 Found 53 hits with Last Name = 'guillon' and Initial = 'j'
Found 53 hits with Last Name = 'guillon' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie
Curated by ChEMBL
| Assay Description
Inhibition constant for human aromatase cytochrome P450 19A1 activity |
Bioorg Med Chem Lett 8: 1041-4 (1999)
BindingDB Entry DOI: 10.7270/Q2445KM7 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50070095
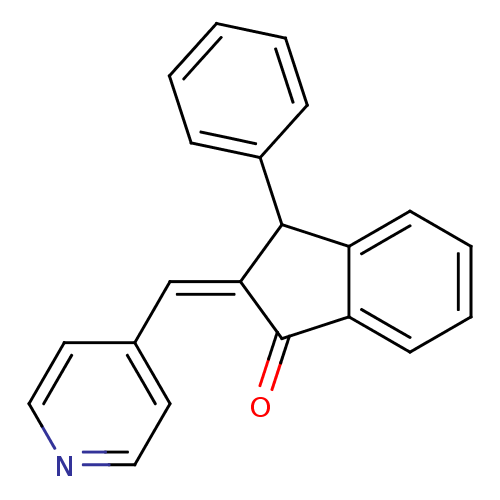
(3-Phenyl-2-[1-pyridin-4-yl-meth-(Z)-ylidene]-indan...)Show InChI InChI=1S/C21H15NO/c23-21-18-9-5-4-8-17(18)20(16-6-2-1-3-7-16)19(21)14-15-10-12-22-13-11-15/h1-14,20H/b19-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie
Curated by ChEMBL
| Assay Description
Inhibition constant for human aromatase cytochrome P450 19A1 activity |
Bioorg Med Chem Lett 8: 1041-4 (1999)
BindingDB Entry DOI: 10.7270/Q2445KM7 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50070097
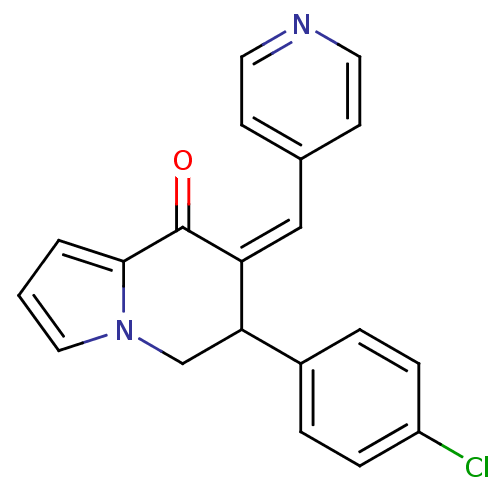
((Z)-6-(4-chlorophenyl)-7-(pyridin-4-ylmethylene)-6...)Show InChI InChI=1S/C20H15ClN2O/c21-16-5-3-15(4-6-16)18-13-23-11-1-2-19(23)20(24)17(18)12-14-7-9-22-10-8-14/h1-12,18H,13H2/b17-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie
Curated by ChEMBL
| Assay Description
Inhibition constant for human aromatase cytochrome P450 19A1 activity |
Bioorg Med Chem Lett 8: 1041-4 (1999)
BindingDB Entry DOI: 10.7270/Q2445KM7 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50215414
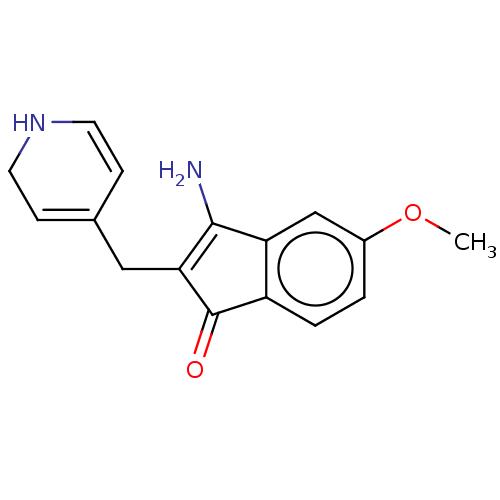
(CHEMBL1096024 | MR-20814)Show SMILES COc1ccc2C(=O)C(CC3=CCNC=C3)=C(N)c2c1 |c:14,t:10,16| Show InChI InChI=1S/C16H16N2O2/c1-20-11-2-3-12-13(9-11)15(17)14(16(12)19)8-10-4-6-18-7-5-10/h2-6,9,18H,7-8,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie
Curated by ChEMBL
| Assay Description
Inhibition constant for human aromatase cytochrome P450 19A1 activity |
Bioorg Med Chem Lett 8: 1041-4 (1999)
BindingDB Entry DOI: 10.7270/Q2445KM7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM15169
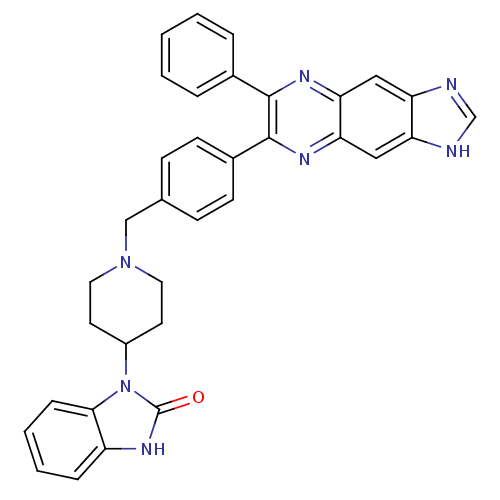
(1-{1-[(4-{7-phenyl-1H-imidazo[4,5-g]quinoxalin-6-y...)Show SMILES O=c1[nH]c2ccccc2n1C1CCN(Cc2ccc(cc2)-c2nc3cc4[nH]cnc4cc3nc2-c2ccccc2)CC1 Show InChI InChI=1S/C34H29N7O/c42-34-39-26-8-4-5-9-31(26)41(34)25-14-16-40(17-15-25)20-22-10-12-24(13-11-22)33-32(23-6-2-1-3-7-23)37-29-18-27-28(36-21-35-27)19-30(29)38-33/h1-13,18-19,21,25H,14-17,20H2,(H,35,36)(H,39,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of Akt2 (unknown origin) |
Eur J Med Chem 113: 214-27 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.047
BindingDB Entry DOI: 10.7270/Q2KD20TJ |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM15169

