

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8336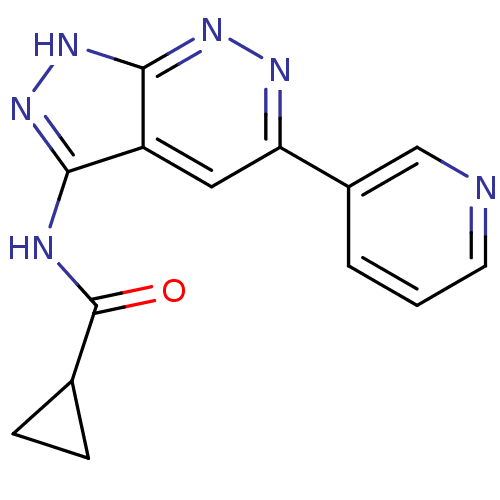 (N-[5-(pyridin-3-yl)-1H-pyrazolo[3,4-c]pyridazin-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | -57.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8337 (N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8339 (N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.190 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50287729 (3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2121-2126 (1996) Article DOI: 10.1016/0960-894X(96)00383-6 BindingDB Entry DOI: 10.7270/Q2NS0TW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8338 (N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.950 | -51.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085043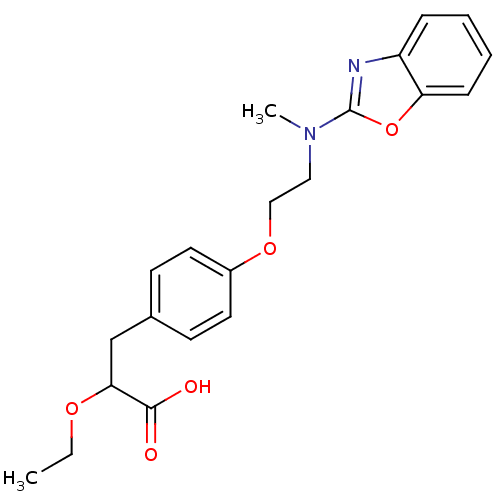 (3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2121-2126 (1996) Article DOI: 10.1016/0960-894X(96)00383-6 BindingDB Entry DOI: 10.7270/Q2NS0TW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085043 (3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2127-2130 (1996) Article DOI: 10.1016/0960-894X(96)00382-4 BindingDB Entry DOI: 10.7270/Q2J1034Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8336 (N-[5-(pyridin-3-yl)-1H-pyrazolo[3,4-c]pyridazin-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | -46.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 21 |
GlaxoSmithKline | Assay Description In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8337 (N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | -46.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 21 |
