(2)7-6-8-19(23)26(33)34/h6-8,10-13H,4-5,9,14-15H2,1-3H3,(H,28,32)(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226467
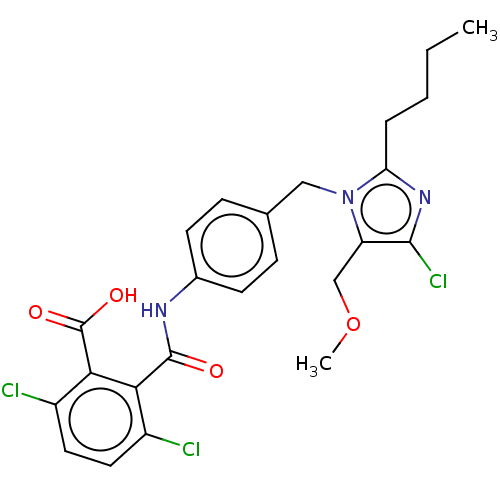
(CHEMBL276956)Show SMILES CCCCc1nc(Cl)c(COC)n1Cc1ccc(NC(=O)c2c(Cl)ccc(Cl)c2C(O)=O)cc1 Show InChI InChI=1S/C24H24Cl3N3O4/c1-3-4-5-19-29-22(27)18(13-34-2)30(19)12-14-6-8-15(9-7-14)28-23(31)20-16(25)10-11-17(26)21(20)24(32)33/h6-11H,3-5,12-13H2,1-2H3,(H,28,31)(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50087583
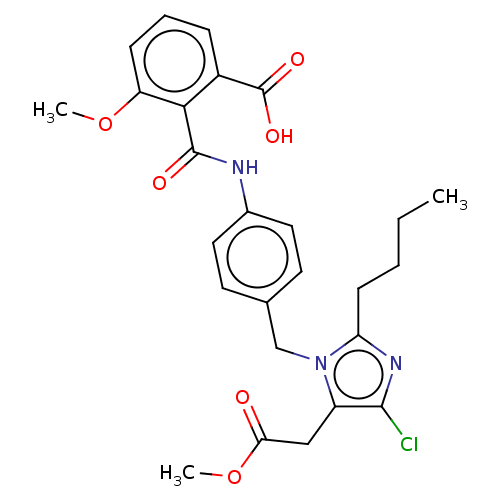
(CHEMBL25031)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2c(OC)cccc2C(O)=O)cc1 Show InChI InChI=1S/C27H28N2O6/c30-24-16-15-23(29(24)18-21-8-4-1-5-9-21)26(33)28-22(27(34)35)17-20-13-11-19(12-14-20)7-3-2-6-10-25(31)32/h1,4-5,8-9,11-14,22-23H,2,6,10,15-18H2,(H,28,33)(H,31,32)(H,34,35)/t22-,23-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50015270
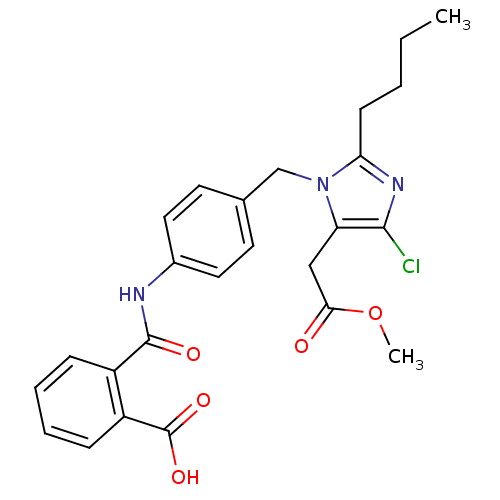
(CHEMBL262958 | N-[4-(2-Butyl-4-chloro-5-methoxycar...)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2ccccc2C(O)=O)cc1 Show InChI InChI=1S/C25H26ClN3O5/c1-3-4-9-21-28-23(26)20(14-22(30)34-2)29(21)15-16-10-12-17(13-11-16)27-24(31)18-7-5-6-8-19(18)25(32)33/h5-8,10-13H,3-4,9,14-15H2,1-2H3,(H,27,31)(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226428
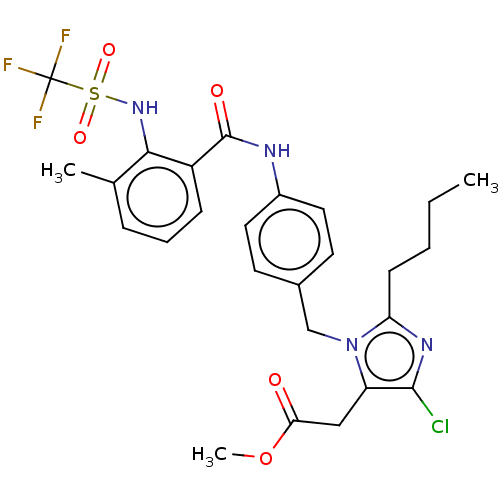
(CHEMBL28011)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2cccc(C)c2NS(=O)(=O)C(F)(F)F)cc1 Show InChI InChI=1S/C26H28ClF3N4O5S/c1-4-5-9-21-32-24(27)20(14-22(35)39-3)34(21)15-17-10-12-18(13-11-17)31-25(36)19-8-6-7-16(2)23(19)33-40(37,38)26(28,29)30/h6-8,10-13,33H,4-5,9,14-15H2,1-3H3,(H,31,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226532
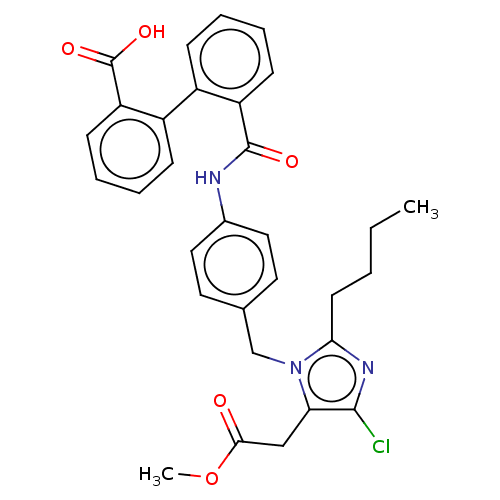
(CHEMBL282491)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2ccccc2-c2ccccc2C(O)=O)cc1 Show InChI InChI=1S/C31H30ClN3O5/c1-3-4-13-27-34-29(32)26(18-28(36)40-2)35(27)19-20-14-16-21(17-15-20)33-30(37)24-11-7-5-9-22(24)23-10-6-8-12-25(23)31(38)39/h5-12,14-17H,3-4,13,18-19H2,1-2H3,(H,33,37)(H,38,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226525