(1-{1-[(4-{7-phenyl-1H-imidazo[4,5-g]quinoxalin-6-y...)Show SMILES O=c1[nH]c2ccccc2n1C1CCN(Cc2ccc(cc2)-c2nc3cc4[nH]cnc4cc3nc2-c2ccccc2)CC1 Show InChI InChI=1S/C34H29N7O/c42-34-39-26-8-4-5-9-31(26)41(34)25-14-16-40(17-15-25)20-22-10-12-24(13-11-22)33-32(23-6-2-1-3-7-23)37-29-18-27-28(36-21-35-27)19-30(29)38-33/h1-13,18-19,21,25H,14-17,20H2,(H,35,36)(H,39,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) |
Eur J Med Chem 113: 214-27 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.047
BindingDB Entry DOI: 10.7270/Q2KD20TJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50305083
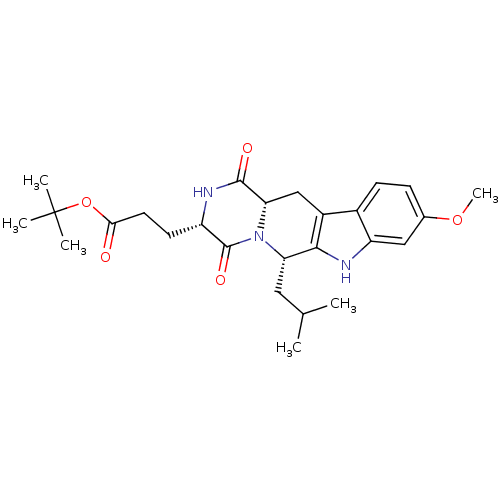
(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50379211
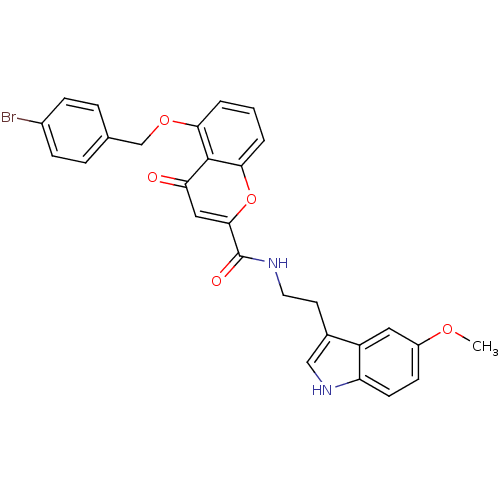
(CHEMBL2011288)Show SMILES COc1ccc2[nH]cc(CCNC(=O)c3cc(=O)c4c(OCc5ccc(Br)cc5)cccc4o3)c2c1 Show InChI InChI=1S/C28H23BrN2O5/c1-34-20-9-10-22-21(13-20)18(15-31-22)11-12-30-28(33)26-14-23(32)27-24(3-2-4-25(27)36-26)35-16-17-5-7-19(29)8-6-17/h2-10,13-15,31H,11-12,16H2,1H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase cytochrome P450 19A1 activity |
Bioorg Med Chem Lett 8: 1041-4 (1999)
BindingDB Entry DOI: 10.7270/Q2445KM7 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50070097