GlaxoSmithKline | Assay Description In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8296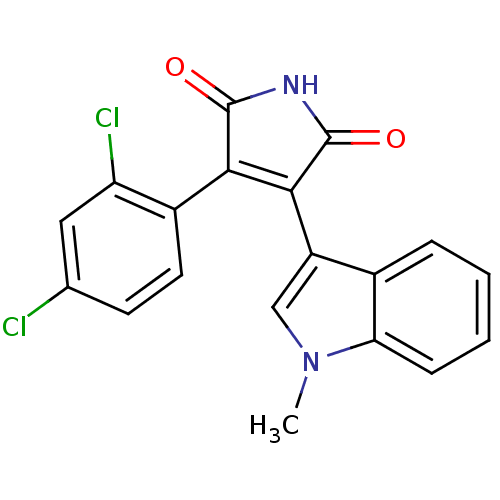 (3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.10 | -45.4 | 34.3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
SmithKline Beecham Pharmaceuticals | Assay Description In vitro kinase inhibition assay using purified GSK-3 alpha from insect cells, was incubated at room temperature with substrate, and test compounds i... | Chem Biol 7: 793-803 (2000) Article DOI: 10.1016/s1074-5521(00)00025-9 BindingDB Entry DOI: 10.7270/Q2M32T0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50287732 (3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2121-2126 (1996) Article DOI: 10.1016/0960-894X(96)00383-6 BindingDB Entry DOI: 10.7270/Q2NS0TW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50049244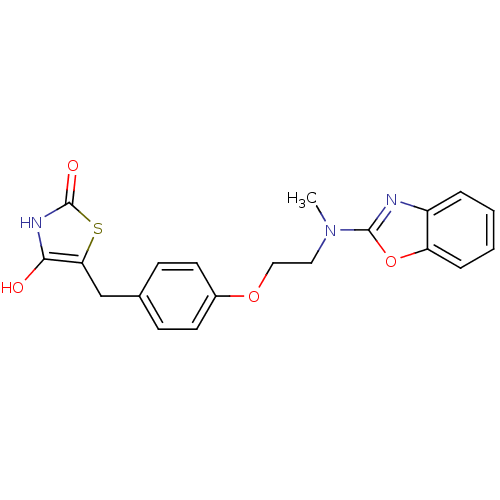 (5-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2121-2126 (1996) Article DOI: 10.1016/0960-894X(96)00383-6 BindingDB Entry DOI: 10.7270/Q2NS0TW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50049244 (5-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2127-2130 (1996) Article DOI: 10.1016/0960-894X(96)00382-4 BindingDB Entry DOI: 10.7270/Q2J1034Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50287733 (2-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2127-2130 (1996) Article DOI: 10.1016/0960-894X(96)00382-4 BindingDB Entry DOI: 10.7270/Q2J1034Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8297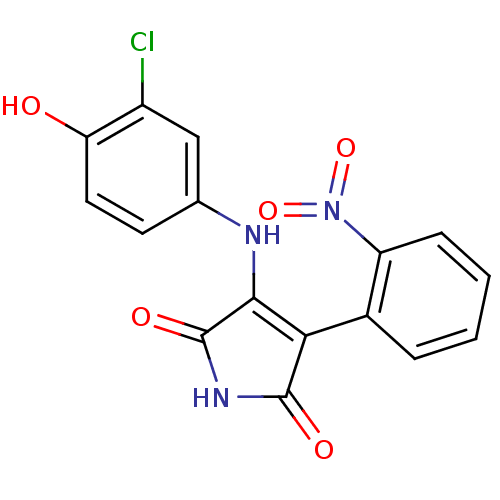 (3-[(3-chloro-4-hydroxyphenyl)amino]-4-(2-nitrophen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 30.8 | -42.4 | 77.5 | n/a | n/a | n/a | n/a | 7.0 | 22 |