(CHEMBL26652)Show SMILES CCCCCc1nccn1Cc1ccc(NC(=O)c2ccccc2C(O)=O)cc1 Show InChI InChI=1S/C23H25N3O3/c1-2-3-4-9-21-24-14-15-26(21)16-17-10-12-18(13-11-17)25-22(27)19-7-5-6-8-20(19)23(28)29/h5-8,10-15H,2-4,9,16H2,1H3,(H,25,27)(H,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin ll receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50023646
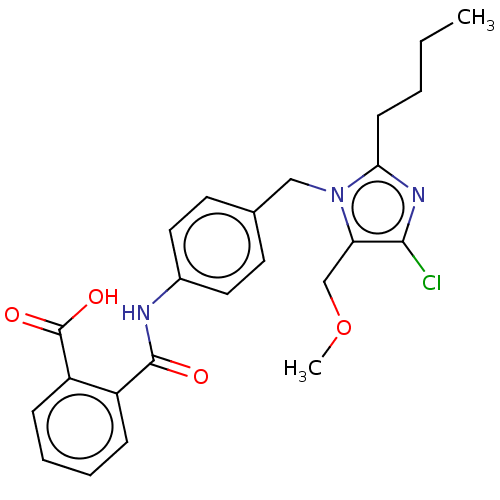
(CHEMBL24364)Show SMILES CCCCc1nc(Cl)c(COC)n1Cc1ccc(NC(=O)c2ccccc2C(O)=O)cc1 Show InChI InChI=1S/C15H16N3O10P/c19-11-5-13(28-12(11)7-27-29(24,25)26)17-6-10(14(20)16-15(17)21)8-1-3-9(4-2-8)18(22)23/h1-4,6,11-13,19H,5,7H2,(H,16,20,21)(H2,24,25,26)/p-2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50087614
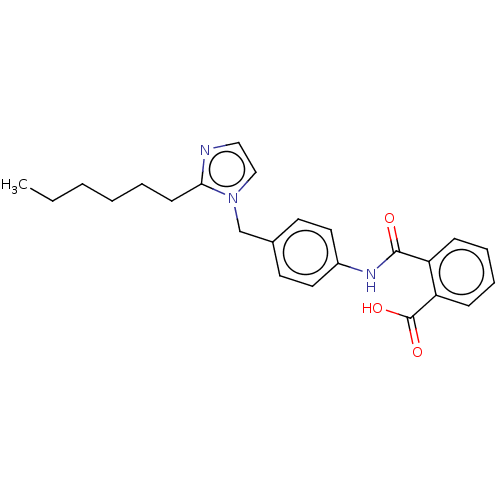
(CHEMBL27981)Show SMILES CCCCCCc1nccn1Cc1ccc(NC(=O)c2ccccc2C(O)=O)cc1 Show InChI InChI=1S/C22H22N2O6/c25-20-9-7-17(24(20)12-14-4-2-1-3-5-14)21(26)23-16(22(27)28)10-15-6-8-18-19(11-15)30-13-29-18/h1-6,8,11,16-17H,7,9-10,12-13H2,(H,23,26)(H,27,28)/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin ll receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226523
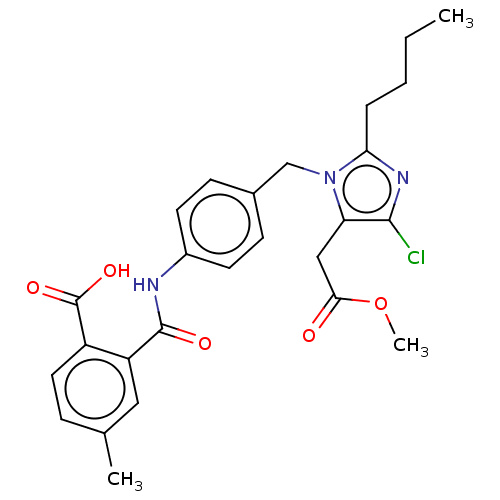
(CHEMBL283071)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2cc(C)ccc2C(O)=O)cc1 Show InChI InChI=1S/C26H28ClN3O5/c1-4-5-6-22-29-24(27)21(14-23(31)35-3)30(22)15-17-8-10-18(11-9-17)28-25(32)20-13-16(2)7-12-19(20)26(33)34/h7-13H,4-6,14-15H2,1-3H3,(H,28,32)(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50108642
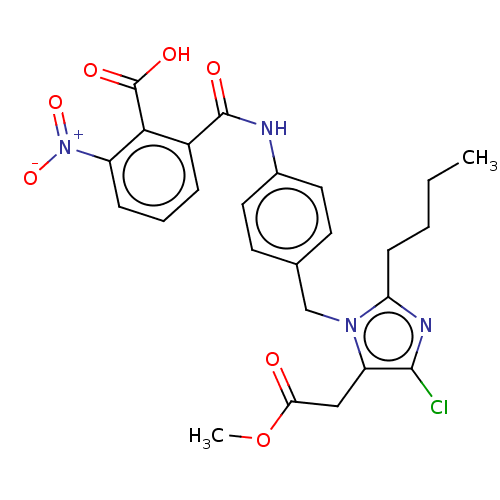
(CHEMBL27725)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2cccc(c2C(O)=O)[N+]([O-])=O)cc1 Show InChI InChI=1S/C25H25ClN4O7/c1-3-4-8-20-28-23(26)19(13-21(31)37-2)29(20)14-15-9-11-16(12-10-15)27-24(32)17-6-5-7-18(30(35)36)22(17)25(33)34/h5-7,9-12H,3-4,8,13-14H2,1-2H3,(H,27,32)(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50222263

(CHEMBL27839)Show SMILES CCCCc1nc(CC(=O)OC)c(Cl)n1Cc1ccc(NC(=O)c2ccccc2C(O)=O)cc1 Show InChI InChI=1S/C25H26ClN3O5/c1-3-4-9-21-28-20(14-22(30)34-2)23(26)29(21)15-16-10-12-17(13-11-16)27-24(31)18-7-5-6-8-19(18)25(32)33/h5-8,10-13H,3-4,9,14-15H2,1-2H3,(H,27,31)(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226721
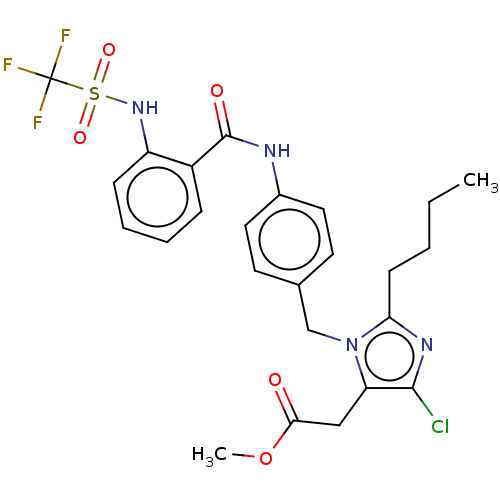
