

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50202412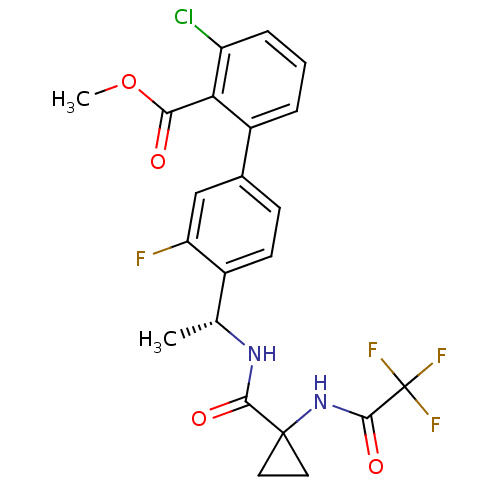 (3-Chloro-3'-fluoro-4'-((R)-1-{[1-(2,2,2-trifluoro-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Inhibition of human bradykinin B1 receptor | Bioorg Med Chem Lett 18: 5027-31 (2008) Article DOI: 10.1016/j.bmcl.2008.08.014 BindingDB Entry DOI: 10.7270/Q2NG4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50127438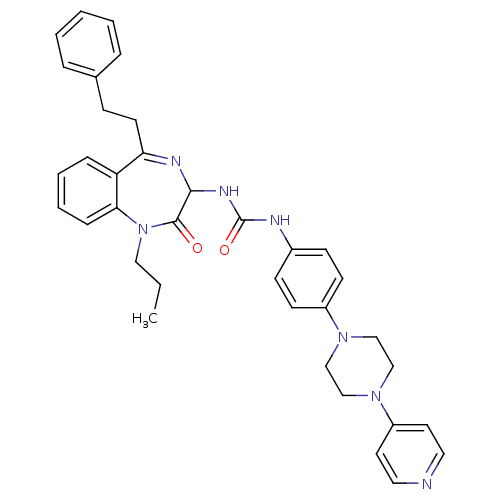 (1-(2-Oxo-5-phenethyl-1-propyl-2,3-dihydro-1H-benzo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Inhibition of human bradykinin B1 receptor | Bioorg Med Chem Lett 18: 5027-31 (2008) Article DOI: 10.1016/j.bmcl.2008.08.014 BindingDB Entry DOI: 10.7270/Q2NG4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 1 nM [3H]- N6 -(phenylisopropyl) adenosine binding to Adenosine A1 receptor in rat fat cell membrane | J Med Chem 32: 1231-7 (1989) BindingDB Entry DOI: 10.7270/Q2PZ57T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50132951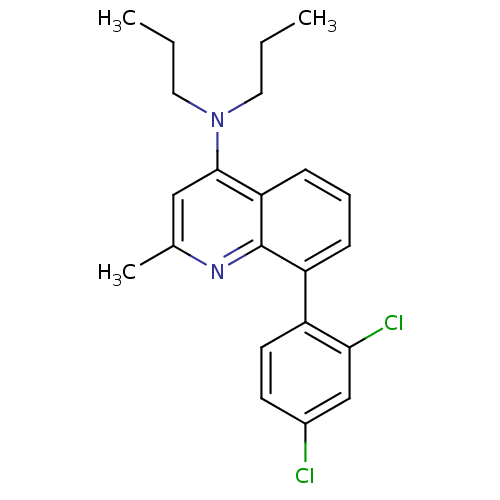 (8-(2,4-dichlorophenyl)-2-methyl-N,N-dipropylquinol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF1 receptor expressed in human IMR32 cells | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 1 nM [3H]- N6-(phenylisopropyl) adenosine binding to Adenosine A1 receptor in rat cerebral cortical membranes | J Med Chem 32: 1231-7 (1989) BindingDB Entry DOI: 10.7270/Q2PZ57T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (RAT) | BDBM50127438 (1-(2-Oxo-5-phenethyl-1-propyl-2,3-dihydro-1H-benzo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat bradykinin B1 receptor | Bioorg Med Chem Lett 18: 5027-31 (2008) Article DOI: 10.1016/j.bmcl.2008.08.014 BindingDB Entry DOI: 10.7270/Q2NG4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50007838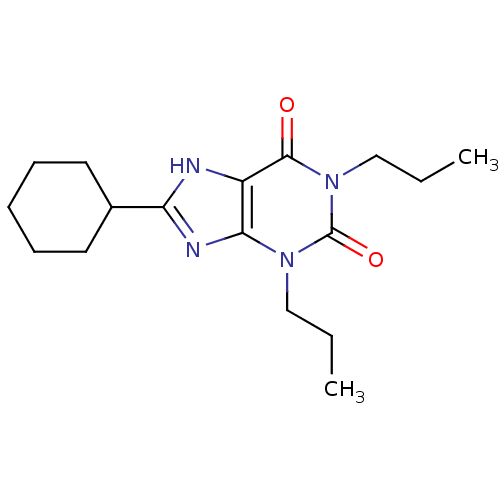 (8-Cyclohexyl-1,3-dipropyl-3,7-dihydro-purine-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 1 nM [3H]- N6-(phenylisopropyl) adenosine binding to Adenosine A1 receptor in rat cerebral cortical membranes | J Med Chem 32: 1231-7 (1989) BindingDB Entry DOI: 10.7270/Q2PZ57T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50007838 (8-Cyclohexyl-1,3-dipropyl-3,7-dihydro-purine-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 1 nM [3H]- N6 -(phenylisopropyl) adenosine binding to Adenosine A1 receptor in rat fat cell membrane | J Med Chem 32: 1231-7 (1989) BindingDB Entry DOI: 10.7270/Q2PZ57T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82013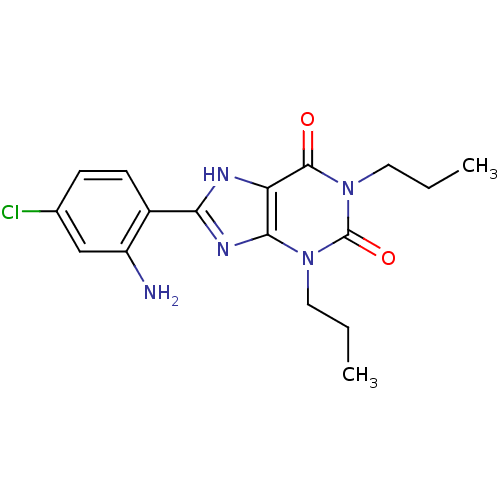 (8-(2-Amino-4-chloro-phenyl)-1,3-dipropyl-3,7-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonism of binding of 1 nM [3H]cyclohexyladenosine to adenosine A1 receptors on rat cortical membranes | J Med Chem 28: 487-92 (1985) BindingDB Entry DOI: 10.7270/Q2RV0P8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82013 (8-(2-Amino-4-chloro-phenyl)-1,3-dipropyl-3,7-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against A1 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50058163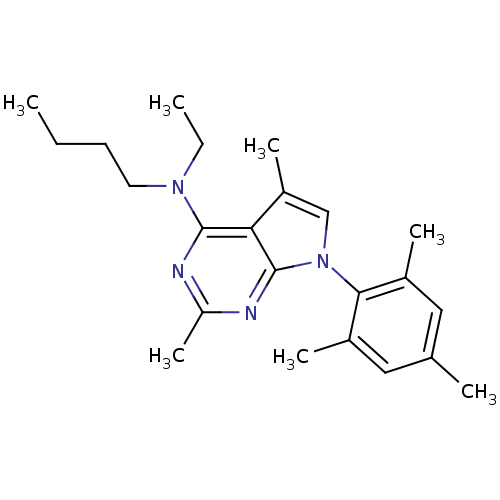 (Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF1 receptor expressed in human IMR32 cells | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82010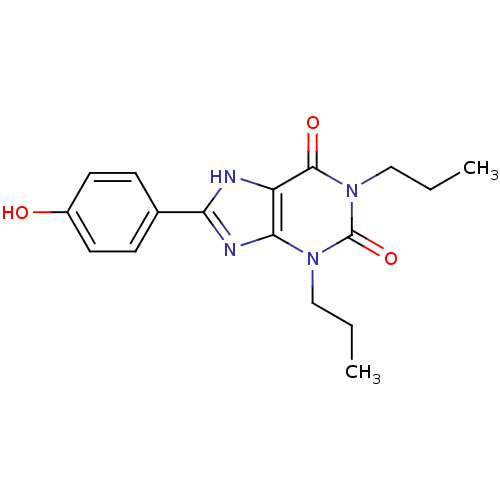 (1,3-Dipropyl-8-(4-hydroxyphenyl)xanthine | 8-(4-Hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against A1 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82010 (1,3-Dipropyl-8-(4-hydroxyphenyl)xanthine | 8-(4-Hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonism of binding of 1 nM [3H]cyclohexyladenosine to adenosine A1 receptors on rat cortical membranes | J Med Chem 28: 487-92 (1985) BindingDB Entry DOI: 