SmithKline Beecham Pharmaceuticals | Assay Description In vitro kinase inhibition assay using purified GSK-3 alpha from insect cells, was incubated at room temperature with substrate, and test compounds i... | Chem Biol 7: 793-803 (2000) Article DOI: 10.1016/s1074-5521(00)00025-9 BindingDB Entry DOI: 10.7270/Q2M32T0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50287727 (3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2121-2126 (1996) Article DOI: 10.1016/0960-894X(96)00383-6 BindingDB Entry DOI: 10.7270/Q2NS0TW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50287730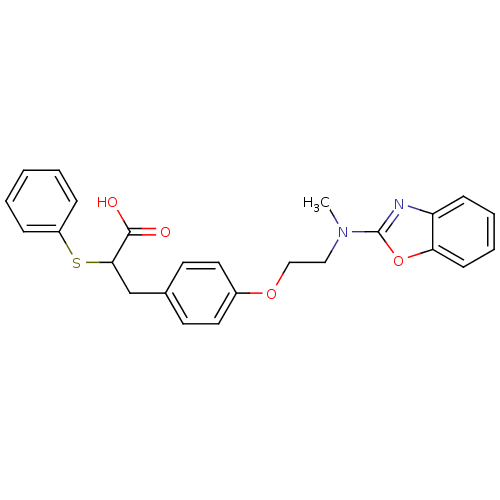 (3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2121-2126 (1996) Article DOI: 10.1016/0960-894X(96)00383-6 BindingDB Entry DOI: 10.7270/Q2NS0TW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8338 (N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 450 | -35.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 21 |
GlaxoSmithKline | Assay Description In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8339 (N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 540 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 21 |
GlaxoSmithKline | Assay Description In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50287728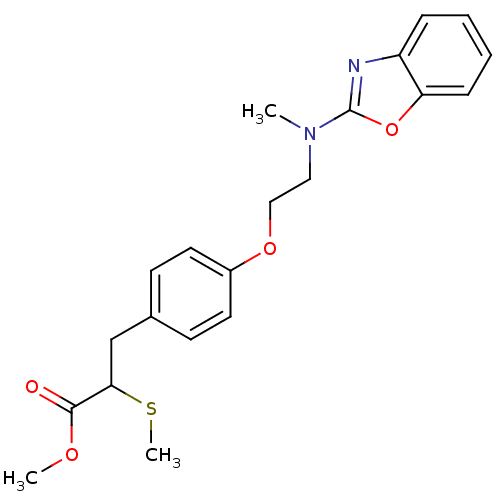 (3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2121-2126 (1996) Article DOI: 10.1016/0960-894X(96)00383-6 BindingDB Entry DOI: 