(CHEMBL24512)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2ccccc2NS(=O)(=O)C(F)(F)F)cc1 Show InChI InChI=1S/C25H26ClF3N4O5S/c1-3-4-9-21-31-23(26)20(14-22(34)38-2)33(21)15-16-10-12-17(13-11-16)30-24(35)18-7-5-6-8-19(18)32-39(36,37)25(27,28)29/h5-8,10-13,32H,3-4,9,14-15H2,1-2H3,(H,30,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226425
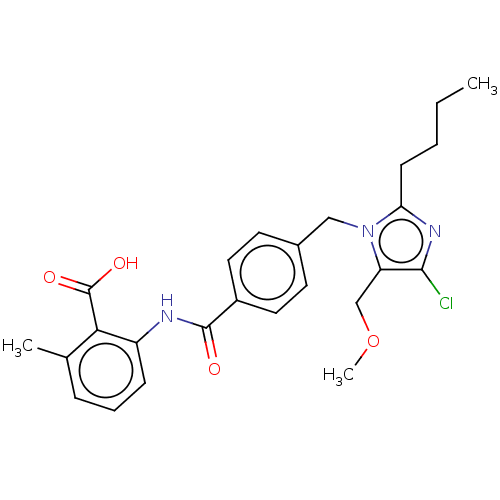
(CHEMBL28391)Show SMILES CCCCc1nc(Cl)c(COC)n1Cc1ccc(cc1)C(=O)Nc1cccc(C)c1C(O)=O Show InChI InChI=1S/C25H28ClN3O4/c1-4-5-9-21-28-23(26)20(15-33-3)29(21)14-17-10-12-18(13-11-17)24(30)27-19-8-6-7-16(2)22(19)25(31)32/h6-8,10-13H,4-5,9,14-15H2,1-3H3,(H,27,30)(H,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226524
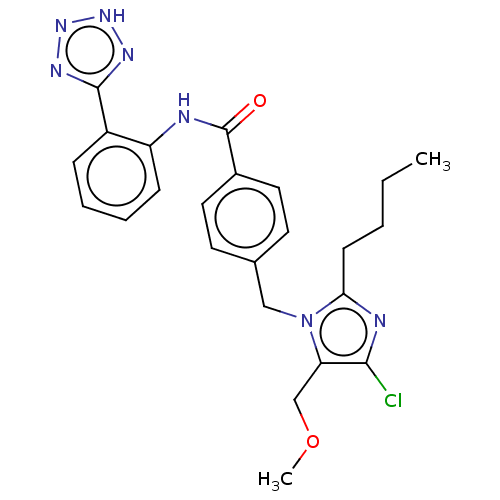
(CHEMBL280868)Show SMILES CCCCc1nc(Cl)c(COC)n1Cc1ccc(cc1)C(=O)Nc1ccccc1-c1nn[nH]n1 Show InChI InChI=1S/C24H26ClN7O2/c1-3-4-9-21-27-22(25)20(15-34-2)32(21)14-16-10-12-17(13-11-16)24(33)26-19-8-6-5-7-18(19)23-28-30-31-29-23/h5-8,10-13H,3-4,9,14-15H2,1-2H3,(H,26,33)(H,28,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50087586
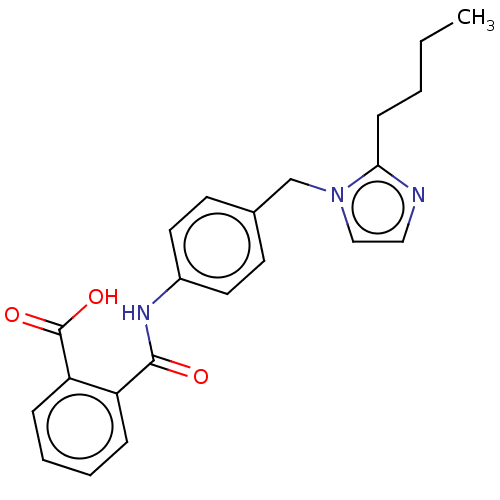
(CHEMBL418756)Show InChI InChI=1S/C27H36N2O7/c1-27(2,3)36-26(35)29-15-7-9-21(18-29)17-23(30)28-22(25(33)34)16-20-13-11-19(12-14-20)8-5-4-6-10-24(31)32/h11-14,21-22H,4,6-7,9-10,15-18H2,1-3H3,(H,28,30)(H,31,32)(H,33,34)/t21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin ll receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226530
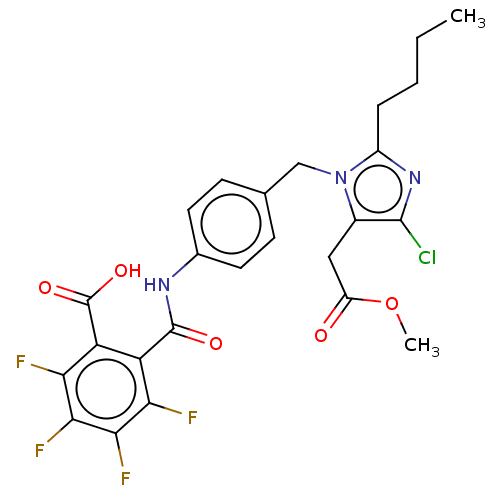
(CHEMBL24934)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2c(F)c(F)c(F)c(F)c2C(O)=O)cc1 Show InChI InChI=1S/C25H22ClF4N3O5/c1-3-4-5-15-32-23(26)14(10-16(34)38-2)33(15)11-12-6-8-13(9-7-12)31-24(35)17-18(25(36)37)20(28)22(30)21(29)19(17)27/h6-9H,3-5,10-11H2,1-2H3,(H,31,35)(H,36,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50087593
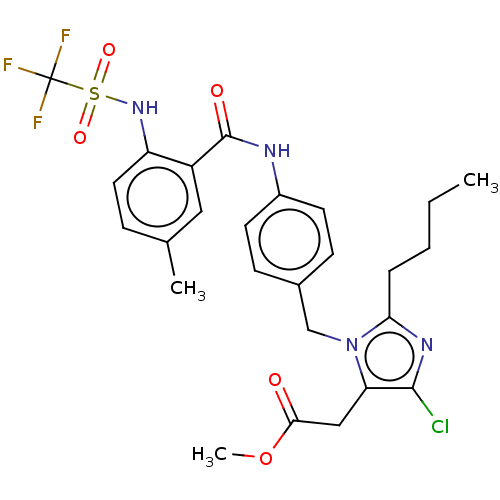
(CHEMBL286028)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2cc(C)ccc2NS(=O)(=O)C(F)(F)F)cc1 Show InChI InChI=1S/C24H32N4O3/c1-2-3-4-5-7-17-10-12-18(13-11-17)15-21(23(30)31)28-22(29)16-19-8-6-9-20(14-19)27-24(25)26/h6,8-14,21H,2-5,7,15-16H2,1H3,(H,28,29)(H,30,31)(H4,25,26,27)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226538
