 Found 267 hits with Last Name = 'gresh' and Initial = 'n'
Found 267 hits with Last Name = 'gresh' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mannose-6-phosphate isomerase
(Saccharomyces cerevisiae) | BDBM50481224

(CHEMBL611642)Show SMILES OC1O[C@H](CC(C(O)=O)C(O)=O)[C@@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C9H14O9/c10-4-3(1-2(7(13)14)8(15)16)18-9(17)6(12)5(4)11/h2-6,9-12,17H,1H2,(H,13,14)(H,15,16)/t3-,4-,5+,6+,9?/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae type I phosphomannose isomerase assessed as D-mannose 6-phosphate to D-fructose 6-phosphate isomerization at p... |
Bioorg Med Chem 17: 7100-7 (2009)
Article DOI: 10.1016/j.bmc.2009.09.005
BindingDB Entry DOI: 10.7270/Q2BR8W03 |
More data for this
Ligand-Target Pair | |
Mannose-6-phosphate isomerase
(Escherichia coli (strain K12)) | BDBM50481224

(CHEMBL611642)Show SMILES OC1O[C@H](CC(C(O)=O)C(O)=O)[C@@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C9H14O9/c10-4-3(1-2(7(13)14)8(15)16)18-9(17)6(12)5(4)11/h2-6,9-12,17H,1H2,(H,13,14)(H,15,16)/t3-,4-,5+,6+,9?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ Paris-Sud
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli type I phosphomannose isomerase assessed as D-mannose 6-phosphate to D-fructose 6-phosphate isomerization at pH 7.1 by... |
Bioorg Med Chem 17: 7100-7 (2009)
Article DOI: 10.1016/j.bmc.2009.09.005
BindingDB Entry DOI: 10.7270/Q2BR8W03 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM19126

((1R,3R,6R,7S,8R,10S,11S,14R,15R,20S)-18-[(2S)-buta...)Show SMILES [H][C@@]12CC[C@]3([H])[C@]4(C[C@@]14C(=O)C[C@]1(C)[C@@H]([C@H](C)N(C)C)[C@H](O)C[C@@]21C)CC[C@@H]1N=C(NC[C@@]31C)[C@@H](C)CC |r,c:32| Show InChI InChI=1S/C31H51N3O2/c1-9-18(2)26-32-17-27(4)21-10-11-22-28(5)14-20(35)25(19(3)34(7)8)29(28,6)15-24(36)31(22)16-30(21,31)13-12-23(27)33-26/h18-23,25,35H,9-17H2,1-8H3,(H,32,33)/t18-,19-,20+,21-,22-,23-,25-,27-,28-,29+,30+,31-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM19125

((1R,3R,6R,7S,8R,10S,11S,14R,15R,20S)-7-[(1S)-1-(di...)Show SMILES [H][C@@]12CC[C@]3([H])[C@]4(C[C@@]14C(=O)C[C@]1(C)[C@@H]([C@H](C)N(C)C)[C@H](O)C[C@@]21C)CC[C@@H]1N=C(NC[C@@]31C)C(C)C |r,c:32| Show InChI InChI=1S/C30H49N3O2/c1-17(2)25-31-16-26(4)20-9-10-21-27(5)13-19(34)24(18(3)33(7)8)28(27,6)14-23(35)30(21)15-29(20,30)12-11-22(26)32-25/h17-22,24,34H,9-16H2,1-8H3,(H,31,32)/t18-,19+,20-,21-,22-,24-,26-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50300042
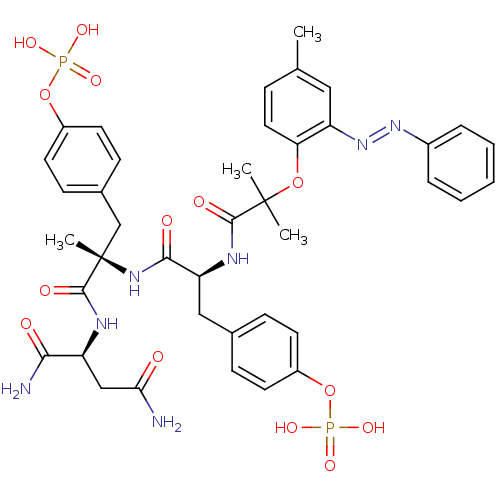
(4-[(2S)-2-{[(1S)-1-{[(1S)-1,2-dicarbamoylethyl]car...)Show SMILES Cc1ccc(OC(C)(C)C(=O)N[C@@H](Cc2ccc(OP(O)(O)=O)cc2)C(=O)N[C@@](C)(Cc2ccc(OP(O)(O)=O)cc2)C(=O)N[C@@H](CC(N)=O)C(N)=O)c(c1)\N=N\c1ccccc1 |r| Show InChI InChI=1S/C40H47N7O14P2/c1-24-10-19-33(30(20-24)47-46-27-8-6-5-7-9-27)59-39(2,3)37(51)44-32(21-25-11-15-28(16-12-25)60-62(53,54)55)36(50)45-40(4,38(52)43-31(35(42)49)22-34(41)48)23-26-13-17-29(18-14-26)61-63(56,57)58/h5-20,31-32H,21-23H2,1-4H3,(H2,41,48)(H2,42,49)(H,43,52)(H,44,51)(H,45,50)(H2,53,54,55)(H2,56,57,58)/b47-46+/t31-,32-,40-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR 176
Curated by ChEMBL
| Assay Description
Inhibition of Grb2 by ELISA |
Eur J Med Chem 45: 244-55 (2010)
Article DOI: 10.1016/j.ejmech.2009.10.003
BindingDB Entry DOI: 10.7270/Q21C1WZD |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50080832
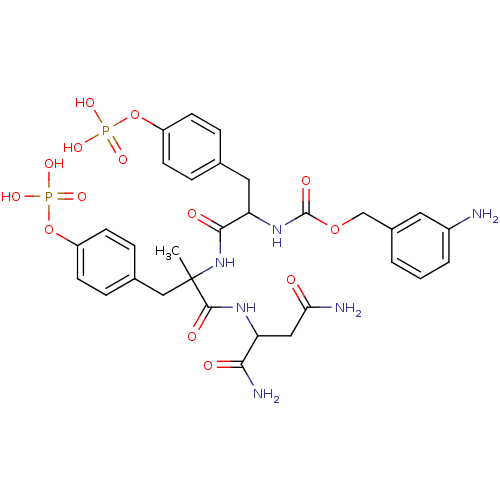
(CHEMBL334099 | [1-[1-(1,2-Dicarbamoyl-ethylcarbamo...)Show SMILES CC(Cc1ccc(OP(O)(O)=O)cc1)(NC(=O)C(Cc1ccc(OP(O)(O)=O)cc1)NC(=O)OCc1cccc(N)c1)C(=O)NC(CC(N)=O)C(N)=O Show InChI InChI=1S/C31H38N6O14P2/c1-31(29(41)35-24(27(34)39)15-26(33)38,16-19-7-11-23(12-8-19)51-53(46,47)48)37-28(40)25(14-18-5-9-22(10-6-18)50-52(43,44)45)36-30(42)49-17-20-3-2-4-21(32)13-20/h2-13,24-25H,14-17,32H2,1H3,(H2,33,38)(H2,34,39)(H,35,41)(H,36,42)(H,37,40)(H2,43,44,45)(H2,46,47,48) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was evaluated for its potency to inhibit the interaction between Growth factor receptor bound protein 2 and phosphotyrosine-containing p... |
J Med Chem 42: 3737-41 (1999)
Article DOI: 10.1021/jm9911074
BindingDB Entry DOI: 10.7270/Q2MW2GB6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM19110