10.7270/Q2NS0TW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50287734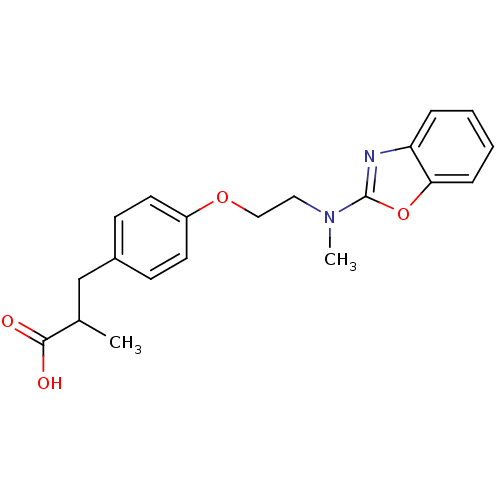 (3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2127-2130 (1996) Article DOI: 10.1016/0960-894X(96)00382-4 BindingDB Entry DOI: 10.7270/Q2J1034Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8327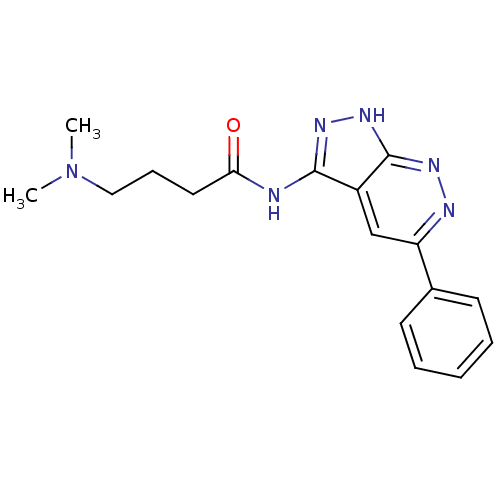 (4-(dimethylamino)-N-{5-phenyl-1H-pyrazolo[3,4-c]py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | -32.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 21 |
GlaxoSmithKline | Assay Description In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085045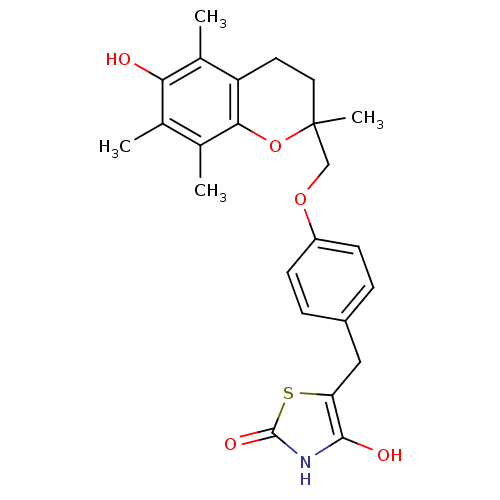 (5-((4-((6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2121-2126 (1996) Article DOI: 10.1016/0960-894X(96)00383-6 BindingDB Entry DOI: 10.7270/Q2NS0TW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50287731 (3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor | Bioorg Med Chem Lett 6: 2121-2126 (1996) Article DOI: 10.1016/0960-894X(96)00383-6 BindingDB Entry DOI: 10.7270/Q2NS0TW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8330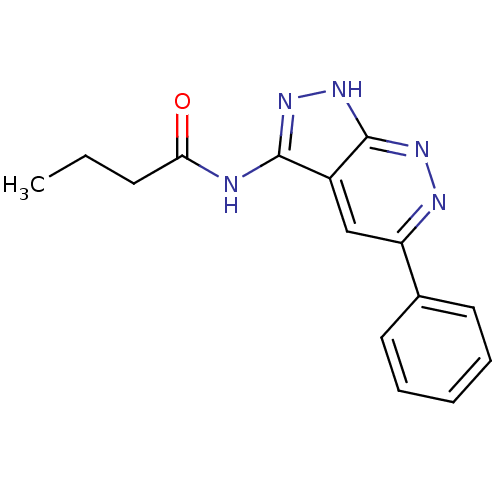 (N-{5-phenyl-1H-pyrazolo[3,4-c]pyridazin-3-yl}butan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8332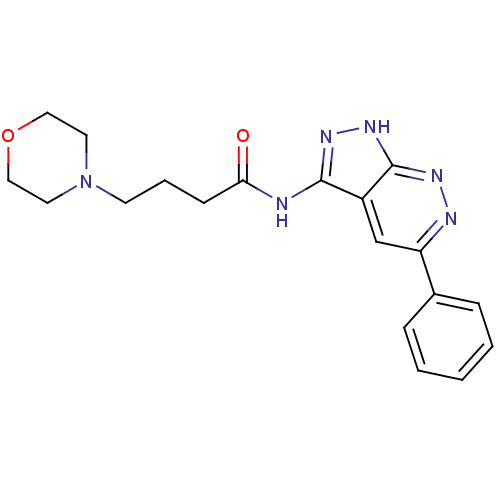 (4-(morpholin-4-yl)-N-{5-phenyl-1H-pyrazolo[3,4-c]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8334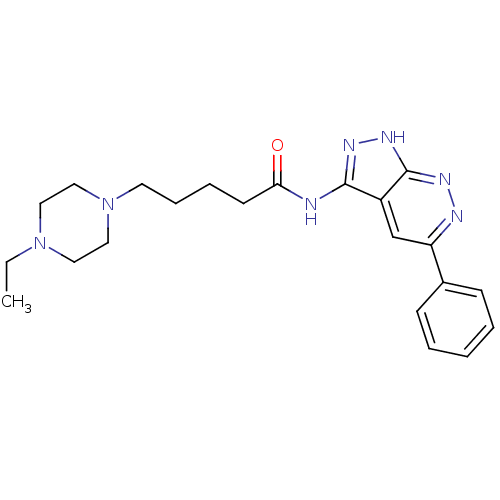 (5-(4-ethylpiperazin-1-yl)-N-{5-phenyl-1H-pyrazolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8335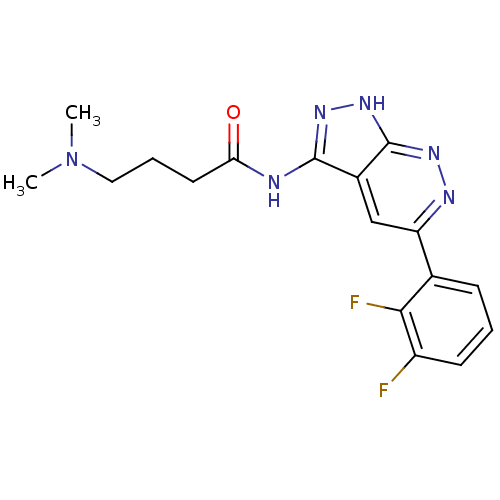 (N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8331 (4-(4-ethylpiperazin-1-yl)-N-{5-phenyl-1H-pyrazolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8333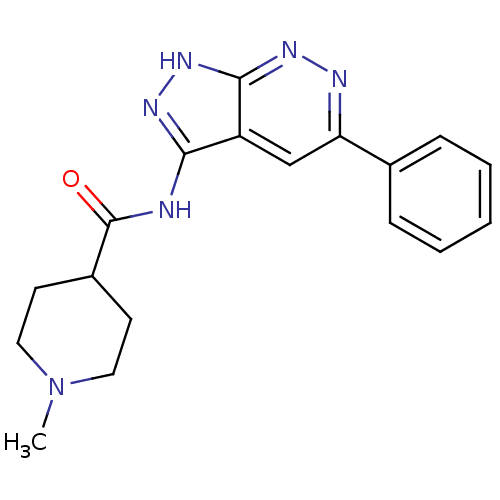 (1-methyl-N-{5-phenyl-1H-pyrazolo[3,4-c]pyridazin-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50082152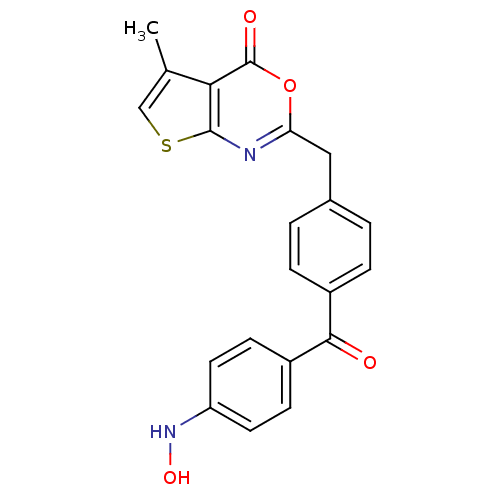 (2-[4-(4-Hydroxyamino-benzoyl)-benzyl]-5-methyl-thi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against Human Cytomegalovirus (hCMV) protease | Bioorg Med Chem Lett 9: 3137-42 (1999) BindingDB Entry DOI: 10.7270/Q20V8D98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50082154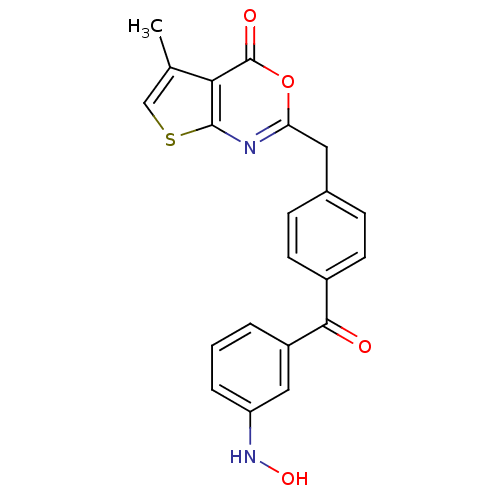 (2-[4-(3-Hydroxyamino-benzoyl)-benzyl]-5-methyl-thi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against Human Cytomegalovirus (hCMV) protease | Bioorg Med Chem Lett 9: 3137-42 (1999) BindingDB Entry DOI: 10.7270/Q20V8D98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8255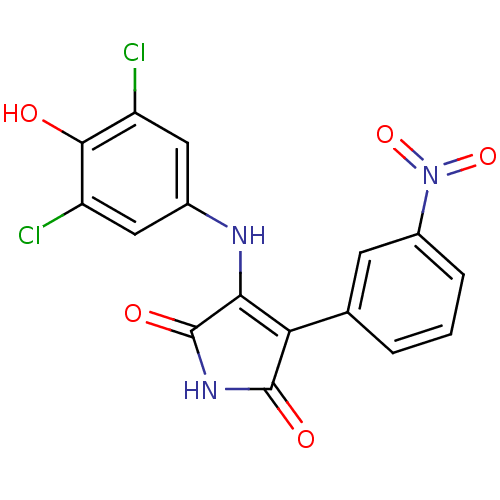 (3-[(3,5-dichloro-4-hydroxyphenyl)amino]-4-(3-nitro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 11: 635-9 (2001) Article DOI: 10.1016/s0960-894x(00)00721-6 BindingDB Entry DOI: 10.7270/Q2QV3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8271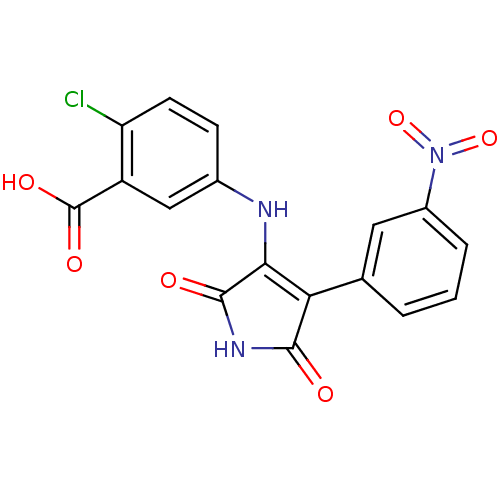 (2-chloro-5-{[4-(3-nitrophenyl)-2,5-dioxo-2,5-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 11: 635-9 (2001) Article DOI: 10.1016/s0960-894x(00)00721-6 BindingDB Entry DOI: 10.7270/Q2QV3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8268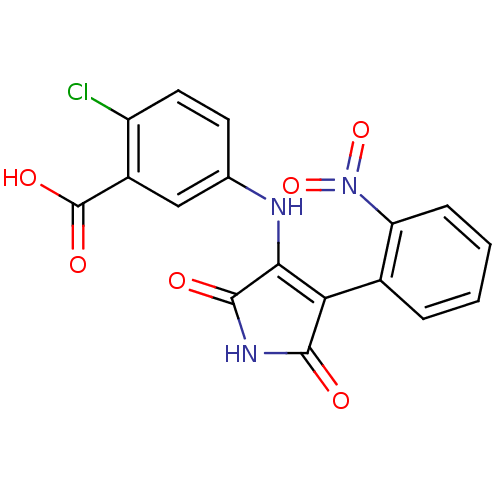 (2-chloro-5-{[4-(2-nitrophenyl)-2,5-dioxo-2,5-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 11: 635-9 (2001) Article DOI: 10.1016/s0960-894x(00)00721-6 BindingDB Entry DOI: 10.7270/Q2QV3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50082153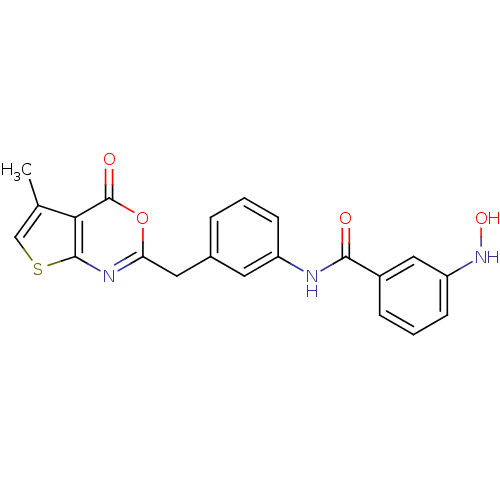 (3-Hydroxyamino-N-[3-(5-methyl-4-oxo-4H-thieno[2,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against Human Cytomegalovirus (hCMV) protease | Bioorg Med Chem Lett 9: 3137-42 (1999) BindingDB Entry DOI: 10.7270/Q20V8D98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8252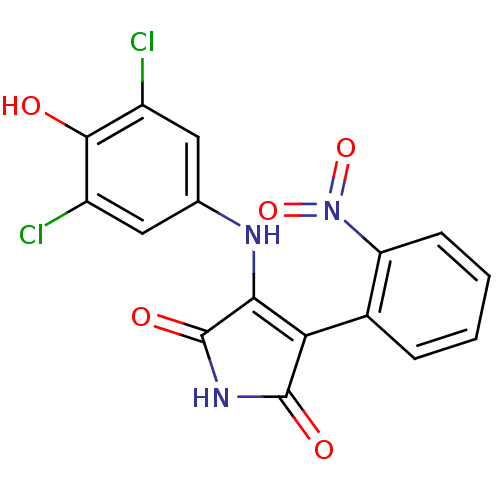 (3-[(3,5-dichloro-4-hydroxyphenyl)amino]-4-(2-nitro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 11: 635-9 (2001) Article DOI: 10.1016/s0960-894x(00)00721-6 BindingDB Entry DOI: 10.7270/Q2QV3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50082157 (5-Methyl-2-[4-(3-nitroso-benzoyl)-benzyl]-thieno[2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against Human Cytomegalovirus (hCMV) protease | Bioorg Med Chem Lett 9: 3137-42 (1999) BindingDB Entry DOI: 10.7270/Q20V8D98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8253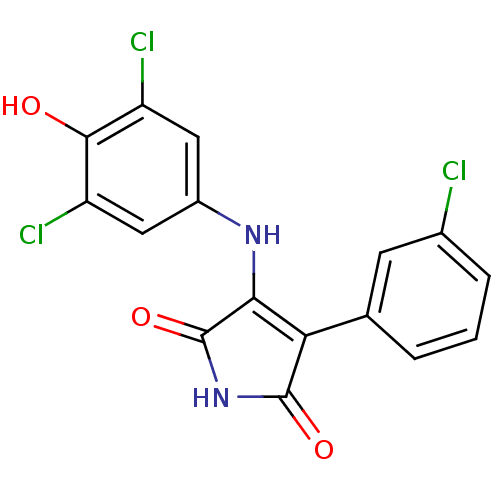 (3-(3-chlorophenyl)-4-[(3,5-dichloro-4-hydroxypheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 11: 635-9 (2001) Article DOI: 10.1016/s0960-894x(00)00721-6 BindingDB Entry DOI: 10.7270/Q2QV3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8247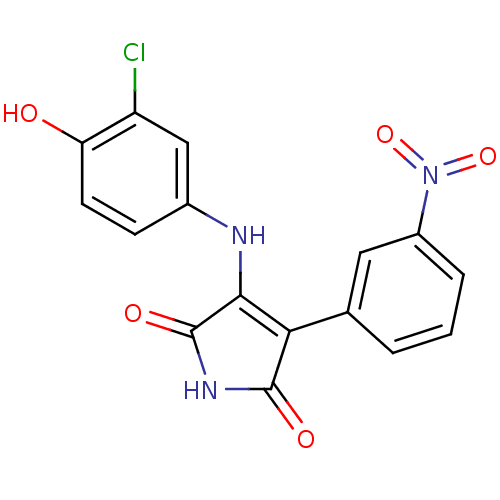 (3-[(3-chloro-4-hydroxyphenyl)amino]-4-(3-nitrophen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 11: 635-9 (2001) Article DOI: 