((Z)-6-(4-chlorophenyl)-7-(pyridin-4-ylmethylene)-6...)Show InChI InChI=1S/C20H15ClN2O/c21-16-5-3-15(4-6-16)18-13-23-11-1-2-19(23)20(24)17(18)12-14-7-9-22-10-8-14/h1-12,18H,13H2/b17-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase cytochrome P450 19A1 activity |
Bioorg Med Chem Lett 8: 1041-4 (1999)
BindingDB Entry DOI: 10.7270/Q2445KM7 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50029743
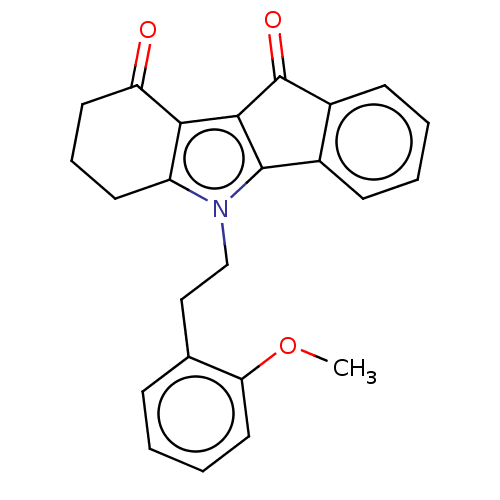
(CHEMBL3353416)Show SMILES COc1ccccc1CCn1c2CCCC(=O)c2c2C(=O)c3ccccc3-c12 Show InChI InChI=1S/C24H21NO3/c1-28-20-12-5-2-7-15(20)13-14-25-18-10-6-11-19(26)21(18)22-23(25)16-8-3-4-9-17(16)24(22)27/h2-5,7-9,12H,6,10-11,13-14H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50029739
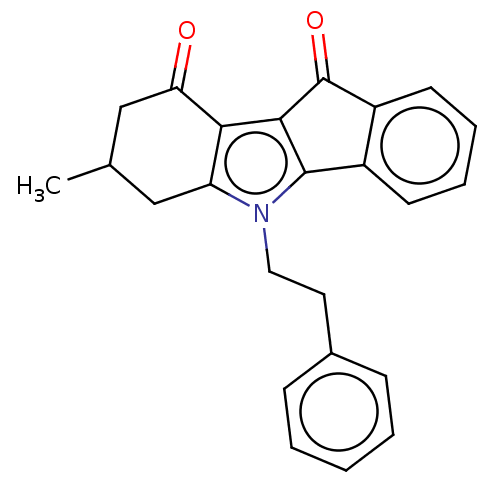
(CHEMBL3353414)Show SMILES CC1Cc2c(c3C(=O)c4ccccc4-c3n2CCc2ccccc2)C(=O)C1 Show InChI InChI=1S/C24H21NO2/c1-15-13-19-21(20(26)14-15)22-23(17-9-5-6-10-18(17)24(22)27)25(19)12-11-16-7-3-2-4-8-16/h2-10,15H,11-14H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50029744
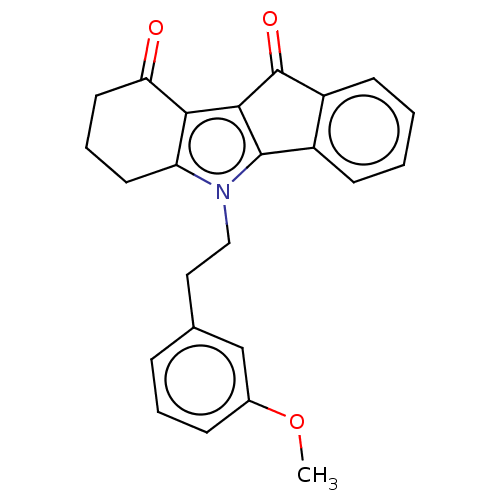
(CHEMBL3353417)Show SMILES COc1cccc(CCn2c3CCCC(=O)c3c3C(=O)c4ccccc4-c23)c1 Show InChI InChI=1S/C24H21NO3/c1-28-16-7-4-6-15(14-16)12-13-25-19-10-5-11-20(26)21(19)22-23(25)17-8-2-3-9-18(17)24(22)27/h2-4,6-9,14H,5,10-13H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50029725