10.7270/Q2RV0P8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50021004 (8-(2,4-Diamino-phenyl)-1,3-dipropyl-3,7-dihydro-pu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against A1 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50231916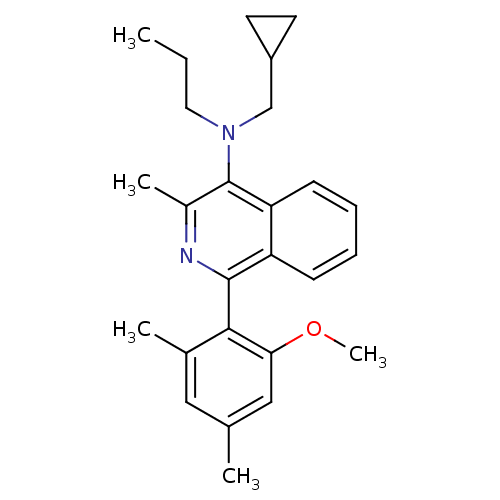 (CHEMBL404351 | N-(cyclopropylmethyl)-1-(2-methoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF1 receptor expressed in human IMR32 cells | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82025 (1,3-Dipropyl-8-phenylxanthine | 8-Phenyl-1,3-dipro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 1 nM [3H]- N6 -(phenylisopropyl) adenosine binding to Adenosine A1 receptor in rat fat cell membrane | J Med Chem 32: 1231-7 (1989) BindingDB Entry DOI: 10.7270/Q2PZ57T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50231921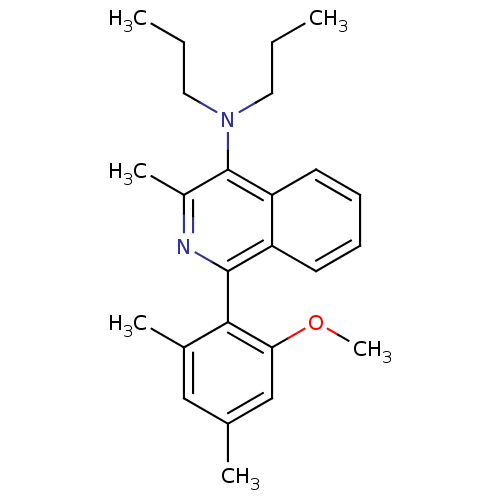 (1-(2-methoxy-4,6-dimethylphenyl)-3-methyl-N,N-dipr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF1 receptor expressed in human IMR32 cells | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50021453 (4-(2,6-Dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H-pu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against A1 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50021453 (4-(2,6-Dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H-pu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against A1 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50231925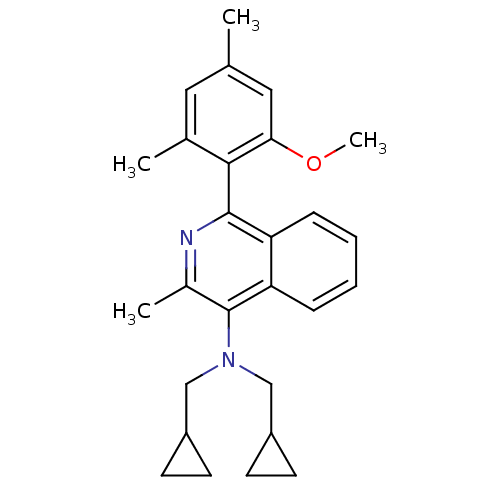 (CHEMBL253914 | N,N-bis(cyclopropylmethyl)-1-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF1 receptor expressed in human IMR32 cells | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50231907 (1-(2,4-bis(2,2-difluoroethyl)-6-methoxyphenyl)-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF1 receptor expressed in human IMR32 cells | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50021456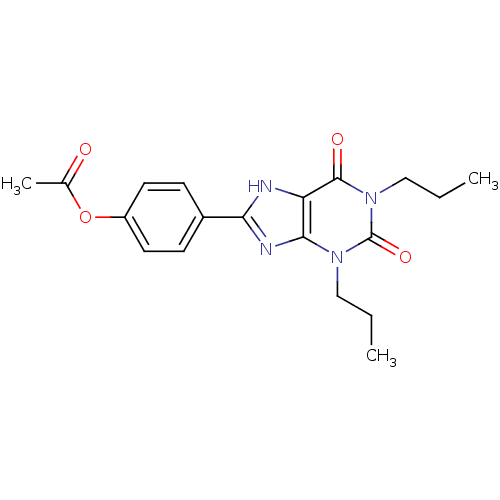 (Acetic acid 4-(2,6-dioxo-1,3-dipropyl-2,3,6,7-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against A1 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50021452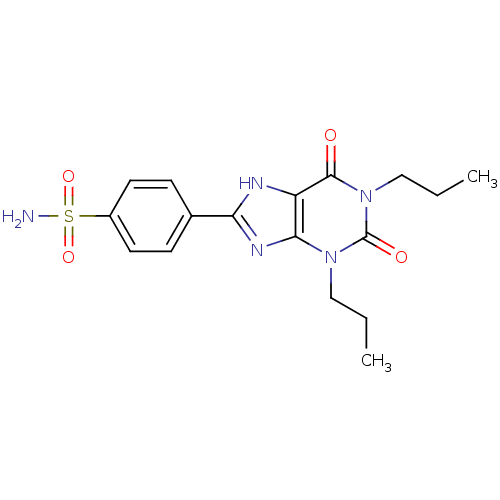 (4-(2,6-Dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H-pu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against A1 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50231927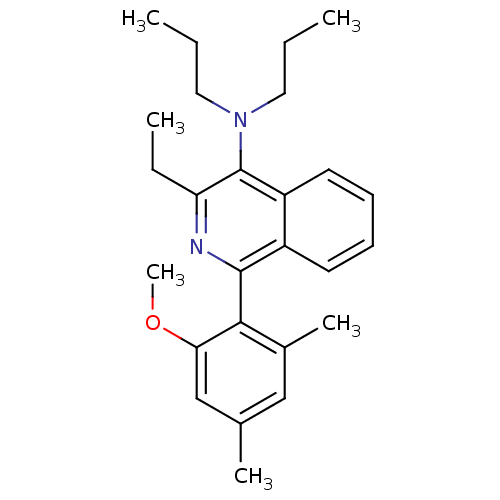 (3-ethyl-1-(2-methoxy-4,6-dimethylphenyl)-N,N-dipro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF1 receptor expressed in human IMR32 cells | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82025 (1,3-Dipropyl-8-phenylxanthine | 8-Phenyl-1,3-dipro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 1 nM [3H]- N6-(phenylisopropyl) adenosine binding to Adenosine A1 receptor in rat cerebral cortical membranes | J Med Chem 32: 1231-7 (1989) BindingDB Entry DOI: 10.7270/Q2PZ57T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM82025 (1,3-Dipropyl-8-phenylxanthine | 8-Phenyl-1,3-dipro...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of the stimulation by 5'-(N-ethylcarbamoyl) adenosine of adenyl cyclase via adenosine A2 receptor in human platelet membranes. | J Med Chem 32: 1231-7 (1989) BindingDB Entry DOI: 10.7270/Q2PZ57T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50021448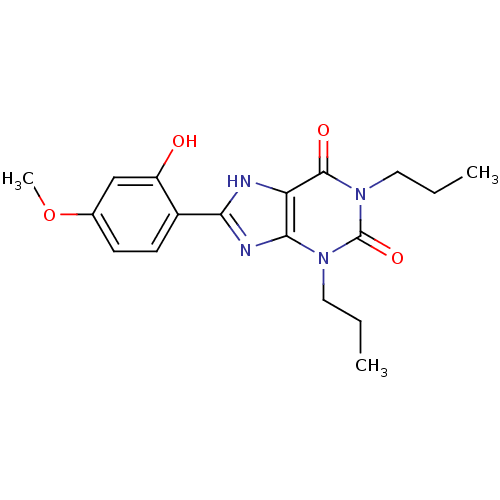 (8-(2-Hydroxy-4-methoxy-phenyl)-1,3-dipropyl-3,7-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against A1 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50321113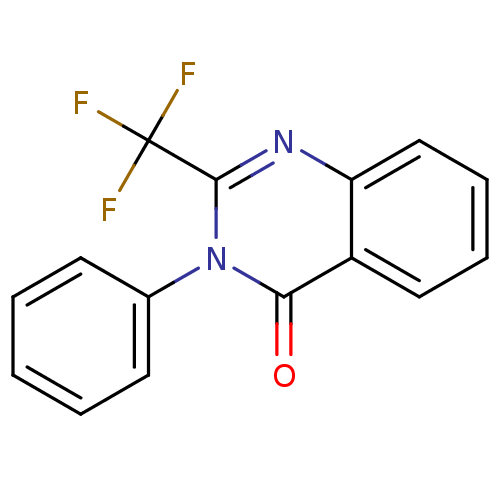 (3-Phenyl-2-(trifluoromethyl)quinazolin-4(3H)-one |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Troms£ Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin after 15 mins by microplate fluorescence analysis in presence of 0.5 to 2 mM substrate FaGLa | Bioorg Med Chem 18: 4317-27 (2010) Article DOI: 10.1016/j.bmc.2010.04.083 BindingDB Entry DOI: 10.7270/Q2QV3MP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50231921 (1-(2-methoxy-4,6-dimethylphenyl)-3-methyl-N,N-dipr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at CRF1 receptor expressed in mouse AtT20 cells assessed as inhibition of sauvagine-stimulated cAMP accumulation | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50021456 (Acetic acid 4-(2,6-dioxo-1,3-dipropyl-2,3,6,7-tetr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against A2 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82025 (1,3-Dipropyl-8-phenylxanthine | 8-Phenyl-1,3-dipro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonism of binding of 1 nM [3H]cyclohexyladenosine to adenosine A1 receptors on rat cortical membranes | J Med Chem 28: 487-92 (1985) BindingDB Entry DOI: 10.7270/Q2RV0P8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50021004 (8-(2,4-Diamino-phenyl)-1,3-dipropyl-3,7-dihydro-pu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against A2 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82025 (1,3-Dipropyl-8-phenylxanthine | 8-Phenyl-1,3-dipro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against A1 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50021455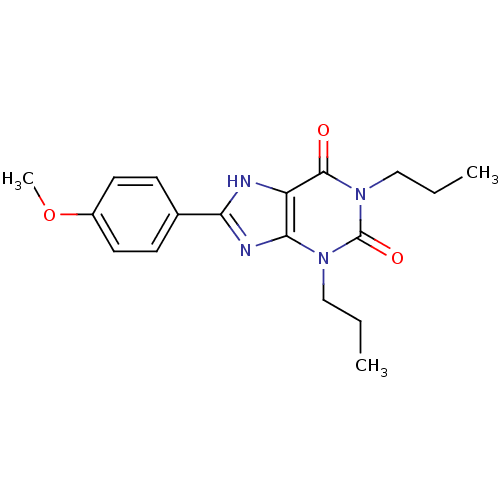 (8-(4-Methoxy-phenyl)-1,3-dipropyl-3,7-dihydro-puri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against A1 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50021449 (8-(2,4-Dihydroxy-phenyl)-1,3-dipropyl-3,7-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against A1 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50231919 (1-(4-(difluoromethoxy)-2-methoxyphenyl)-3-methyl-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF1 receptor expressed in human IMR32 cells | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM50018152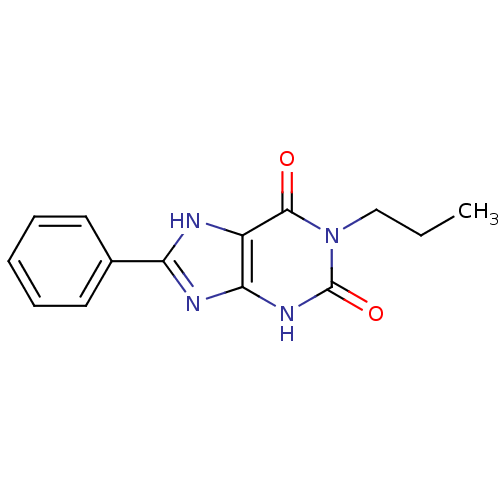 (8-Phenyl-1-propyl-3,7-dihydro-purine-2,6-dione | C...