((3R,6R,7S,8R,10S,11R,14R,15S,20S)-18-[(2S)-butan-2...)Show SMILES [H][C@@]12CC3=CC[C@@H]4N=C(OC[C@@]4(C)[C@]3([H])CC[C@@]1([H])[C@]1(C)C[C@@H](O)[C@H]([C@H](C)N(C)C)[C@@]1(C)CC2=O)[C@@H](C)CC |r,c:7,t:3| Show InChI InChI=1S/C31H50N2O3/c1-9-18(2)28-32-26-13-10-20-14-21-23(12-11-22(20)29(26,4)17-36-28)30(5)16-25(35)27(19(3)33(7)8)31(30,6)15-24(21)34/h10,18-19,21-23,25-27,35H,9,11-17H2,1-8H3/t18-,19-,21+,22+,23+,25+,26-,27-,29-,30-,31+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS
| Assay Description
Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM19126

((1R,3R,6R,7S,8R,10S,11S,14R,15R,20S)-18-[(2S)-buta...)Show SMILES [H][C@@]12CC[C@]3([H])[C@]4(C[C@@]14C(=O)C[C@]1(C)[C@@H]([C@H](C)N(C)C)[C@H](O)C[C@@]21C)CC[C@@H]1N=C(NC[C@@]31C)[C@@H](C)CC |r,c:32| Show InChI InChI=1S/C31H51N3O2/c1-9-18(2)26-32-17-27(4)21-10-11-22-28(5)14-20(35)25(19(3)34(7)8)29(28,6)15-24(36)31(22)16-30(21,31)13-12-23(27)33-26/h18-23,25,35H,9-17H2,1-8H3,(H,32,33)/t18-,19-,20+,21-,22-,23-,25-,27-,28-,29+,30+,31-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50424266
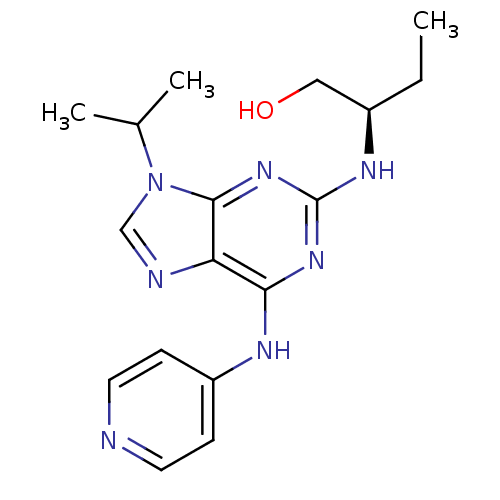
(CHEMBL2314466)Show SMILES CC[C@H](CO)Nc1nc(Nc2ccncc2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C17H23N7O/c1-4-12(9-25)21-17-22-15(20-13-5-7-18-8-6-13)14-16(23-17)24(10-19-14)11(2)3/h5-8,10-12,25H,4,9H2,1-3H3,(H2,18,20,21,22,23)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM19125