(CHEMBL1957192)Show InChI InChI=1S/C23H19NO2/c25-19-12-6-11-18-20(19)21-22(16-9-4-5-10-17(16)23(21)26)24(18)14-13-15-7-2-1-3-8-15/h1-5,7-10H,6,11-14H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50029742

(CHEMBL3353415)Show SMILES O=C1c2ccccc2-c2c1c1c(CC(CC1=O)c1ccccc1)n2CCc1ccccc1 Show InChI InChI=1S/C29H23NO2/c31-25-18-21(20-11-5-2-6-12-20)17-24-26(25)27-28(22-13-7-8-14-23(22)29(27)32)30(24)16-15-19-9-3-1-4-10-19/h1-14,21H,15-18H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50029900

(CHEMBL3353422)Show SMILES CC(C)n1c2CCC(Cc3ccccc3)(Cc3ccccc3)C(=O)c2c2C(=O)c3ccccc3-c12 Show InChI InChI=1S/C32H29NO2/c1-21(2)33-26-17-18-32(19-22-11-5-3-6-12-22,20-23-13-7-4-8-14-23)31(35)27(26)28-29(33)24-15-9-10-16-25(24)30(28)34/h3-16,21H,17-20H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50029746

(CHEMBL3353418)Show SMILES COc1ccc(CCn2c3CCCC(=O)c3c3C(=O)c4ccccc4-c23)cc1 Show InChI InChI=1S/C24H21NO3/c1-28-16-11-9-15(10-12-16)13-14-25-19-7-4-8-20(26)21(19)22-23(25)17-5-2-3-6-18(17)24(22)27/h2-3,5-6,9-12H,4,7-8,13-14H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50029730
(CHEMBL1957193)Show InChI InChI=1S/C24H21NO2/c26-20-14-6-13-19-21(20)22-23(17-11-4-5-12-18(17)24(22)27)25(19)15-7-10-16-8-2-1-3-9-16/h1-5,8-9,11-12H,6-7,10,13-15H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50070095

(3-Phenyl-2-[1-pyridin-4-yl-meth-(Z)-ylidene]-indan...)Show InChI InChI=1S/C21H15NO/c23-21-18-9-5-4-8-17(18)20(16-6-2-1-3-7-16)19(21)14-15-10-12-22-13-11-15/h1-14,20H/b19-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase cytochrome P450 19A1 activity |
Bioorg Med Chem Lett 8: 1041-4 (1999)
BindingDB Entry DOI: 10.7270/Q2445KM7 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50029735

(CHEMBL1957202)Show SMILES O=C1c2ccccc2-c2c1c1c(CC(CC1=O)c1ccccc1)n2Cc1ccccc1 Show InChI InChI=1S/C28H21NO2/c30-24-16-20(19-11-5-2-6-12-19)15-23-25(24)26-27(21-13-7-8-14-22(21)28(26)31)29(23)17-18-9-3-1-4-10-18/h1-14,20H,15-17H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50029841

