

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM8726 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Immunology Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum ENR in presence of EGCG by dilution assay | J Med Chem 50: 765-75 (2007) Article DOI: 10.1021/jm061154d BindingDB Entry DOI: 10.7270/Q2QJ7J4Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM8726 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0521 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Immunology Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum ENR in presence of ECG by dilution assay | J Med Chem 50: 765-75 (2007) Article DOI: 10.1021/jm061154d BindingDB Entry DOI: 10.7270/Q2QJ7J4Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM8726 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Immunology Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum ENR in presence of EGC by dilution assay | J Med Chem 50: 765-75 (2007) Article DOI: 10.1021/jm061154d BindingDB Entry DOI: 10.7270/Q2QJ7J4Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM8726 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.281 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Immunology Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum ENR in presence of quercetin by dilution assay | J Med Chem 50: 765-75 (2007) Article DOI: 10.1021/jm061154d BindingDB Entry DOI: 10.7270/Q2QJ7J4Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50413787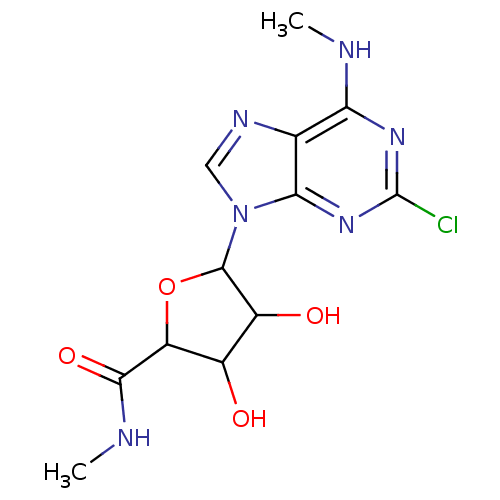 (CHEMBL449380) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells | Eur J Med Chem 44: 1377-82 (2009) Article DOI: 10.1016/j.ejmech.2008.09.022 BindingDB Entry DOI: 10.7270/Q20C4X03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474629 (CHEMBL415006) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand co-expressed with delta and kappa opioid receptor... | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells | Eur J Med Chem 44: 1377-82 (2009) Article DOI: 10.1016/j.ejmech.2008.09.022 BindingDB