((1R,3R,6R,7S,8R,10S,11S,14R,15R,20S)-7-[(1S)-1-(di...)Show SMILES [H][C@@]12CC[C@]3([H])[C@]4(C[C@@]14C(=O)C[C@]1(C)[C@@H]([C@H](C)N(C)C)[C@H](O)C[C@@]21C)CC[C@@H]1N=C(NC[C@@]31C)C(C)C |r,c:32| Show InChI InChI=1S/C30H49N3O2/c1-17(2)25-31-16-26(4)20-9-10-21-27(5)13-19(34)24(18(3)33(7)8)28(27,6)14-23(35)30(21)15-29(20,30)12-11-22(26)32-25/h17-22,24,34H,9-16H2,1-8H3,(H,31,32)/t18-,19+,20-,21-,22-,24-,26-,27-,28+,29+,30-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50424264
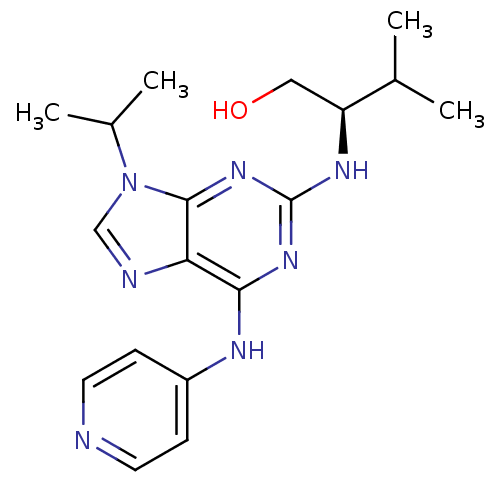
(CHEMBL2314464)Show SMILES CC(C)[C@H](CO)Nc1nc(Nc2ccncc2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C18H25N7O/c1-11(2)14(9-26)22-18-23-16(21-13-5-7-19-8-6-13)15-17(24-18)25(10-20-15)12(3)4/h5-8,10-12,14,26H,9H2,1-4H3,(H2,19,21,22,23,24)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50424270
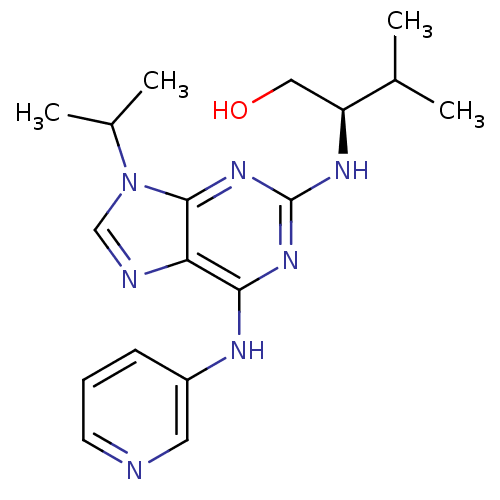
(CHEMBL2314471)Show SMILES CC(C)[C@H](CO)Nc1nc(Nc2cccnc2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C18H25N7O/c1-11(2)14(9-26)22-18-23-16(21-13-6-5-7-19-8-13)15-17(24-18)25(10-20-15)12(3)4/h5-8,10-12,14,26H,9H2,1-4H3,(H2,21,22,23,24)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50424272
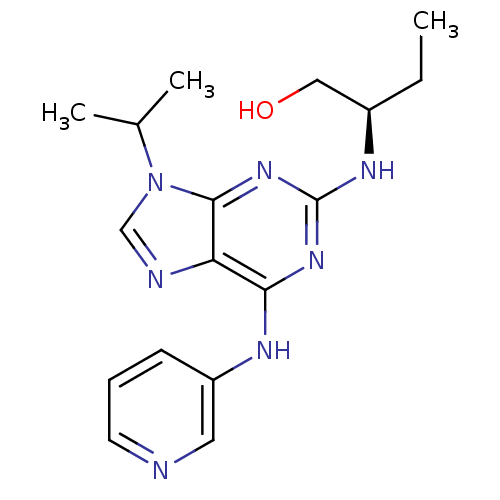
(CHEMBL2314473)Show SMILES CC[C@H](CO)Nc1nc(Nc2cccnc2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C17H23N7O/c1-4-12(9-25)21-17-22-15(20-13-6-5-7-18-8-13)14-16(23-17)24(10-19-14)11(2)3/h5-8,10-12,25H,4,9H2,1-3H3,(H2,20,21,22,23)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM19111

((3R,6R,7S,8R,10S,11R,14R,15S,20S)-18-(butan-2-yl)-...)Show SMILES [H][C@@]12CC3=CC[C@@H]4N=C(OC[C@@]4(C)[C@]3([H])CC[C@@]1([H])[C@]1(C)C[C@@H](O)[C@H]([C@H](C)N(C)C)[C@@]1(C)CC2=O)C(C)CC |r,c:7,t:3| Show InChI InChI=1S/C31H50N2O3/c1-9-18(2)28-32-26-13-10-20-14-21-23(12-11-22(20)29(26,4)17-36-28)30(5)16-25(35)27(19(3)33(7)8)31(30,6)15-24(21)34/h10,18-19,21-23,25-27,35H,9,11-17H2,1-8H3/t18?,19-,21+,22+,23+,25+,26-,27-,29-,30-,31+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS
| Assay Description
Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM19129

((3R,6R,7S,8R,10S,11R,14R,15R,20S)-18-[(2S)-butan-2...)Show SMILES [H][C@@]12CC3=CC[C@@H]4N=C(NC[C@@]4(C)[C@]3([H])CC[C@@]1([H])[C@]1(C)C[C@@H](O)[C@H]([C@H](C)N(C)C)[C@@]1(C)CC2=O)[C@@H](C)CC |r,c:7,t:3| Show InChI InChI=1S/C31H51N3O2/c1-9-18(2)28-32-17-29(4)22-11-12-23-21(14-20(22)10-13-26(29)33-28)24(35)15-31(6)27(19(3)34(7)8)25(36)16-30(23,31)5/h10,18-19,21-23,25-27,36H,9,11-17H2,1-8H3,(H,32,33)/t18-,19-,21+,22+,23+,25+,26-,27-,29-,30-,31+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM19101

((3R,6R,7S,8R,10S,11R,14R,15S,20S)-7-[(1S)-1-(dimet...)Show SMILES [H][C@@]12CC3=CC[C@@H]4N=C(OC[C@@]4(C)[C@]3([H])CC[C@@]1([H])[C@]1(C)C[C@@H](O)[C@H]([C@H](C)N(C)C)[C@@]1(C)CC2=O)C(C)C |r,c:7,t:3| Show InChI InChI=1S/C30H48N2O3/c1-17(2)27-31-25-12-9-19-13-20-22(11-10-21(19)28(25,4)16-35-27)29(5)15-24(34)26(18(3)32(7)8)30(29,6)14-23(20)33/h9,17-18,20-22,24-26,34H,10-16H2,1-8H3/t18-,20+,21+,22+,24+,25-,26-,28-,29-,30+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS
| Assay Description
Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50424294
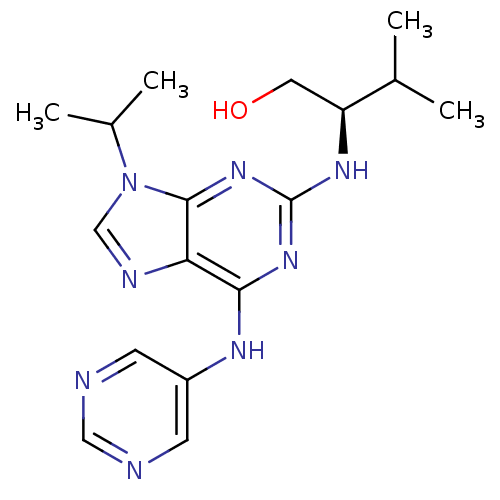
(CHEMBL2314447)Show SMILES CC(C)[C@H](CO)Nc1nc(Nc2cncnc2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C17H24N8O/c1-10(2)13(7-26)22-17-23-15(21-12-5-18-8-19-6-12)14-16(24-17)25(9-20-14)11(3)4/h5-6,8-11,13,26H,7H2,1-4H3,(H2,21,22,23,24)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50424282