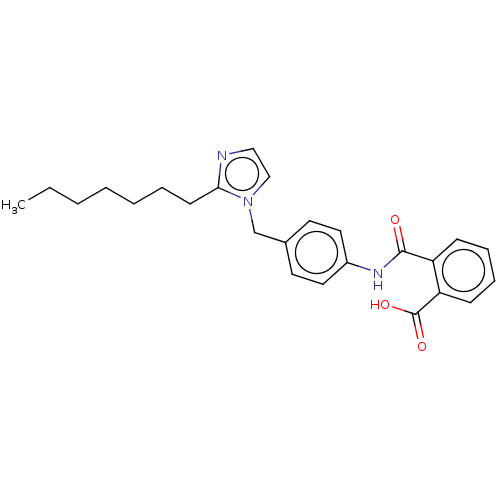
(CHEMBL281514)Show SMILES CCCCCCCc1nccn1Cc1ccc(NC(=O)c2ccccc2C(O)=O)cc1 Show InChI InChI=1S/C25H29N3O3/c1-2-3-4-5-6-11-23-26-16-17-28(23)18-19-12-14-20(15-13-19)27-24(29)21-9-7-8-10-22(21)25(30)31/h7-10,12-17H,2-6,11,18H2,1H3,(H,27,29)(H,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50218592
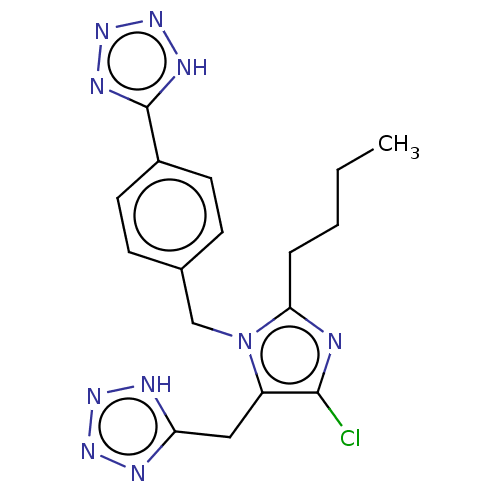
(CHEMBL282283)Show SMILES CCCCc1nc(Cl)c(Cc2nnn[nH]2)n1Cc1ccc(cc1)-c1nnn[nH]1 Show InChI InChI=1S/C17H19ClN10/c1-2-3-4-15-19-16(18)13(9-14-20-24-25-21-14)28(15)10-11-5-7-12(8-6-11)17-22-26-27-23-17/h5-8H,2-4,9-10H2,1H3,(H,20,21,24,25)(H,22,23,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226474
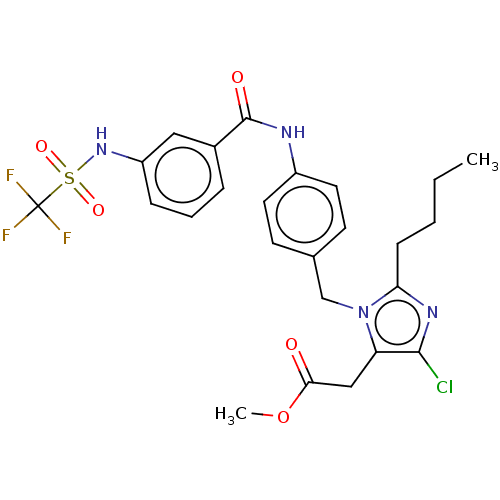
(CHEMBL26727)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2cccc(NS(=O)(=O)C(F)(F)F)c2)cc1 Show InChI InChI=1S/C25H26ClF3N4O5S/c1-3-4-8-21-31-23(26)20(14-22(34)38-2)33(21)15-16-9-11-18(12-10-16)30-24(35)17-6-5-7-19(13-17)32-39(36,37)25(27,28)29/h5-7,9-13,32H,3-4,8,14-15H2,1-2H3,(H,30,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50087580
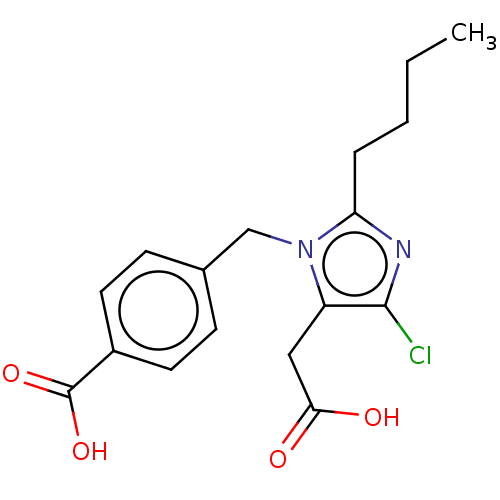
(CHEMBL25686)Show InChI InChI=1S/C22H28N2O4/c1-2-3-4-12-28-19-10-8-16(9-11-19)14-20(22(26)27)24-21(25)15-17-6-5-7-18(23)13-17/h5-11,13,20H,2-4,12,14-15,23H2,1H3,(H,24,25)(H,26,27)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226515
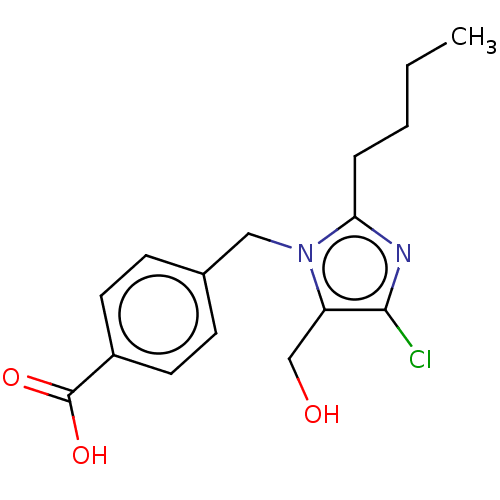
(CHEMBL416384)Show InChI InChI=1S/C16H19ClN2O3/c1-2-3-4-14-18-15(17)13(10-20)19(14)9-11-5-7-12(8-6-11)16(21)22/h5-8,20H,2-4,9-10H2,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226514

(CHEMBL283983)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NS(=O)(=O)C(F)(F)F)cc1 Show InChI InChI=1S/C18H21ClF3N3O4S/c1-3-4-5-15-23-17(19)14(10-16(26)29-2)25(15)11-12-6-8-13(9-7-12)24-30(27,28)18(20,21)22/h6-9,24H,3-5,10-11H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226473
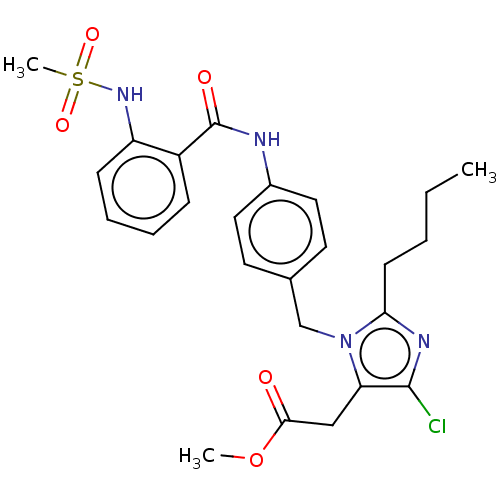
(CHEMBL26712)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2ccccc2NS(C)(=O)=O)cc1 Show InChI InChI=1S/C25H29ClN4O5S/c1-4-5-10-22-28-24(26)21(15-23(31)35-2)30(22)16-17-11-13-18(14-12-17)27-25(32)19-8-6-7-9-20(19)29-36(3,33)34/h6-9,11-14,29H,4-5,10,15-16H2,1-3H3,(H,27,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50223499