Entry DOI: 10.7270/Q20C4X03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50413786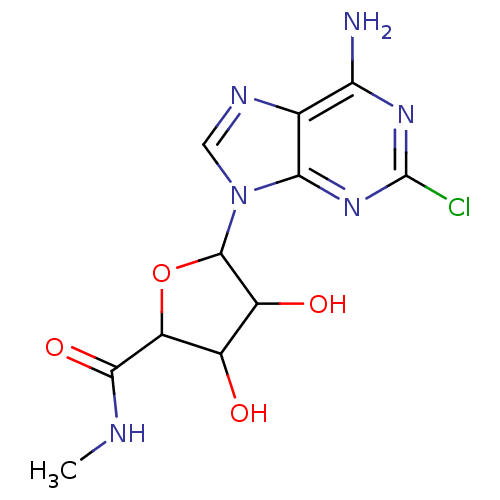 (CHEMBL483954) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells | Eur J Med Chem 44: 1377-82 (2009) Article DOI: 10.1016/j.ejmech.2008.09.022 BindingDB Entry DOI: 10.7270/Q20C4X03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM529895 (US11203594, Example 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose CDK4/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitors by us... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474628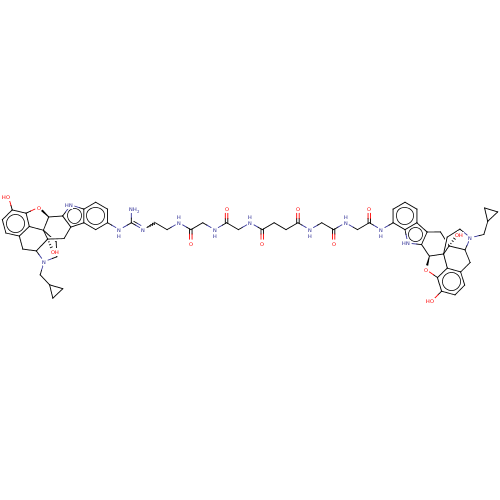 (CHEMBL386810) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand co-expressed with delta and kappa opioid receptor... | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50413798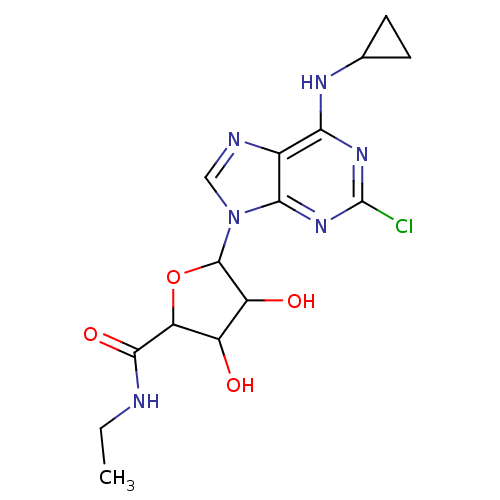 (CHEMBL488149) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells | Eur J Med Chem 44: 1377-82 (2009) Article DOI: 10.1016/j.ejmech.2008.09.022 BindingDB Entry DOI: 10.7270/Q20C4X03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM8726 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.798 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Immunology Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum ENR in presence of butein by dilution assay | J Med Chem 50: 765-75 (2007) Article DOI: 10.1021/jm061154d BindingDB Entry DOI: 10.7270/Q2QJ7J4Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM529784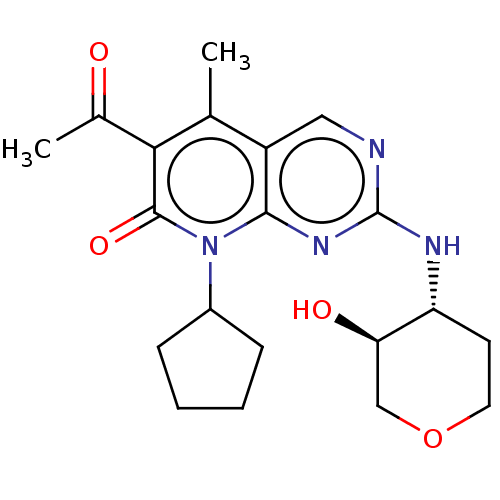 (US11203594, Example 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose CDK4/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitors by us... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50413804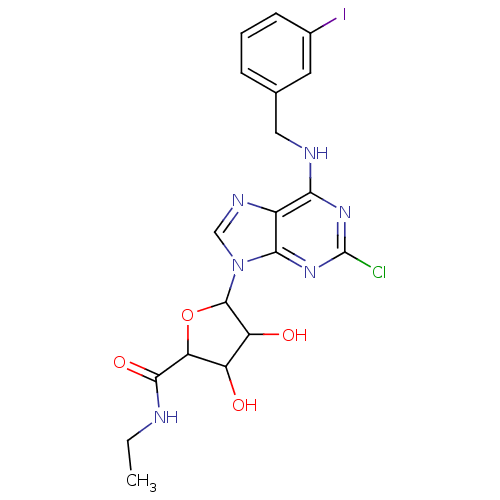 (CHEMBL486171) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells | Eur J Med Chem 44: 1377-82 (2009) Article DOI: 10.1016/j.ejmech.2008.09.022 BindingDB Entry DOI: 10.7270/Q20C4X03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM529904 (US11203594, Example 14 | US11203594, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CDK4/Cyclin D1 and CDK6/Cylcin D3: The Chelation-Enhanced Fluorescence (CHEF) monitors the phosphorylation state in real time where the level of fluo... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM8726 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Immunology Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum ENR in presence of EGCG | J Med Chem 50: 765-75 (2007) Article DOI: 10.1021/jm061154d BindingDB Entry DOI: 10.7270/Q2QJ7J4Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50413790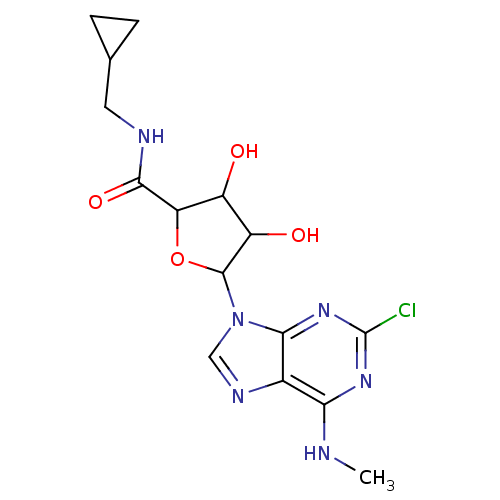 (CHEMBL483969) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells | Eur J Med Chem 44: 1377-82 (2009) Article DOI: 10.1016/j.ejmech.2008.09.022 BindingDB Entry DOI: 10.7270/Q20C4X03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM529893 (US11203594, Example 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CDK4/Cyclin D1 and CDK6/Cylcin D3: The Chelation-Enhanced Fluorescence (CHEF) monitors the phosphorylation state in real time where the level of fluo... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474624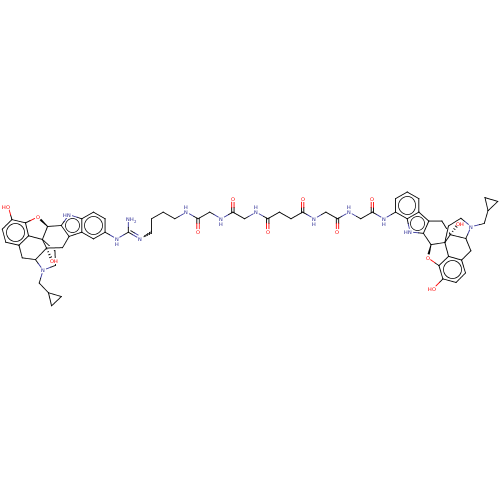 (CHEMBL409172) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand singly expressed with delta or kappa receptor | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50100462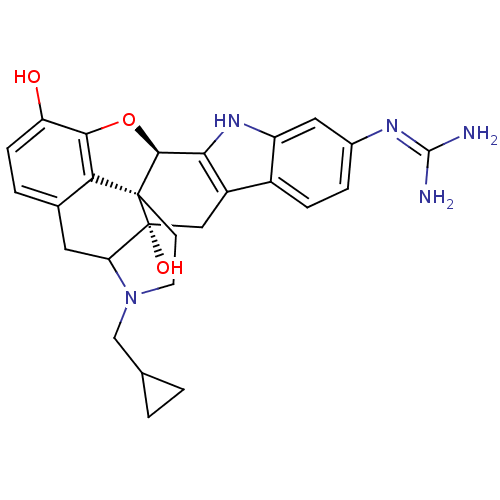 (6`-guanidino-17-(cyclopropylmethyl)-6,7-didehydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Effect on binding to wild-type opioid receptor kappa 1 using [3H]diprenorphine in transiently expressed rat HEK293 cells | J Med Chem 44: 2073-9 (2001) BindingDB Entry DOI: 10.7270/Q2416XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50100462 (6`-guanidino-17-(cyclopropylmethyl)-6,7-didehydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards rat opioid receptor kappa 1 was determined using [3H]diprenorphine radioligand | J Med Chem 44: 2073-9 (2001) BindingDB