(CHEMBL2314436)Show SMILES CC(C)[C@H](CO)Nc1nc(Nc2ccnnc2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C17H24N8O/c1-10(2)13(8-26)22-17-23-15(21-12-5-6-19-20-7-12)14-16(24-17)25(9-18-14)11(3)4/h5-7,9-11,13,26H,8H2,1-4H3,(H2,19,21,22,23,24)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50424296
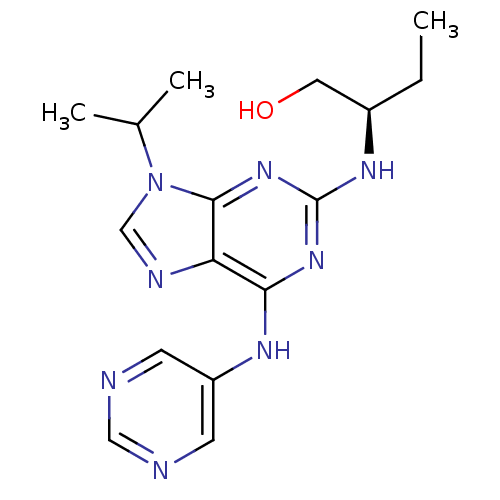
(CHEMBL2314449)Show SMILES CC[C@H](CO)Nc1nc(Nc2cncnc2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C16H22N8O/c1-4-11(7-25)21-16-22-14(20-12-5-17-8-18-6-12)13-15(23-16)24(9-19-13)10(2)3/h5-6,8-11,25H,4,7H2,1-3H3,(H2,20,21,22,23)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50424283
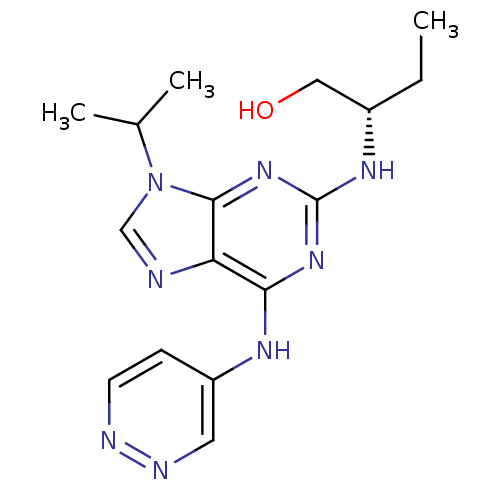
(CHEMBL2314435)Show SMILES CC[C@@H](CO)Nc1nc(Nc2ccnnc2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C16H22N8O/c1-4-11(8-25)21-16-22-14(20-12-5-6-18-19-7-12)13-15(23-16)24(9-17-13)10(2)3/h5-7,9-11,25H,4,8H2,1-3H3,(H2,18,20,21,22,23)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50424265

(CHEMBL2314467)Show SMILES CC[C@@H](CO)Nc1nc(Nc2ccncc2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C17H23N7O/c1-4-12(9-25)21-17-22-15(20-13-5-7-18-8-6-13)14-16(23-17)24(10-19-14)11(2)3/h5-8,10-12,25H,4,9H2,1-3H3,(H2,18,20,21,22,23)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
Inhibition of BuChE activity was determined by the spectroscopic method of Ellman using butyrylthiocholine iodide as substrate, in 96-well microtiter... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50424284
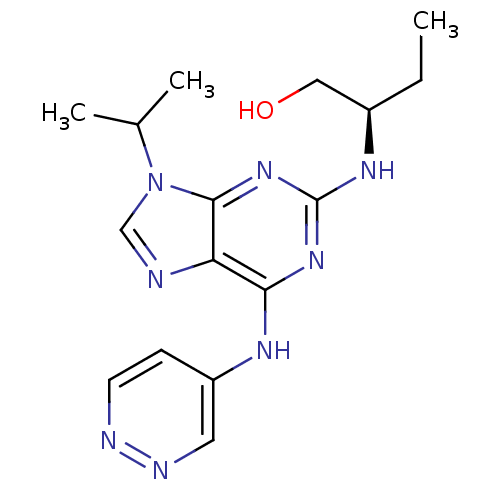
(CHEMBL2314437)Show SMILES CC[C@H](CO)Nc1nc(Nc2ccnnc2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C16H22N8O/c1-4-11(8-25)21-16-22-14(20-12-5-6-18-19-7-12)13-15(23-16)24(9-17-13)10(2)3/h5-7,9-11,25H,4,8H2,1-3H3,(H2,18,20,21,22,23)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50267086

(Adociaquinone B | CHEMBL476648)Show SMILES C[C@@]12CCCc3coc(c13)C(=O)c1cc3C(=O)C4=NCCS(=O)(=O)C4C(=O)c3cc21 |r,t:19| Show InChI InChI=1S/C22H17NO6S/c1-22-4-2-3-10-9-29-20(15(10)22)18(25)13-7-11-12(8-14(13)22)19(26)21-16(17(11)24)23-5-6-30(21,27)28/h7-9,21H,2-6H2,1H3/t21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDC25B |
Bioorg Med Chem 16: 9040-9 (2008)
Article DOI: 10.1016/j.bmc.2008.08.009
BindingDB Entry DOI: 10.7270/Q2833SZB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50424295