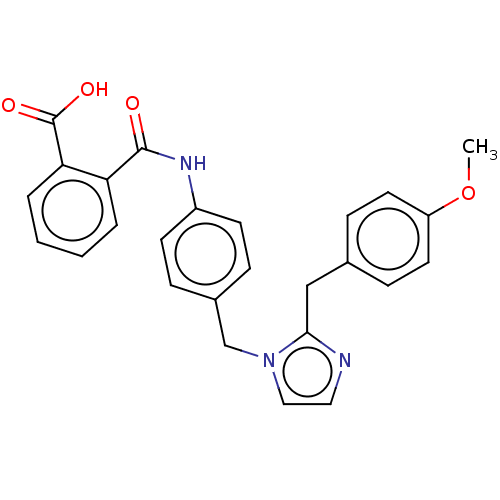
(CHEMBL25701)Show SMILES COc1ccc(Cc2nccn2Cc2ccc(NC(=O)c3ccccc3C(O)=O)cc2)cc1 Show InChI InChI=1S/C26H23N3O4/c1-33-21-12-8-18(9-13-21)16-24-27-14-15-29(24)17-19-6-10-20(11-7-19)28-25(30)22-4-2-3-5-23(22)26(31)32/h2-15H,16-17H2,1H3,(H,28,30)(H,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin ll receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226468
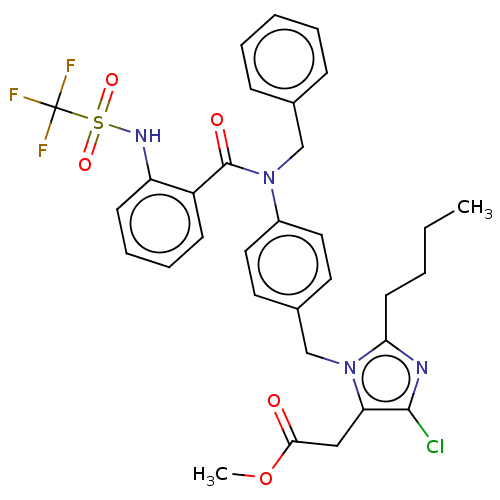
(CHEMBL26673)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(cc1)N(Cc1ccccc1)C(=O)c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C32H32ClF3N4O5S/c1-3-4-14-28-37-30(33)27(19-29(41)45-2)40(28)21-23-15-17-24(18-16-23)39(20-22-10-6-5-7-11-22)31(42)25-12-8-9-13-26(25)38-46(43,44)32(34,35)36/h5-13,15-18,38H,3-4,14,19-21H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226722
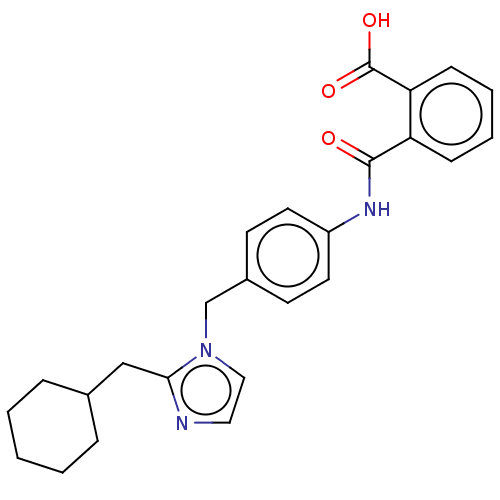
(CHEMBL281690)Show SMILES OC(=O)c1ccccc1C(=O)Nc1ccc(Cn2ccnc2CC2CCCCC2)cc1 Show InChI InChI=1S/C25H27N3O3/c29-24(21-8-4-5-9-22(21)25(30)31)27-20-12-10-19(11-13-20)17-28-15-14-26-23(28)16-18-6-2-1-3-7-18/h4-5,8-15,18H,1-3,6-7,16-17H2,(H,27,29)(H,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin ll receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50087591
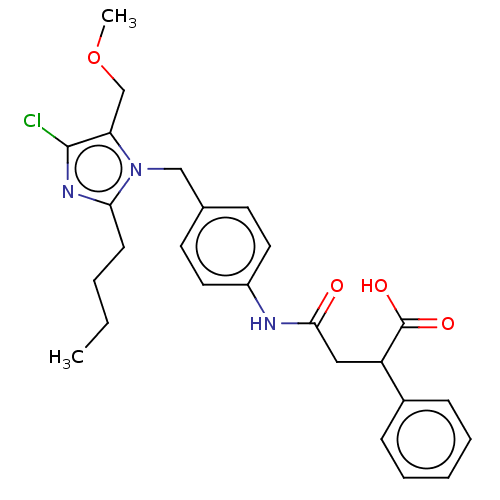
(CHEMBL26765)Show SMILES CCCCc1nc(Cl)c(COC)n1Cc1ccc(NC(=O)CC(C(O)=O)c2ccccc2)cc1 Show InChI InChI=1S/C20H21Cl2NO5/c1-19(12-20(19,21)22)18(28)23-15(17(26)27)11-14-9-7-13(8-10-14)5-3-2-4-6-16(24)25/h7-10,15H,2,4,6,11-12H2,1H3,(H,23,28)(H,24,25)(H,26,27)/t15-,19?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226540

(CHEMBL280193)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(cc1)N1C(=O)c2ccccc2C1=O Show InChI InChI=1S/C25H24ClN3O4/c1-3-4-9-21-27-23(26)20(14-22(30)33-2)28(21)15-16-10-12-17(13-11-16)29-24(31)18-7-5-6-8-19(18)25(29)32/h5-8,10-13H,3-4,9,14-15H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226478
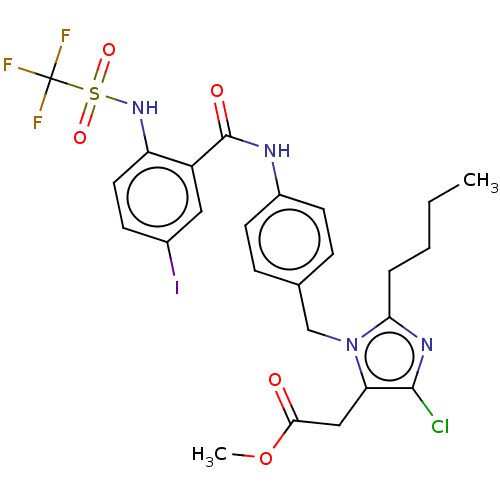