(CHEMBL3353420)Show SMILES [#6]-[#6](-[#6])-n1c2-[#6]-[#6]-[#6]-[#6](=O)-c2c2-[#6](=O)-c3cccc(-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])c3-c12 Show InChI InChI=1S/C23H25NO3/c1-13(2)11-12-27-18-10-5-7-15-19(18)22-21(23(15)26)20-16(24(22)14(3)4)8-6-9-17(20)25/h5,7,10-11,14H,6,8-9,12H2,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM15169

(1-{1-[(4-{7-phenyl-1H-imidazo[4,5-g]quinoxalin-6-y...)Show SMILES O=c1[nH]c2ccccc2n1C1CCN(Cc2ccc(cc2)-c2nc3cc4[nH]cnc4cc3nc2-c2ccccc2)CC1 Show InChI InChI=1S/C34H29N7O/c42-34-39-26-8-4-5-9-31(26)41(34)25-14-16-40(17-15-25)20-22-10-12-24(13-11-22)33-32(23-6-2-1-3-7-23)37-29-18-27-28(36-21-35-27)19-30(29)38-33/h1-13,18-19,21,25H,14-17,20H2,(H,35,36)(H,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of Akt3 (unknown origin) |
Eur J Med Chem 113: 214-27 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.047
BindingDB Entry DOI: 10.7270/Q2KD20TJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50602549

(CHEMBL5183354)Show SMILES COC[C@H](Cc1ccccc1)NC[C@@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50602549

(CHEMBL5183354)Show SMILES COC[C@H](Cc1ccccc1)NC[C@@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50602549

(CHEMBL5183354)Show SMILES COC[C@H](Cc1ccccc1)NC[C@@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50029749

(CHEMBL3353419)Show SMILES [#6]-[#6](-[#6])-n1c2-[#6]-[#6]-[#6]-[#6](=O)-c2c2-[#6](=O)-c3c(cccc3-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])-c12 Show InChI InChI=1S/C23H25NO3/c1-13(2)11-12-27-18-10-5-7-15-19(18)23(26)21-20-16(8-6-9-17(20)25)24(14(3)4)22(15)21/h5,7,10-11,14H,6,8-9,12H2,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50215414

(CHEMBL1096024 | MR-20814)Show SMILES COc1ccc2C(=O)C(CC3=CCNC=C3)=C(N)c2c1 |c:14,t:10,16| Show InChI InChI=1S/C16H16N2O2/c1-20-11-2-3-12-13(9-11)15(17)14(16(12)19)8-10-4-6-18-7-5-10/h2-6,9,18H,7-8,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase cytochrome P450 19A1 activity |
Bioorg Med Chem Lett 8: 1041-4 (1999)
BindingDB Entry DOI: 10.7270/Q2445KM7 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50029731

(CHEMBL3353413)Show InChI InChI=1S/C19H19NO2/c1-10(2)20-14-8-11(3)9-15(21)16(14)17-18(20)12-6-4-5-7-13(12)19(17)22/h4-7,10-11H,8-9H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50602550

(CHEMBL5177406)Show SMILES CCCCCNC[C@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50602549

(CHEMBL5183354)Show SMILES COC[C@H](Cc1ccccc1)NC[C@@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50602553

(CHEMBL5204309)Show SMILES CCCCCCCNC[C@@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50602551

(CHEMBL5181317)Show SMILES CCCCCNC[C@@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50377446

((S,R)-MEFLOQUINE)Show SMILES O[C@@H]([C@@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50377446

((S,R)-MEFLOQUINE)Show SMILES O[C@@H]([C@@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50151865