(CHEMBL2314448)Show SMILES CC[C@@H](CO)Nc1nc(Nc2cncnc2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C16H22N8O/c1-4-11(7-25)21-16-22-14(20-12-5-17-8-18-6-12)13-15(23-16)24(9-19-13)10(2)3/h5-6,8-11,25H,4,7H2,1-3H3,(H2,20,21,22,23)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50244787
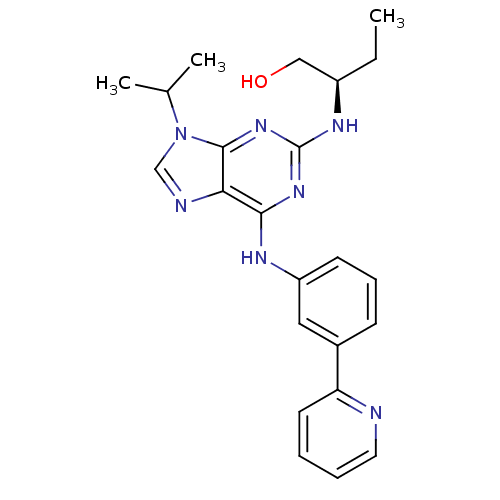
((R)-2-(1-Hydroxybut-2-ylamino)-6-[3-(2-pyridyl)phe...)Show SMILES CC[C@H](CO)Nc1nc(Nc2cccc(c2)-c2ccccn2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C23H27N7O/c1-4-17(13-31)27-23-28-21(20-22(29-23)30(14-25-20)15(2)3)26-18-9-7-8-16(12-18)19-10-5-6-11-24-19/h5-12,14-15,17,31H,4,13H2,1-3H3,(H2,26,27,28,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50424271
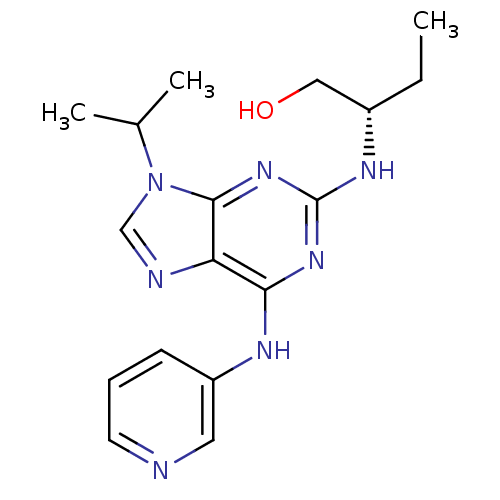
(CHEMBL2314472)Show SMILES CC[C@@H](CO)Nc1nc(Nc2cccnc2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C17H23N7O/c1-4-12(9-25)21-17-22-15(20-13-6-5-7-18-8-13)14-16(23-17)24(10-19-14)11(2)3/h5-8,10-12,25H,4,9H2,1-3H3,(H2,20,21,22,23)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50424279
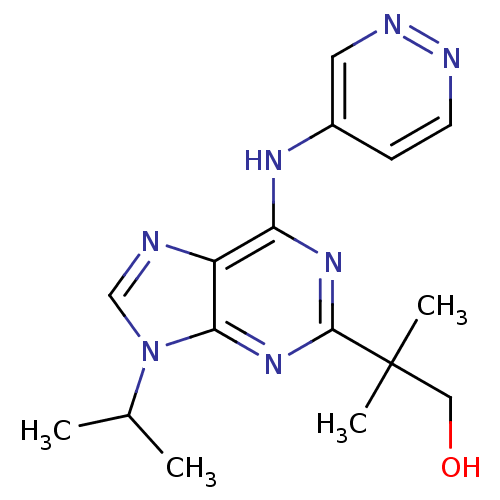
(CHEMBL2314434)Show InChI InChI=1S/C16H21N7O/c1-10(2)23-9-17-12-13(20-11-5-6-18-19-7-11)21-15(22-14(12)23)16(3,4)8-24/h5-7,9-10,24H,8H2,1-4H3,(H,18,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM19112

((3R,6R,7S,8R,10S,11R,14R,15S,20S)-7-[(1S)-1-(dimet...)Show SMILES [H][C@@]12CC3=CC[C@@H]4N=C(OC[C@@]4(C)[C@]3([H])CC[C@@]1([H])[C@]1(C)C[C@@H](O)[C@H]([C@H](C)N(C)C)[C@@]1(C)CC2=O)C(CC)CC |r,c:7,t:3| Show InChI InChI=1S/C32H52N2O3/c1-9-20(10-2)29-33-27-14-11-21-15-22-24(13-12-23(21)30(27,4)18-37-29)31(5)17-26(36)28(19(3)34(7)8)32(31,6)16-25(22)35/h11,19-20,22-24,26-28,36H,9-10,12-18H2,1-8H3/t19-,22+,23+,24+,26+,27-,28-,30-,31-,32+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS
| Assay Description
Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM19104

((2S)-N-[(1R,6S,7S,8R,11R,12S,14R,15S,16R)-15-[(1S)...)Show SMILES [H][C@@]12CC3=CC[C@H](NC(=O)[C@@H](C)CC)[C@@](C)(CO)[C@]3([H])CC[C@@]1([H])[C@]1(C)C[C@@H](O)[C@H]([C@H](C)N(C)C)[C@@]1(C)CC2=O |r,t:3| Show InChI InChI=1S/C31H52N2O4/c1-9-18(2)28(37)32-26-13-10-20-14-21-23(12-11-22(20)29(26,4)17-34)30(5)16-25(36)27(19(3)33(7)8)31(30,6)15-24(21)35/h10,18-19,21-23,25-27,34,36H,9,11-17H2,1-8H3,(H,32,37)/t18-,19-,21+,22+,23+,25+,26-,27-,29-,30-,31+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS
| Assay Description
Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM19122
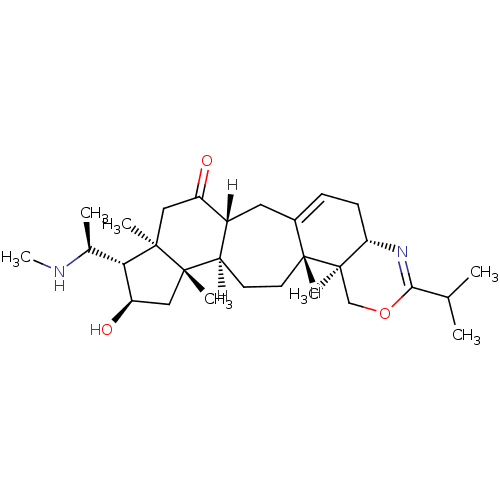
((3R,6R,7S,8R,10S,11R,14R,15S,20S)-8-hydroxy-6,10,1...)Show SMILES [H][C@@]12CC3=CC[C@@H]4N=C(OC[C@@]4(C)[C@]3([H])CC[C@@]1([H])[C@]1(C)C[C@@H](O)[C@H]([C@H](C)NC)[C@@]1(C)CC2=O)C(C)C |r,c:7,t:3| Show InChI InChI=1S/C29H46N2O3/c1-16(2)26-31-24-11-8-18-12-19-21(10-9-20(18)27(24,4)15-34-26)28(5)14-23(33)25(17(3)30-7)29(28,6)13-22(19)32/h8,16-17,19-21,23-25,30,33H,9-15H2,1-7H3/t17-,19+,20+,21+,23+,24-,25-,27-,28-,29+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM19120