(CHEMBL27977)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2cc(I)ccc2NS(=O)(=O)C(F)(F)F)cc1 Show InChI InChI=1S/C25H25ClF3IN4O5S/c1-3-4-5-21-32-23(26)20(13-22(35)39-2)34(21)14-15-6-9-17(10-7-15)31-24(36)18-12-16(30)8-11-19(18)33-40(37,38)25(27,28)29/h6-12,33H,3-5,13-14H2,1-2H3,(H,31,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226426
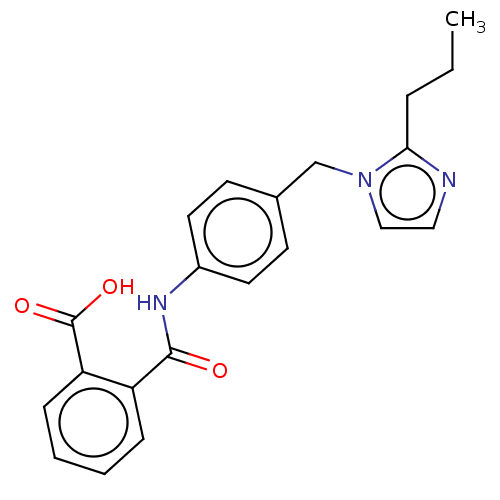
(CHEMBL285829)Show InChI InChI=1S/C21H21N3O3/c1-2-5-19-22-12-13-24(19)14-15-8-10-16(11-9-15)23-20(25)17-6-3-4-7-18(17)21(26)27/h3-4,6-13H,2,5,14H2,1H3,(H,23,25)(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin ll receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50087582
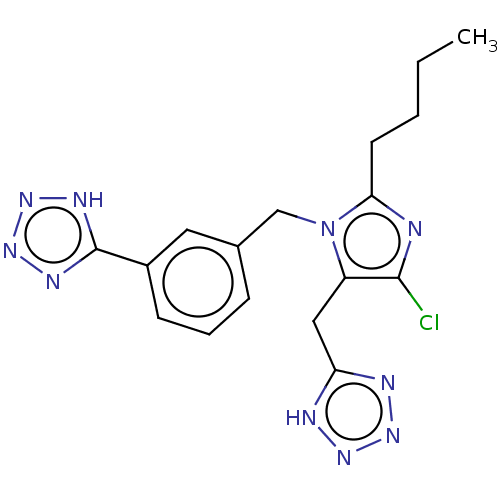
(CHEMBL281068)Show SMILES CCCCc1nc(Cl)c(Cc2nnn[nH]2)n1Cc1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C20H26N4O5S/c1-3-4-5-10-29-14-8-6-13(7-9-14)11-15(19(26)27)22-18(25)17(24-28-2)16-12-30-20(21)23-16/h6-9,12,15H,3-5,10-11H2,1-2H3,(H2,21,23)(H,22,25)(H,26,27)/b24-17+/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226534
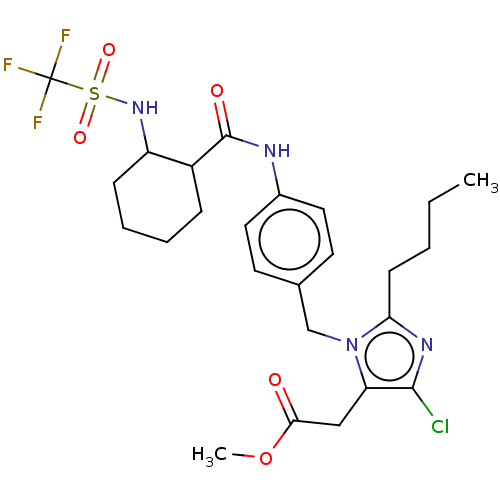
(CHEMBL282307)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)C2CCCCC2NS(=O)(=O)C(F)(F)F)cc1 Show InChI InChI=1S/C25H32ClF3N4O5S/c1-3-4-9-21-31-23(26)20(14-22(34)38-2)33(21)15-16-10-12-17(13-11-16)30-24(35)18-7-5-6-8-19(18)32-39(36,37)25(27,28)29/h10-13,18-19,32H,3-9,14-15H2,1-2H3,(H,30,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226534

(CHEMBL282307)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)C2CCCCC2NS(=O)(=O)C(F)(F)F)cc1 Show InChI InChI=1S/C25H32ClF3N4O5S/c1-3-4-9-21-31-23(26)20(14-22(34)38-2)33(21)15-16-10-12-17(13-11-16)30-24(35)18-7-5-6-8-19(18)32-39(36,37)25(27,28)29/h10-13,18-19,32H,3-9,14-15H2,1-2H3,(H,30,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226529
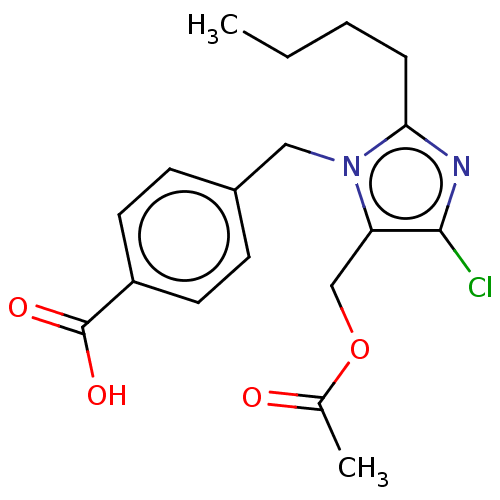
(CHEMBL27454)Show InChI InChI=1S/C18H21ClN2O4/c1-3-4-5-16-20-17(19)15(11-25-12(2)22)21(16)10-13-6-8-14(9-7-13)18(23)24/h6-9H,3-5,10-11H2,1-2H3,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226513
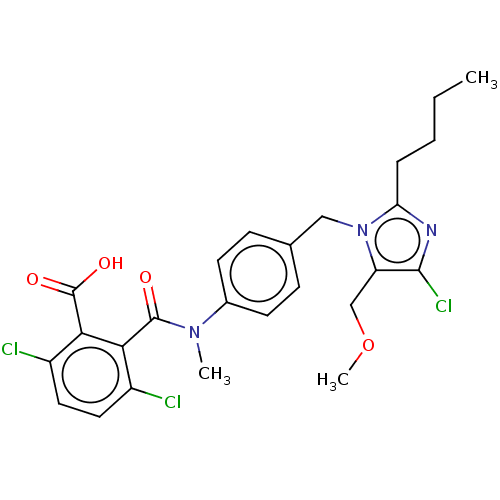