Entry DOI: 10.7270/Q2416XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM204859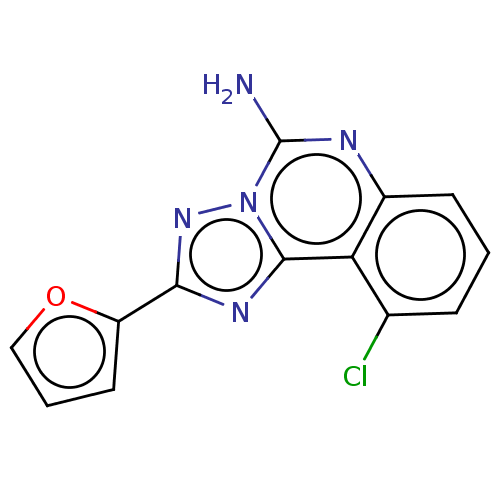 (US9227979, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM529784 (US11203594, Example 2) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the CDK6/Cyclin D3 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitor... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM529895 (US11203594, Example 5) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the CDK6/Cyclin D3 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitor... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474624 (CHEMBL409172) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand co-expressed with delta and kappa opioid receptor... | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM529894 (US11203594, Example 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CDK4/Cyclin D1 and CDK6/Cylcin D3: The Chelation-Enhanced Fluorescence (CHEF) monitors the phosphorylation state in real time where the level of fluo... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50413791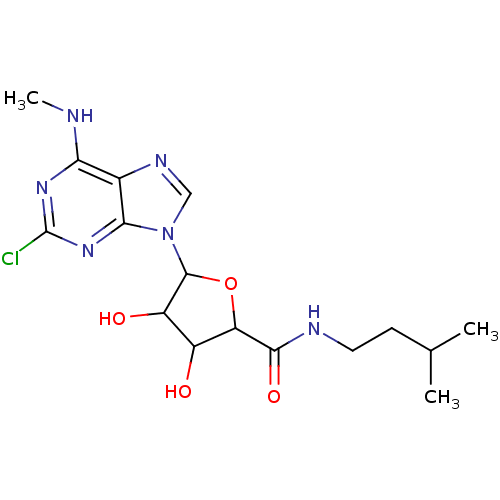 (CHEMBL483975) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells | Eur J Med Chem 44: 1377-82 (2009) Article DOI: 10.1016/j.ejmech.2008.09.022 BindingDB Entry DOI: 10.7270/Q20C4X03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM529656 (US11203594, Example 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CDK4/Cyclin D1 and CDK6/Cylcin D3: The Chelation-Enhanced Fluorescence (CHEF) monitors the phosphorylation state in real time where the level of fluo... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM529919 (US11203594, Example 29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CDK4/Cyclin D1 and CDK6/Cylcin D3: The Chelation-Enhanced Fluorescence (CHEF) monitors the phosphorylation state in real time where the level of fluo... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM529656 (US11203594, Example 1) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CDK4/Cyclin D1 and CDK6/Cylcin D3: The Chelation-Enhanced Fluorescence (CHEF) monitors the phosphorylation state in real time where the level of fluo... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM529894 (US11203594, Example 4) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CDK4/Cyclin D1 and CDK6/Cylcin D3: The Chelation-Enhanced Fluorescence (CHEF) monitors the phosphorylation state in real time where the level of fluo... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM529902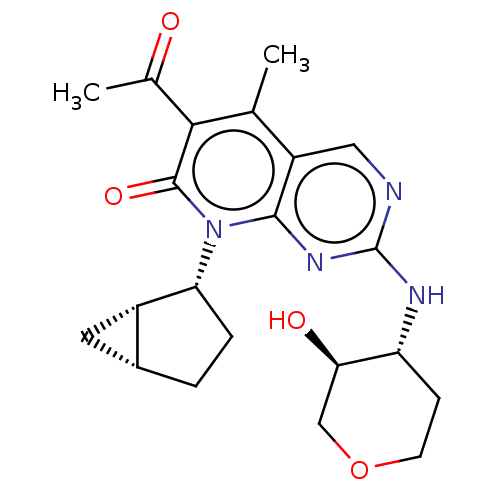 (US11203594, Example 12 | US11203594, Example 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CDK4/Cyclin D1 and CDK6/Cylcin D3: The Chelation-Enhanced Fluorescence (CHEF) monitors the phosphorylation state in real time where the level of fluo... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM529893 (US11203594, Example 3) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CDK4/Cyclin D1 and CDK6/Cylcin D3: The Chelation-Enhanced Fluorescence (CHEF) monitors the phosphorylation state in real time where the level of fluo... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50413816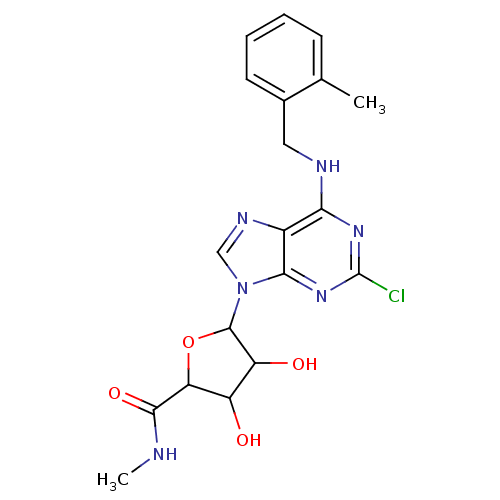 (CHEMBL516346) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells | Eur J Med Chem 44: 1377-82 (2009) Article DOI: 10.1016/j.ejmech.2008.09.022 BindingDB Entry DOI: 10.7270/Q20C4X03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50492667 (CHEMBL2407709) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase 9 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay | Eur J Med Chem 66: 372-9 (2013) Article DOI: 10.1016/j.ejmech.2013.06.003 BindingDB Entry DOI: 10.7270/Q2K93BF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50413797 (CHEMBL488148) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells | Eur J Med Chem 44: 1377-82 (2009) Article DOI: 10.1016/j.ejmech.2008.09.022 BindingDB Entry DOI: 10.7270/Q20C4X03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50413817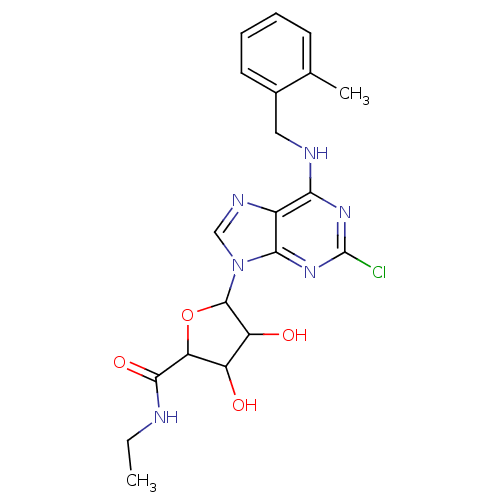 (CHEMBL473502) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells | Eur J Med Chem 44: 1377-82 (2009) Article DOI: 10.1016/j.ejmech.2008.09.022 BindingDB Entry DOI: 10.7270/Q20C4X03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM529904 (US11203594, Example 14 | US11203594, Example 15) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CDK4/Cyclin D1 and CDK6/Cylcin D3: The Chelation-Enhanced Fluorescence (CHEF) monitors the phosphorylation state in real time where the level of fluo... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM529906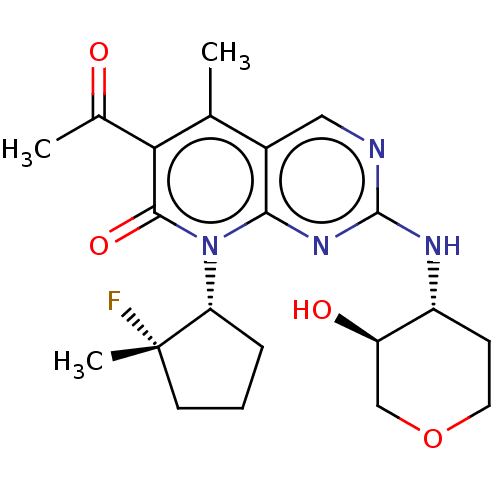 (US11203594, Example 16 | US11203594, Example 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CDK4/Cyclin D1 and CDK6/Cylcin D3: The Chelation-Enhanced Fluorescence (CHEF) monitors the phosphorylation state in real time where the level of fluo... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM204870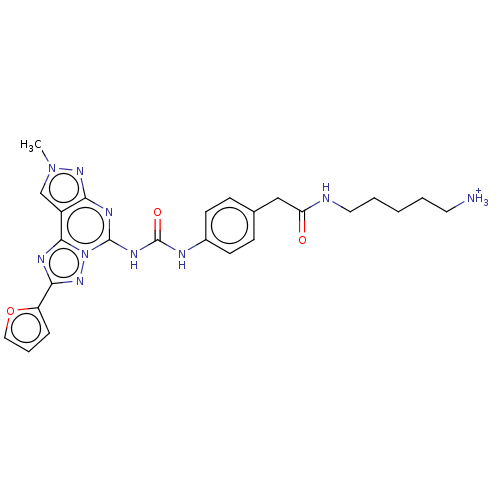 (EA7/MRS5816 | US9227979, 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM529898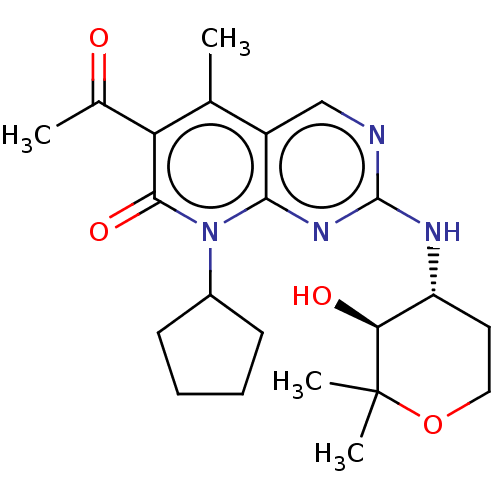 (US11203594, Example 8 | US11203594, Example 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CDK4/Cyclin D1 and CDK6/Cylcin D3: The Chelation-Enhanced Fluorescence (CHEF) monitors the phosphorylation state in real time where the level of fluo... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM529900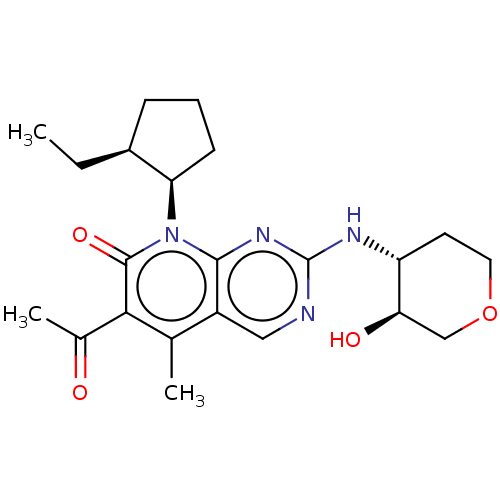 (US11203594, Example 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CDK4/Cyclin D1 and CDK6/Cylcin D3: The Chelation-Enhanced Fluorescence (CHEF) monitors the phosphorylation state in real time where the level of fluo... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM204863 (EA12/MRS5821 | US9227979, 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50100464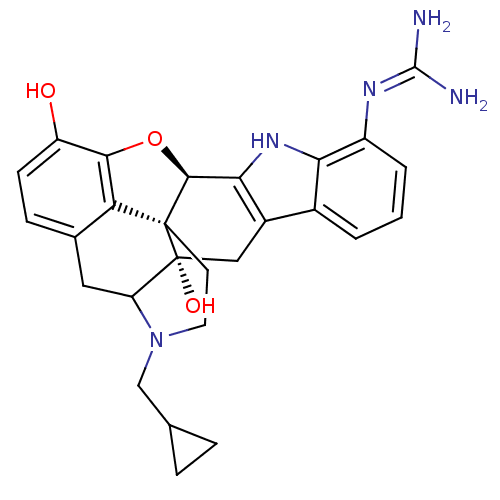 (7`-guanidino-17-(cyclopropylmethyl)-6,7-didehydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards mouse