10.1016/s0960-894x(00)00721-6 BindingDB Entry DOI: 10.7270/Q2QV3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50082155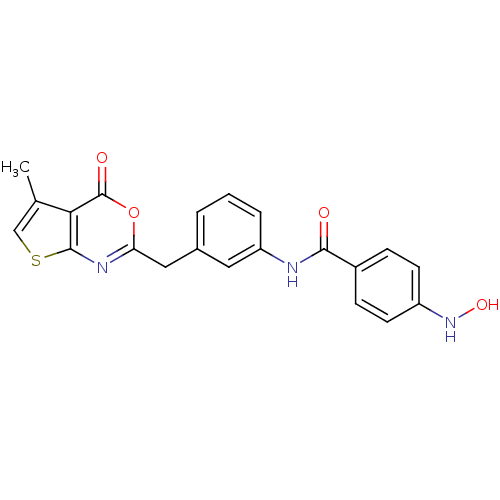 (4-Hydroxyamino-N-[3-(5-methyl-4-oxo-4H-thieno[2,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against Human Cytomegalovirus (hCMV) protease | Bioorg Med Chem Lett 9: 3137-42 (1999) BindingDB Entry DOI: 10.7270/Q20V8D98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8229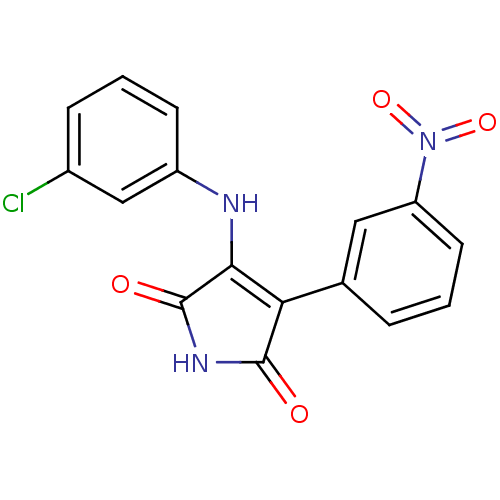 (3-[(3-chlorophenyl)amino]-4-(3-nitrophenyl)-2,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 7.0 | 22 |
SmithKline Beecham Pharmaceuticals | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 11: 635-9 (2001) Article DOI: 10.1016/s0960-894x(00)00721-6 BindingDB Entry DOI: 10.7270/Q2QV3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8258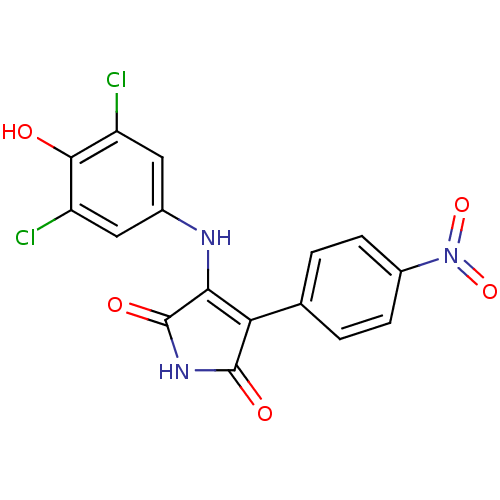 (3-[(3,5-dichloro-4-hydroxyphenyl)amino]-4-(4-nitro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 11: 635-9 (2001) Article DOI: 10.1016/s0960-894x(00)00721-6 BindingDB Entry DOI: 10.7270/Q2QV3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8267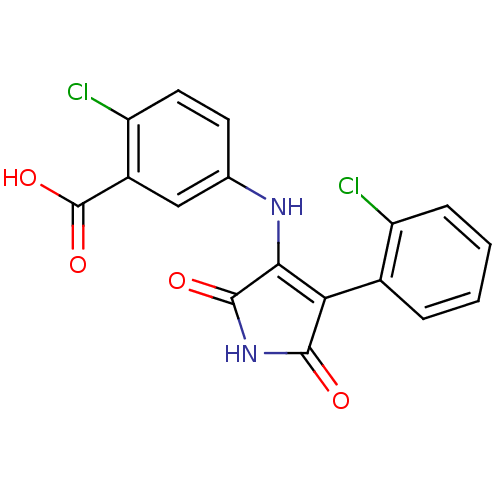 (2-chloro-5-{[4-(2-chlorophenyl)-2,5-dioxo-2,5-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 11: 635-9 (2001) Article DOI: 10.1016/s0960-894x(00)00721-6 BindingDB Entry DOI: 10.7270/Q2QV3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8269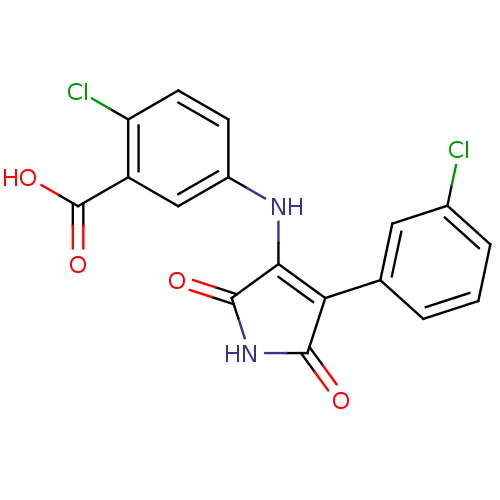 (2-chloro-5-{[4-(3-chlorophenyl)-2,5-dioxo-2,5-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 11: 635-9 (2001) Article DOI: 10.1016/s0960-894x(00)00721-6 