(CHEMBL25677)Show SMILES CCCCc1nc(Cl)c(COC)n1Cc1ccc(cc1)N(C)C(=O)c1c(Cl)ccc(Cl)c1C(O)=O Show InChI InChI=1S/C25H26Cl3N3O4/c1-4-5-6-20-29-23(28)19(14-35-3)31(20)13-15-7-9-16(10-8-15)30(2)24(32)21-17(26)11-12-18(27)22(21)25(33)34/h7-12H,4-6,13-14H2,1-3H3,(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226477
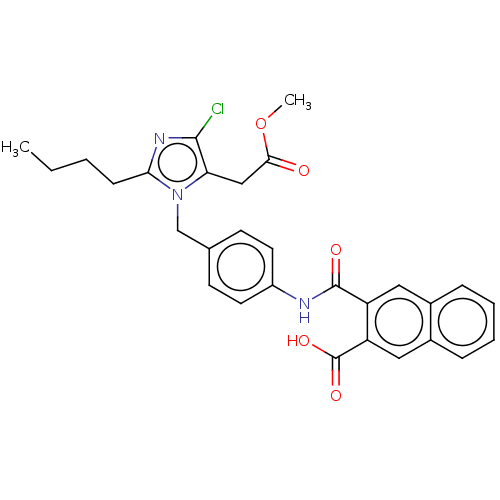
(CHEMBL281761)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2cc3ccccc3cc2C(O)=O)cc1 Show InChI InChI=1S/C29H28ClN3O5/c1-3-4-9-25-32-27(30)24(16-26(34)38-2)33(25)17-18-10-12-21(13-11-18)31-28(35)22-14-19-7-5-6-8-20(19)15-23(22)29(36)37/h5-8,10-15H,3-4,9,16-17H2,1-2H3,(H,31,35)(H,36,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226519
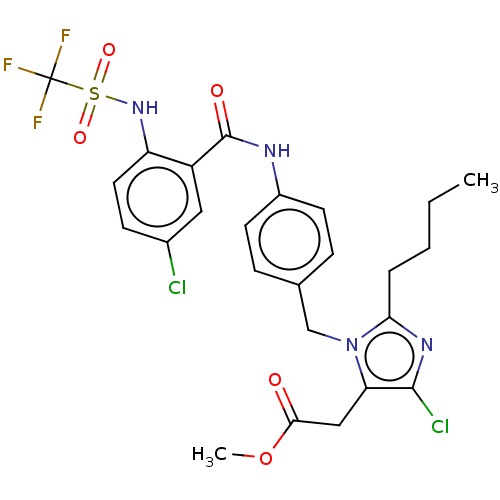
(CHEMBL26935)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2cc(Cl)ccc2NS(=O)(=O)C(F)(F)F)cc1 Show InChI InChI=1S/C25H25Cl2F3N4O5S/c1-3-4-5-21-32-23(27)20(13-22(35)39-2)34(21)14-15-6-9-17(10-7-15)31-24(36)18-12-16(26)8-11-19(18)33-40(37,38)25(28,29)30/h6-12,33H,3-5,13-14H2,1-2H3,(H,31,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226471
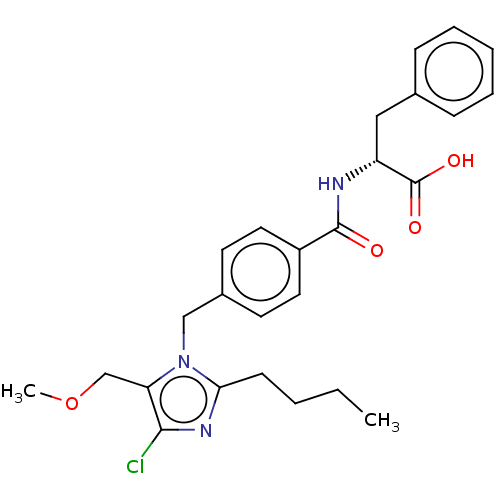
(CHEMBL25171)Show SMILES CCCCc1nc(Cl)c(COC)n1Cc1ccc(cc1)C(=O)N[C@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C26H30ClN3O4/c1-3-4-10-23-29-24(27)22(17-34-2)30(23)16-19-11-13-20(14-12-19)25(31)28-21(26(32)33)15-18-8-6-5-7-9-18/h5-9,11-14,21H,3-4,10,15-17H2,1-2H3,(H,28,31)(H,32,33)/t21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226470
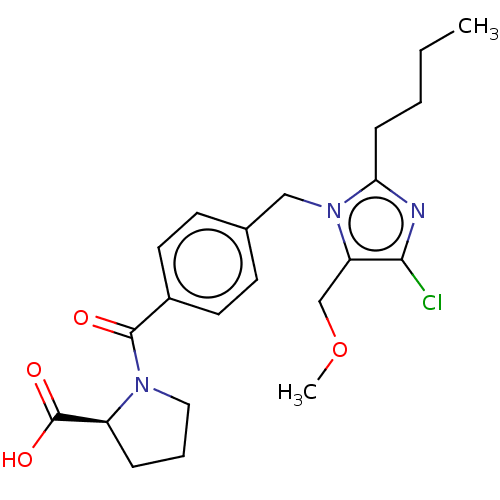
(CHEMBL28013)Show SMILES CCCCc1nc(Cl)c(COC)n1Cc1ccc(cc1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C22H28ClN3O4/c1-3-4-7-19-24-20(23)18(14-30-2)26(19)13-15-8-10-16(11-9-15)21(27)25-12-5-6-17(25)22(28)29/h8-11,17H,3-7,12-14H2,1-2H3,(H,28,29)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226531
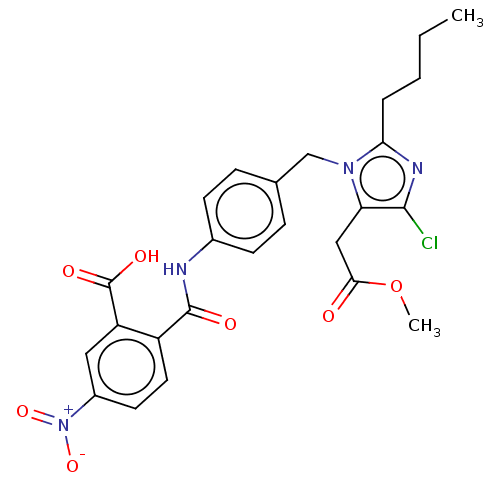
(CHEMBL28425)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2ccc(cc2C(O)=O)[N+]([O-])=O)cc1 Show InChI InChI=1S/C25H25ClN4O7/c1-3-4-5-21-28-23(26)20(13-22(31)37-2)29(21)14-15-6-8-16(9-7-15)27-24(32)18-11-10-17(30(35)36)12-19(18)25(33)34/h6-12H,3-5,13-14H2,1-2H3,(H,27,32)(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50087590
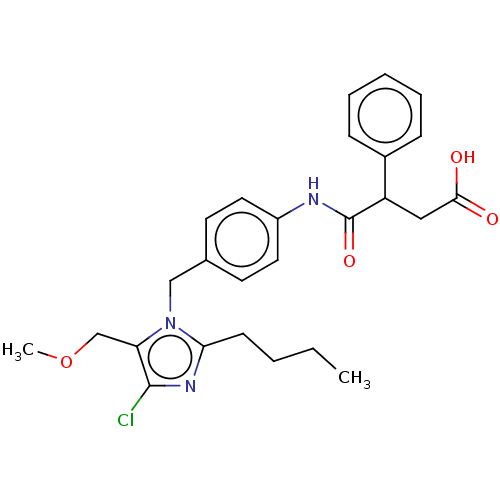