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 1 nM [3H]- N6 -(phenylisopropyl) adenosine binding to Adenosine A1 receptor in rat fat cell membrane | J Med Chem 32: 1231-7 (1989) BindingDB Entry DOI: 10.7270/Q2PZ57T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50231930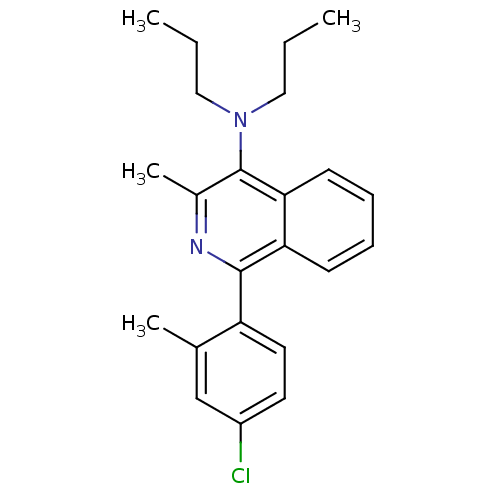 (1-(4-chloro-2-methylphenyl)-3-methyl-N,N-dipropyli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF1 receptor expressed in human IMR32 cells | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50231907 (1-(2,4-bis(2,2-difluoroethyl)-6-methoxyphenyl)-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at CRF1 receptor expressed in mouse AtT20 cells assessed as inhibition of sauvagine-stimulated cAMP accumulation | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM81925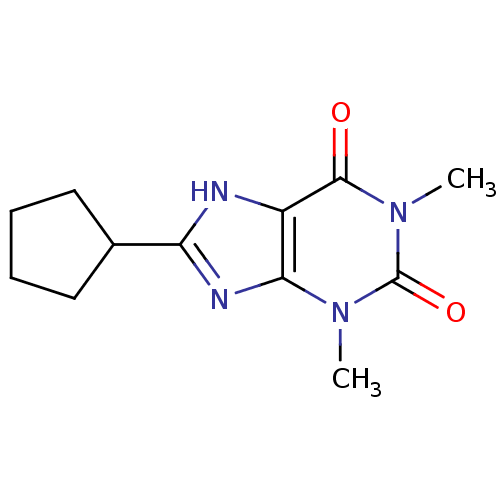 (8-Cyclopentyl-1,3-dimethyl-3,7-dihydro-purine-2,6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 1 nM [3H]- N6-(phenylisopropyl) adenosine binding to Adenosine A1 receptor in rat cerebral cortical membranes | J Med Chem 32: 1231-7 (1989) BindingDB Entry DOI: 10.7270/Q2PZ57T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50231910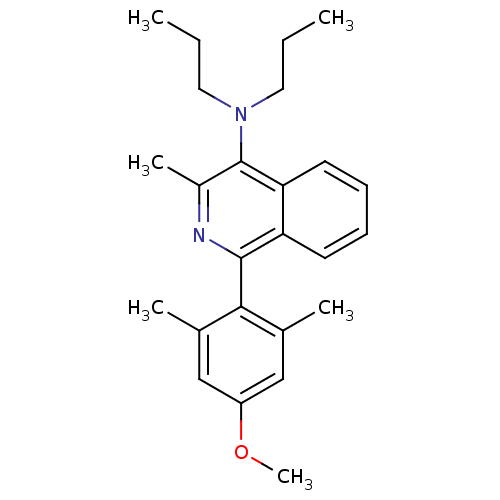 (1-(4-methoxy-2,6-dimethylphenyl)-3-methyl-N,N-dipr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF1 receptor expressed in human IMR32 cells | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50021450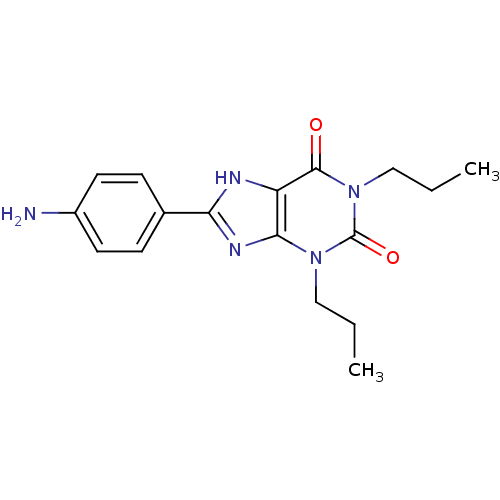 (8-(4-Amino-phenyl)-1,3-dipropyl-3,7-dihydro-purine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against A1 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50021450 (8-(4-Amino-phenyl)-1,3-dipropyl-3,7-dihydro-purine...