BindingDB Entry DOI: 10.7270/Q2QV3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8263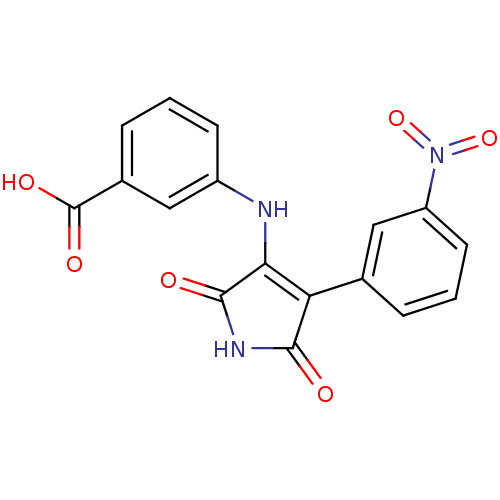 (3-{[4-(3-nitrophenyl)-2,5-dioxo-2,5-dihydro-1H-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 11: 635-9 (2001) Article DOI: 10.1016/s0960-894x(00)00721-6 BindingDB Entry DOI: 10.7270/Q2QV3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8251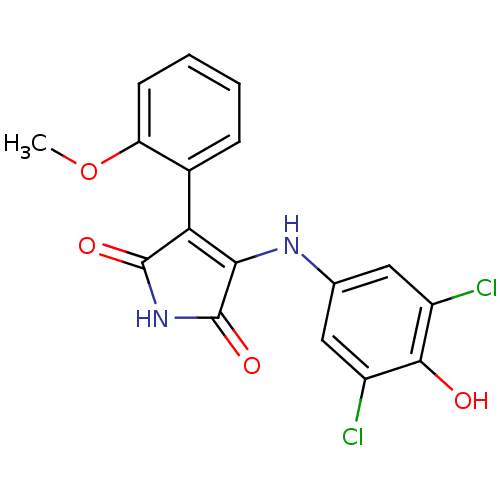 (3-[(3,5-dichloro-4-hydroxyphenyl)amino]-4-(2-metho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 11: 635-9 (2001) Article DOI: 10.1016/s0960-894x(00)00721-6 BindingDB Entry DOI: 10.7270/Q2QV3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8257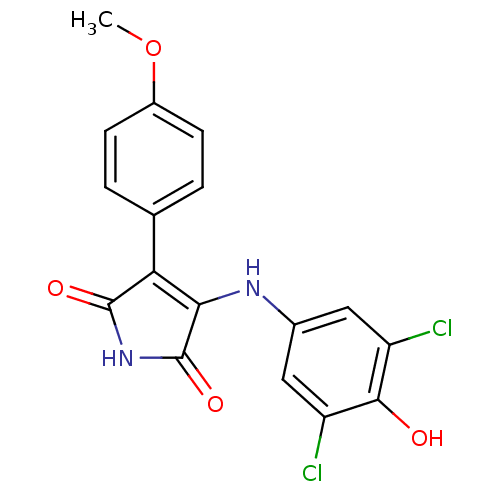 (3-[(3,5-dichloro-4-hydroxyphenyl)amino]-4-(4-metho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 11: 635-9 (2001) Article DOI: 10.1016/s0960-894x(00)00721-6 BindingDB Entry DOI: 10.7270/Q2QV3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8270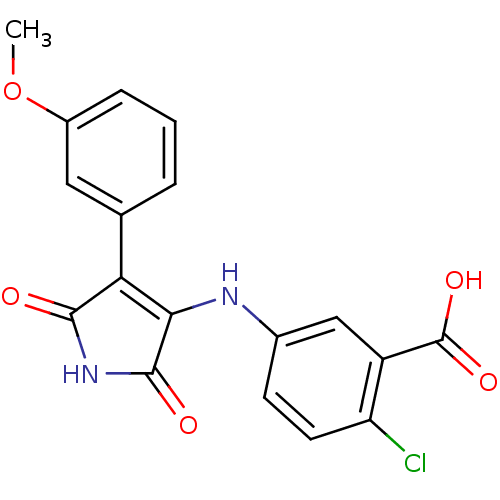 (2-chloro-5-{[4-(3-methoxyphenyl)-2,5-dioxo-2,5-dih...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 11: 635-9 (2001) Article DOI: 10.1016/s0960-894x(00)00721-6 BindingDB Entry DOI: 10.7270/Q2QV3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8256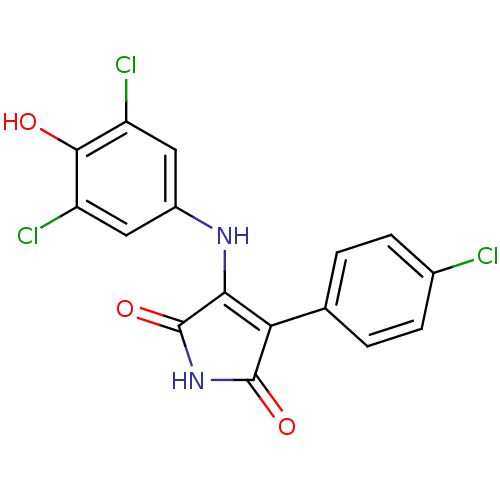 (3-(4-chlorophenyl)-4-[(3,5-dichloro-4-hydroxypheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 11: 635-9 (2001) Article DOI: 10.1016/s0960-894x(00)00721-6 BindingDB Entry DOI: 10.7270/Q2QV3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 158 total ) | Next | Last >> |