(CHEMBL172 | MEFLOQUINE)Show SMILES O[C@H]([C@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50029733
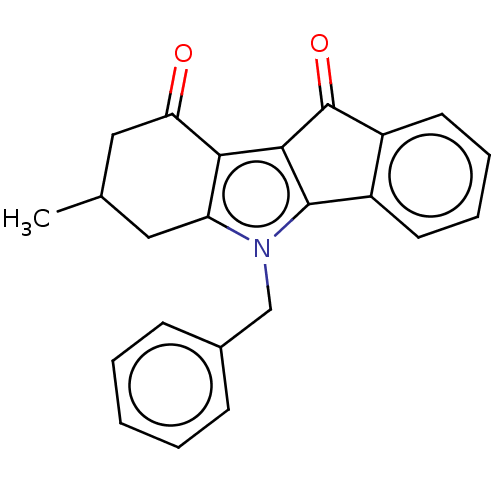
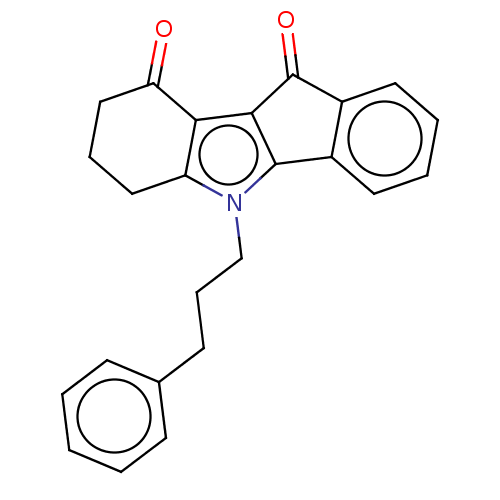
(CHEMBL1957203)Show SMILES CC1Cc2c(c3C(=O)c4ccccc4-c3n2Cc2ccccc2)C(=O)C1 Show InChI InChI=1S/C23H19NO2/c1-14-11-18-20(19(25)12-14)21-22(16-9-5-6-10-17(16)23(21)26)24(18)13-15-7-3-2-4-8-15/h2-10,14H,11-13H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50602549

(CHEMBL5183354)Show SMILES COC[C@H](Cc1ccccc1)NC[C@@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50151865

(CHEMBL172 | MEFLOQUINE)Show SMILES O[C@H]([C@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50377446

((S,R)-MEFLOQUINE)Show SMILES O[C@@H]([C@@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50151865

(CHEMBL172 | MEFLOQUINE)Show SMILES O[C@H]([C@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50029898

(CHEMBL3353421)Show InChI InChI=1S/C19H19NO2/c1-10(2)20-14-9-8-11(3)18(21)15(14)16-17(20)12-6-4-5-7-13(12)19(16)22/h4-7,10-11H,8-9H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50602552
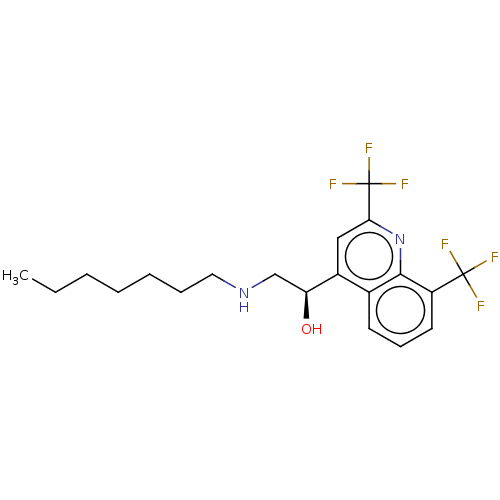
(CHEMBL5191501)Show SMILES CCCCCCCNC[C@H](O)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50029690

(CHEMBL1957191)Show InChI InChI=1S/C22H17NO2/c24-18-12-6-11-17-19(18)20-21(15-9-4-5-10-16(15)22(20)25)23(17)13-14-7-2-1-3-8-14/h1-5,7-10H,6,11-13H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50151865

(CHEMBL172 | MEFLOQUINE)Show SMILES O[C@H]([C@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50151865

(CHEMBL172 | MEFLOQUINE)Show SMILES O[C@H]([C@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50366114
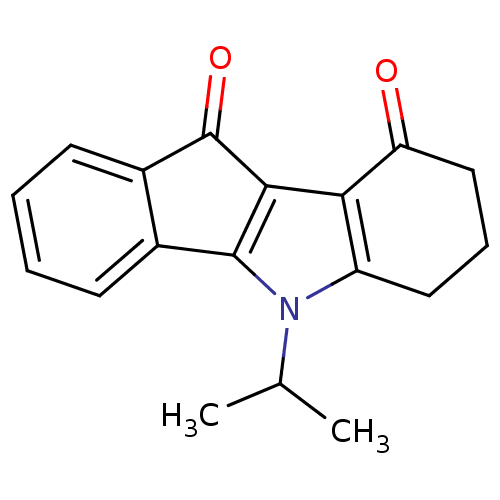
(CHEMBL1957190)Show InChI InChI=1S/C18H17NO2/c1-10(2)19-13-8-5-9-14(20)15(13)16-17(19)11-6-3-4-7-12(11)18(16)21/h3-4,6-7,10H,5,8-9H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in HEK293 cells assessed as reduction in mitoxantrone efflux after 30 mins by FACS method |
J Med Chem 58: 265-77 (2015)
Article DOI: 10.1021/jm500943z
BindingDB Entry DOI: 10.7270/Q2V40WST |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50377446