(CHEMBL282090)Show SMILES CCCCc1nc(Cl)c(COC)n1Cc1ccc(NC(=O)C(CC(O)=O)c2ccccc2)cc1 Show InChI InChI=1S/C22H24N4O6S/c1-31-18(27)7-5-3-4-6-14-8-10-15(11-9-14)12-16(21(29)30)24-20(28)19(26-32-2)17-13-33-22(23)25-17/h8-11,13,16H,3,5,7,12H2,1-2H3,(H2,23,25)(H,24,28)(H,29,30)/b26-19-/t16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50025538

(CHEMBL27401)Show SMILES CCCCc1nc(Cl)c(COC)n1Cc1ccc(cc1)C(=O)Nc1ccccc1C(O)=O Show InChI InChI=1S/C35H59N5O5/c1-21(2)16-17-36-33(43)26(10)37-30(42)19-25(9)28(20-27-14-12-11-13-15-27)38-34(44)32(24(7)8)40-35(45)31(23(5)6)39-29(41)18-22(3)4/h11-15,21-26,28,31-32H,16-20H2,1-10H3,(H,36,43)(H,37,42)(H,38,44)(H,39,41)(H,40,45)/t25-,26?,28?,31+,32?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226419
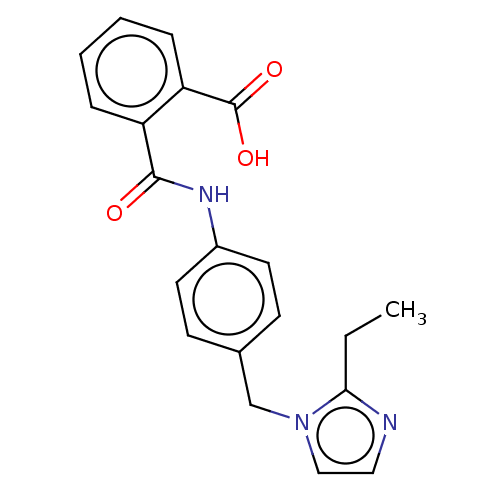
(CHEMBL24947)Show InChI InChI=1S/C20H19N3O3/c1-2-18-21-11-12-23(18)13-14-7-9-15(10-8-14)22-19(24)16-5-3-4-6-17(16)20(25)26/h3-12H,2,13H2,1H3,(H,22,24)(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin ll receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50223881

(CHEMBL284175)Show SMILES OC(=O)c1ccccc1C(=O)Nc1ccc(Cn2ccnc2CCc2ccccc2)cc1 Show InChI InChI=1S/C26H23N3O3/c30-25(22-8-4-5-9-23(22)26(31)32)28-21-13-10-20(11-14-21)18-29-17-16-27-24(29)15-12-19-6-2-1-3-7-19/h1-11,13-14,16-17H,12,15,18H2,(H,28,30)(H,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin ll receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226472
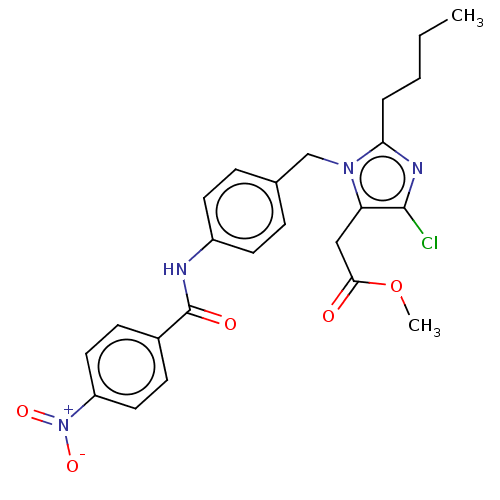
(CHEMBL24880)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(NC(=O)c2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C24H25ClN4O5/c1-3-4-5-21-27-23(25)20(14-22(30)34-2)28(21)15-16-6-10-18(11-7-16)26-24(31)17-8-12-19(13-9-17)29(32)33/h6-13H,3-5,14-15H2,1-2H3,(H,26,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50223882
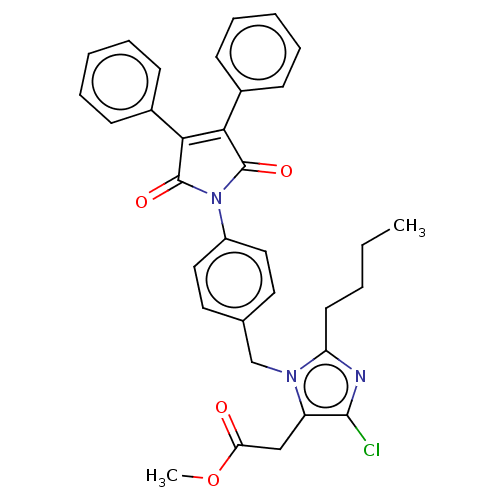
(CHEMBL434586)Show SMILES CCCCc1nc(Cl)c(CC(=O)OC)n1Cc1ccc(cc1)N1C(=O)C(=C(C1=O)c1ccccc1)c1ccccc1 |c:27| Show InChI InChI=1S/C33H30ClN3O4/c1-3-4-15-27-35-31(34)26(20-28(38)41-2)36(27)21-22-16-18-25(19-17-22)37-32(39)29(23-11-7-5-8-12-23)30(33(37)40)24-13-9-6-10-14-24/h5-14,16-19H,3-4,15,20-21H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data