((3R,6R,7S,8R,10S,11R,14R,15S,20S)-7-[(1S)-1-(dimet...)Show SMILES [H][C@@]12CC3=CC[C@@H]4N=C(OC[C@@]4(C)[C@]3([H])CC[C@@]1([H])[C@]1(C)C[C@@H](OC(=O)C(C)(C)C)[C@H]([C@H](C)N(C)C)[C@@]1(C)CC2=O)C(C)C |r,c:7,t:3| Show InChI InChI=1S/C35H56N2O4/c1-20(2)30-36-28-15-12-22-16-23-25(14-13-24(22)33(28,7)19-40-30)34(8)18-27(41-31(39)32(4,5)6)29(21(3)37(10)11)35(34,9)17-26(23)38/h12,20-21,23-25,27-29H,13-19H2,1-11H3/t21-,23+,24+,25+,27+,28-,29-,33-,34-,35+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM19121

(N-[(6S,7S,8R,11S,12S,14R,15S,16R)-14-hydroxy-7-(hy...)Show SMILES [H][C@@]12CC[C@]3([H])[C@]4(C[C@@]14C(=O)C[C@]1(C)[C@@H]([C@H](C)NC)[C@H](O)C[C@@]21C)CC[C@H](NC(=O)C(C)C)[C@@]3(C)CO |r| Show InChI InChI=1S/C29H48N2O4/c1-16(2)24(35)31-21-10-11-28-14-29(28)20(9-8-19(28)25(21,4)15-32)26(5)12-18(33)23(17(3)30-7)27(26,6)13-22(29)34/h16-21,23,30,32-33H,8-15H2,1-7H3,(H,31,35)/t17-,18+,19-,20-,21-,23-,25-,26-,27+,28+,29-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM19118

((1S,6R,7S,8R,10S,11S,14R,15S,20S)-7-[(1S)-1-(dimet...)Show SMILES [H][C@@]12CC[C@]3([H])C(=C[C@@H]1CC[C@@H]1N=C(OC[C@@]21C)C(C)C)C(=O)C[C@]1(C)[C@@H]([C@H](C)N(C)C)[C@H](O)C[C@@]31C |r,wU:11.12,24.27,26.29,16.19,4.4,wD:35.40,27.30,32.35,8.9,1.0,c:6,13,(-2.23,3.97,;-2.23,5.51,;-.9,4.74,;.43,5.51,;.43,7.05,;1.47,5.9,;-.9,7.82,;-2.23,8.59,;-2.23,7.05,;-3.57,7.82,;-4.9,7.05,;-4.9,5.51,;-6.23,4.74,;-6.23,3.2,;-4.9,2.43,;-3.57,3.2,;-3.57,4.74,;-4.9,3.97,;-7.57,2.43,;-8.9,3.2,;-7.57,.89,;-.9,9.36,;-2.23,10.13,;.43,10.13,;1.77,9.36,;1.77,10.9,;3.23,9.83,;4,11.17,;3.23,12.5,;5.54,11.17,;6.31,9.83,;6.31,12.5,;4.14,8.59,;5.63,8.19,;3.23,7.34,;1.77,7.82,;2.17,6.33,)| Show InChI InChI=1S/C30H48N2O3/c1-17(2)27-31-25-12-9-19-13-20-22(11-10-21(19)28(25,4)16-35-27)29(5)15-24(34)26(18(3)32(7)8)30(29,6)14-23(20)33/h13,17-19,21-22,24-26,34H,9-12,14-16H2,1-8H3/t18-,19?,21+,22+,24+,25-,26-,28-,29-,30+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50080831

(CHEMBL434413 | [1-[1-(1,2-Dicarbamoyl-ethylcarbamo...)Show SMILES NC(=O)CC(NC(=O)C1(CCCCC1)NC(=O)C(Cc1ccc(OP(O)(O)=O)cc1)NC(=O)OCc1cccc(N)c1)C(N)=O Show InChI InChI=1S/C28H37N6O10P/c29-19-6-4-5-18(13-19)16-43-27(39)33-22(14-17-7-9-20(10-8-17)44-45(40,41)42)25(37)34-28(11-2-1-3-12-28)26(38)32-21(24(31)36)15-23(30)35/h4-10,13,21-22H,1-3,11-12,14-16,29H2,(H2,30,35)(H2,31,36)(H,32,38)(H,33,39)(H,34,37)(H2,40,41,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was evaluated for its potency to inhibit the interaction between Growth factor receptor bound protein 2 and phosphotyrosine-containing p... |
J Med Chem 42: 3737-41 (1999)
Article DOI: 10.1021/jm9911074
BindingDB Entry DOI: 10.7270/Q2MW2GB6 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM19105
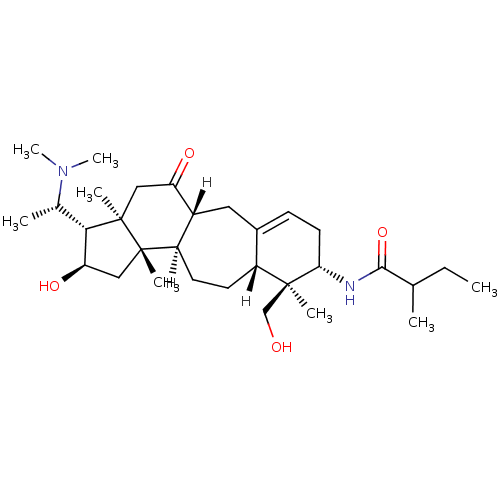
(N-[(1R,6S,7S,8R,11R,12S,14R,15S,16R)-15-[(1S)-1-(d...)Show SMILES [H][C@@]12CC3=CC[C@H](NC(=O)C(C)CC)[C@@](C)(CO)[C@]3([H])CC[C@@]1([H])[C@]1(C)C[C@@H](O)[C@H]([C@H](C)N(C)C)[C@@]1(C)CC2=O |r,t:3| Show InChI InChI=1S/C31H52N2O4/c1-9-18(2)28(37)32-26-13-10-20-14-21-23(12-11-22(20)29(26,4)17-34)30(5)16-25(36)27(19(3)33(7)8)31(30,6)15-24(21)35/h10,18-19,21-23,25-27,34,36H,9,11-17H2,1-8H3,(H,32,37)/t18?,19-,21+,22+,23+,25+,26-,27-,29-,30-,31+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CNRS
| Assay Description
Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50420297