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against A2 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50231909 (1-(4-methoxy-2-methylphenyl)-3-methyl-N,N-dipropyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF1 receptor expressed in human IMR32 cells | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50231926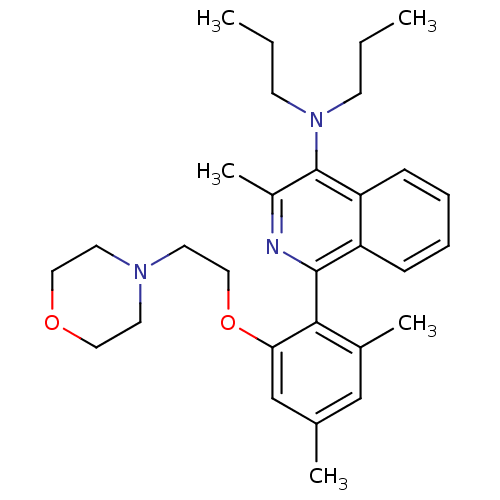 (1-(2,4-dimethyl-6-(2-morpholinoethoxy)phenyl)-3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF1 receptor expressed in human IMR32 cells | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50231912 (1-(2-(2-(4-(dipropylamino)-3-methylisoquinolin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF1 receptor expressed in human IMR32 cells | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50021457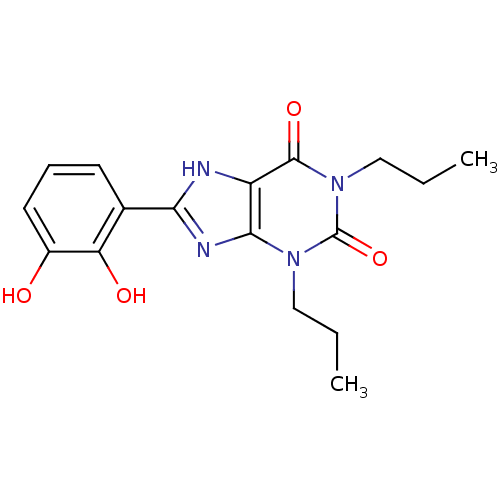 (8-(2,3-Dihydroxy-phenyl)-1,3-dipropyl-3,7-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against A1 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50021460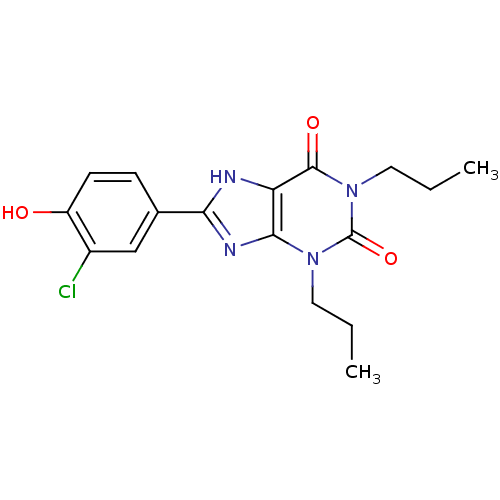 (8-(3-Chloro-4-hydroxy-phenyl)-1,3-dipropyl-3,7-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against A1 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50231923 (1-(2-ethyl-4-methoxyphenyl)-3-methyl-N,N-dipropyli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF1 receptor expressed in human IMR32 cells | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50018152 (8-Phenyl-1-propyl-3,7-dihydro-purine-2,6-dione | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of 1 nM [3H]- N6 -(phenylisopropyl) adenosine binding to Adenosine A1 receptor in rat fat cell membrane | J Med Chem 32: 1231-7 (1989) BindingDB Entry DOI: 10.7270/Q2PZ57T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1548 total ) | Next | Last >> |