((S,R)-MEFLOQUINE)Show SMILES O[C@@H]([C@@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50377446

((S,R)-MEFLOQUINE)Show SMILES O[C@@H]([C@@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113981
BindingDB Entry DOI: 10.7270/Q2668J7B |
More data for this
Ligand-Target Pair | |
Histidine-rich protein PFHRP-II
(Plasmodium falciparum) | BDBM50411865
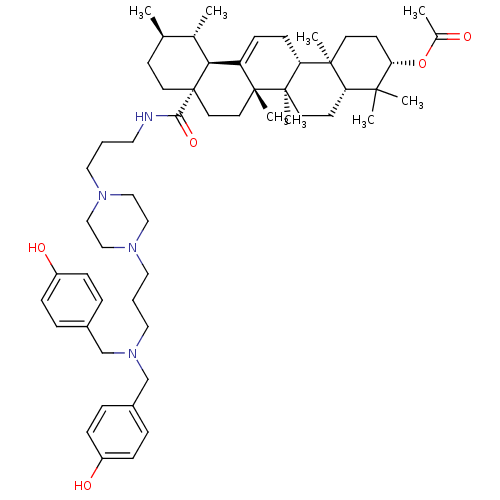
(CHEMBL261832)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](OC(C)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(=O)NCCCN1CCN(CCCN(Cc2ccc(O)cc2)Cc2ccc(O)cc2)CC1 |c:9| Show InChI InChI=1S/C56H84N4O5/c1-39-21-26-56(28-27-54(7)46(50(56)40(39)2)19-20-48-53(6)24-23-49(65-41(3)61)52(4,5)47(53)22-25-55(48,54)8)51(64)57-29-9-30-58-33-35-59(36-34-58)31-10-32-60(37-42-11-15-44(62)16-12-42)38-43-13-17-45(63)18-14-43/h11-19,39-40,47-50,62-63H,9-10,20-38H2,1-8H3,(H,57,64)/t39-,40+,47+,48-,49+,50+,53+,54-,55-,56+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.25E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of beta-hematin formation |
Bioorg Med Chem 16: 771-82 (2008)
Article DOI: 10.1016/j.bmc.2007.10.031
BindingDB Entry DOI: 10.7270/Q23N24MK |
More data for this
Ligand-Target Pair | |
Histidine-rich protein PFHRP-II
(Plasmodium falciparum) | BDBM22985
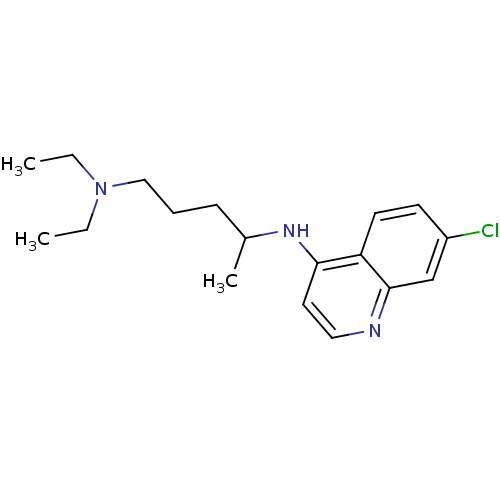
(Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...)Show InChI InChI=1S/C18H26ClN3/c1-4-22(5-2)12-6-7-14(3)21-17-10-11-20-18-13-15(19)8-9-16(17)18/h8-11,13-14H,4-7,12H2,1-3H3,(H,20,21) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul (UFRGS)
Curated by ChEMBL
| Assay Description
Inhibition of beta-hematin formation |
Bioorg Med Chem 16: 771-82 (2008)
Article DOI: 10.1016/j.bmc.2007.10.031
BindingDB Entry DOI: 10.7270/Q23N24MK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data