(CHEMBL2089032)Show SMILES CC(C)n1cnc2c(NCc3ccc(cc3)-c3ccccn3)nc(NC[C@@H](O)CO)nc12 |r| Show InChI InChI=1S/C23H27N7O2/c1-15(2)30-14-27-20-21(28-23(29-22(20)30)26-12-18(32)13-31)25-11-16-6-8-17(9-7-16)19-5-3-4-10-24-19/h3-10,14-15,18,31-32H,11-13H2,1-2H3,(H2,25,26,28,29)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK9/cyclin T expressed in baculovirus-infected insect cells |
Eur J Med Chem 56: 210-216 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.033
BindingDB Entry DOI: 10.7270/Q2ST7R34 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform alpha
(Homo sapiens (Human)) | BDBM50420301
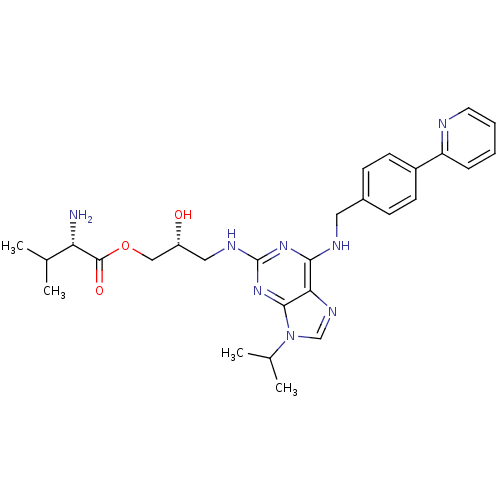
(CHEMBL2089036)Show SMILES CC(C)[C@H](N)C(=O)OC[C@H](O)CNc1nc(NCc2ccc(cc2)-c2ccccn2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C28H36N8O3/c1-17(2)23(29)27(38)39-15-21(37)14-32-28-34-25(24-26(35-28)36(16-33-24)18(3)4)31-13-19-8-10-20(11-9-19)22-7-5-6-12-30-22/h5-12,16-18,21,23,37H,13-15,29H2,1-4H3,(H2,31,32,34,35)/t21-,23+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1 after 30 mins by liquid scintillation counter |
Eur J Med Chem 56: 210-216 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.033
BindingDB Entry DOI: 10.7270/Q2ST7R34 |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50080836

(4-[2-[2-(3-Amino-benzyloxycarbonylamino)-3-(4-phos...)Show SMILES CC(Cc1ccc(cc1)C(O)=O)(NC(=O)C(Cc1ccc(OP(O)(O)=O)cc1)NC(=O)OCc1cccc(N)c1)C(=O)NC(CC(N)=O)C(N)=O Show InChI InChI=1S/C32H37N6O12P/c1-32(16-19-5-9-21(10-6-19)29(42)43,30(44)36-24(27(35)40)15-26(34)39)38-28(41)25(14-18-7-11-23(12-8-18)50-51(46,47)48)37-31(45)49-17-20-3-2-4-22(33)13-20/h2-13,24-25H,14-17,33H2,1H3,(H2,34,39)(H2,35,40)(H,36,44)(H,37,45)(H,38,41)(H,42,43)(H2,46,47,48) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was evaluated for its potency to inhibit the interaction between Growth factor receptor bound protein 2 and phosphotyrosine-containing p... |
J Med Chem 42: 3737-41 (1999)
Article DOI: 10.1021/jm9911074
BindingDB Entry DOI: 10.7270/Q2MW2GB6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
Inhibition of AChE activity was determined by the spectroscopic method of Ellman using acetylthiocholine iodide as substrate, in 96-well microtiter p... |
J Med Chem 50: 5311-23 (2007)
Article DOI: 10.1021/jm070536w
BindingDB Entry DOI: 10.7270/Q22B8W82 |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50420301

(CHEMBL2089036)Show SMILES CC(C)[C@H](N)C(=O)OC[C@H](O)CNc1nc(NCc2ccc(cc2)-c2ccccn2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C28H36N8O3/c1-17(2)23(29)27(38)39-15-21(37)14-32-28-34-25(24-26(35-28)36(16-33-24)18(3)4)31-13-19-8-10-20(11-9-19)22-7-5-6-12-30-22/h5-12,16-18,21,23,37H,13-15,29H2,1-4H3,(H2,31,32,34,35)/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK9/cyclin T expressed in baculovirus-infected insect cells |
Eur J Med Chem 56: 210-216 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.033
BindingDB Entry DOI: 10.7270/Q2ST7R34 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform alpha
(Homo sapiens (Human)) | BDBM50420297

(CHEMBL2089032)Show SMILES CC(C)n1cnc2c(NCc3ccc(cc3)-c3ccccn3)nc(NC[C@@H](O)CO)nc12 |r| Show InChI InChI=1S/C23H27N7O2/c1-15(2)30-14-27-20-21(28-23(29-22(20)30)26-12-18(32)13-31)25-11-16-6-8-17(9-7-16)19-5-3-4-10-24-19/h3-10,14-15,18,31-32H,11-13H2,1-2H3,(H2,25,26,28,29)/t18-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1 after 30 mins by liquid scintillation counter |
Eur J Med Chem 56: 210-216 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.033
BindingDB Entry DOI: 10.7270/Q2ST7R34 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50424292

(CHEMBL2314445)Show InChI InChI=1S/C16H21N7O/c1-10(2)23-9-19-12-13(20-11-5-17-8-18-6-11)21-15(22-14(12)23)16(3,4)7-24/h5-6,8-10,24H,7H2,1-4H3,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50420301

(CHEMBL2089036)Show SMILES CC(C)[C@H](N)C(=O)OC[C@H](O)CNc1nc(NCc2ccc(cc2)-c2ccccn2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C28H36N8O3/c1-17(2)23(29)27(38)39-15-21(37)14-32-28-34-25(24-26(35-28)36(16-33-24)18(3)4)31-13-19-8-10-20(11-9-19)22-7-5-6-12-30-22/h5-12,16-18,21,23,37H,13-15,29H2,1-4H3,(H2,31,32,34,35)/t21-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 expressed in Escherichia coli |
Eur J Med Chem 56: 210-216 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.033
BindingDB Entry DOI: 10.7270/Q2ST7R34 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50420297