delta-opioid receptor was determined using [3H]diprenorphine as radioligand | J Med Chem 44: 2073-9 (2001) BindingDB Entry DOI: 10.7270/Q2416XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM529912 (US11203594, Example 22) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The purpose of the CDK6/Cyclin D3 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitor... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50413789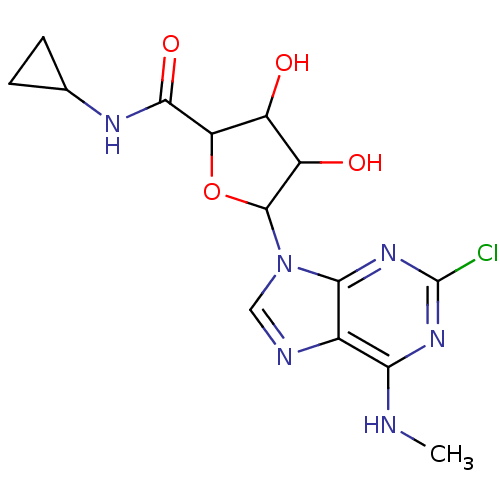 (CHEMBL521312) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells | Eur J Med Chem 44: 1377-82 (2009) Article DOI: 10.1016/j.ejmech.2008.09.022 BindingDB Entry DOI: 10.7270/Q20C4X03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50413803 (CHEMBL487754) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells | Eur J Med Chem 44: 1377-82 (2009) Article DOI: 10.1016/j.ejmech.2008.09.022 BindingDB Entry DOI: 10.7270/Q20C4X03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50413806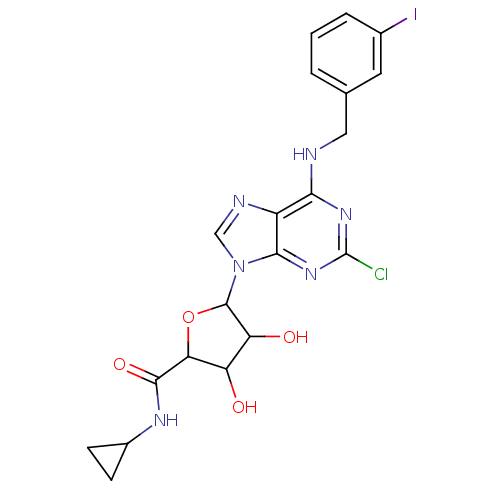 (CHEMBL486374) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sobhasaria Engineering College Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MEAC from human adenosine A3 receptor expressed in CHO cells | Eur J Med Chem 44: 1377-82 (2009) Article DOI: 10.1016/j.ejmech.2008.09.022 BindingDB Entry DOI: 10.7270/Q20C4X03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM529902 (US11203594, Example 12 | US11203594, Example 13) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CDK4/Cyclin D1 and CDK6/Cylcin D3: The Chelation-Enhanced Fluorescence (CHEF) monitors the phosphorylation state in real time where the level of fluo... | Citation and Details BindingDB Entry DOI: 10.7270/Q24B34GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474626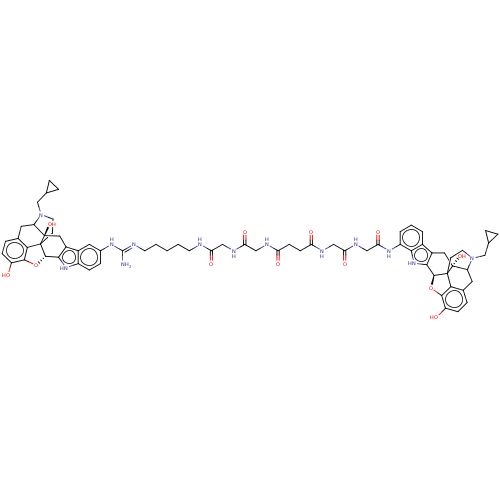 (CHEMBL414603) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand singly expressed with delta or kappa receptor | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4259 total ) | Next | Last >> |