(CHEMBL2089032)Show SMILES CC(C)n1cnc2c(NCc3ccc(cc3)-c3ccccn3)nc(NC[C@@H](O)CO)nc12 |r| Show InChI InChI=1S/C23H27N7O2/c1-15(2)30-14-27-20-21(28-23(29-22(20)30)26-12-18(32)13-31)25-11-16-6-8-17(9-7-16)19-5-3-4-10-24-19/h3-10,14-15,18,31-32H,11-13H2,1-2H3,(H2,25,26,28,29)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 expressed in Escherichia coli |
Eur J Med Chem 56: 210-216 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.033
BindingDB Entry DOI: 10.7270/Q2ST7R34 |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50080834
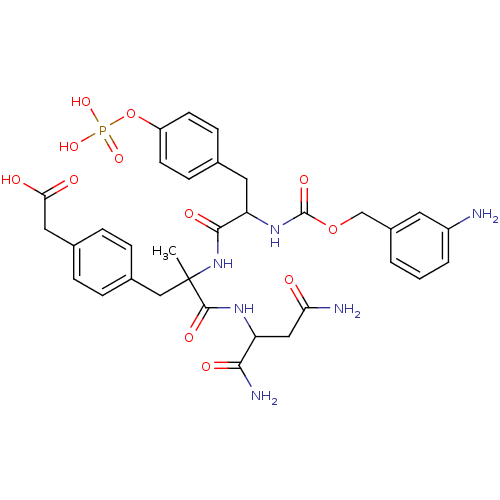
(CHEMBL409455 | {4-[2-[2-(3-Amino-benzyloxycarbonyl...)Show SMILES CC(Cc1ccc(CC(O)=O)cc1)(NC(=O)C(Cc1ccc(OP(O)(O)=O)cc1)NC(=O)OCc1cccc(N)c1)C(=O)NC(CC(N)=O)C(N)=O Show InChI InChI=1S/C33H39N6O12P/c1-33(31(45)37-25(29(36)43)16-27(35)40,17-21-7-5-20(6-8-21)15-28(41)42)39-30(44)26(14-19-9-11-24(12-10-19)51-52(47,48)49)38-32(46)50-18-22-3-2-4-23(34)13-22/h2-13,25-26H,14-18,34H2,1H3,(H2,35,40)(H2,36,43)(H,37,45)(H,38,46)(H,39,44)(H,41,42)(H2,47,48,49) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 198 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was evaluated for its potency to inhibit the interaction between Growth factor receptor bound protein 2 and phosphotyrosine-containing p... |
J Med Chem 42: 3737-41 (1999)
Article DOI: 10.1021/jm9911074
BindingDB Entry DOI: 10.7270/Q2MW2GB6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50424263
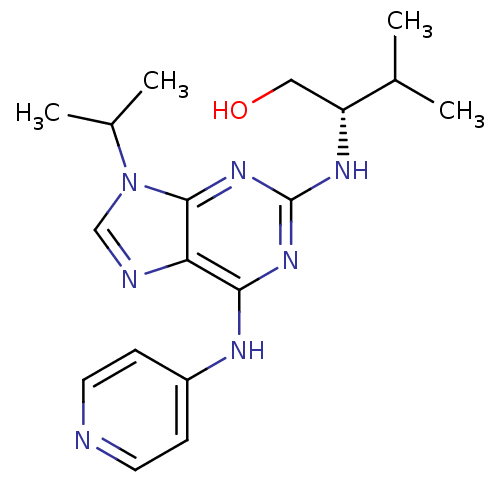
(CHEMBL2314465)Show SMILES CC(C)[C@@H](CO)Nc1nc(Nc2ccncc2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C18H25N7O/c1-11(2)14(9-26)22-18-23-16(21-13-5-7-19-8-6-13)15-17(24-18)25(10-20-15)12(3)4/h5-8,10-12,14,26H,9H2,1-4H3,(H2,19,21,22,23,24)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM7533
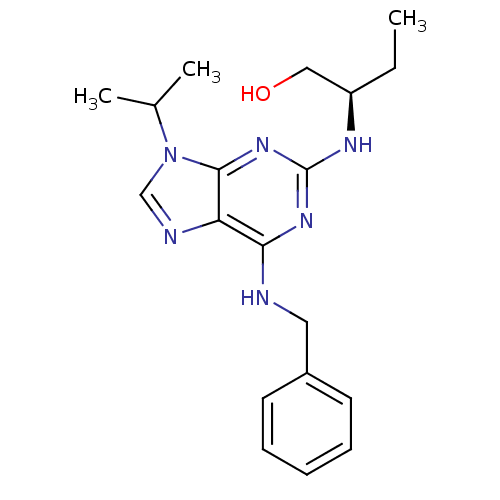
((2R)-2-[[6-(benzylamino)-9-isopropyl-purin-2-yl]am...)Show SMILES CC[C@H](CO)Nc1nc(NCc2ccccc2)c2ncn(C(C)C)c2n1 |r| Show InChI InChI=1S/C19H26N6O/c1-4-15(11-26)22-19-23-17(20-10-14-8-6-5-7-9-14)16-18(24-19)25(12-21-16)13(2)3/h5-9,12-13,15,26H,4,10-11H2,1-3H3,(H2,20,22,23,24)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50424280
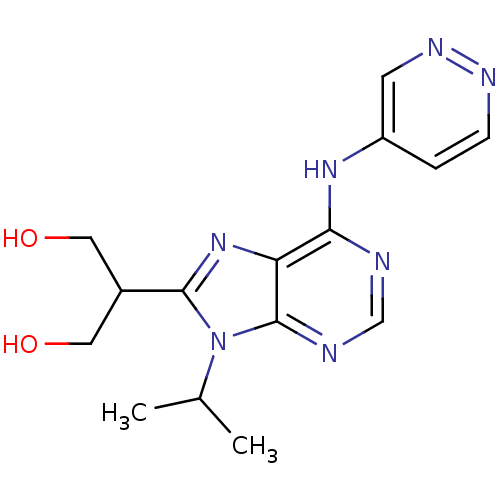
(CHEMBL2311568)Show InChI InChI=1S/C15H19N7O2/c1-9(2)22-14(10(6-23)7-24)21-12-13(16-8-17-15(12)22)20-11-3-4-18-19-5-11/h3-5,8-10,23-24H,6-7H2,1-2H3,(H,16,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CDK5/p25 after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 125-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.141
BindingDB Entry DOI: 10.7270/Q2J38TVS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data