 Found 19005 hits with Last Name = 'han' and Initial = 'f'
Found 19005 hits with Last Name = 'han' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50082842
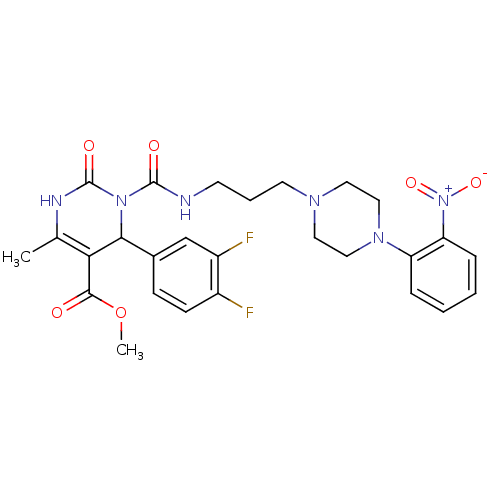
(4-(3,4-Difluoro-phenyl)-6-methyl-3-{3-[4-(2-nitro-...)Show SMILES COC(=O)C1=C(C)NC(=O)N(C1c1ccc(F)c(F)c1)C(=O)NCCCN1CCN(CC1)c1ccccc1[N+]([O-])=O |c:4| Show InChI InChI=1S/C27H30F2N6O6/c1-17-23(25(36)41-2)24(18-8-9-19(28)20(29)16-18)34(27(38)31-17)26(37)30-10-5-11-32-12-14-33(15-13-32)21-6-3-4-7-22(21)35(39)40/h3-4,6-9,16,24H,5,10-15H2,1-2H3,(H,30,37)(H,31,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. |
J Med Chem 42: 4794-803 (1999)
BindingDB Entry DOI: 10.7270/Q25B01P0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50308541
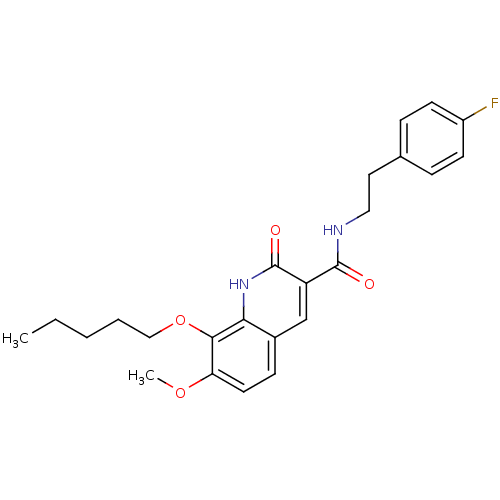
(7-Methoxy-2-oxo-8-pentyloxy-1,2-dihydroquinoline-3...)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)[nH]c12 Show InChI InChI=1S/C24H27FN2O4/c1-3-4-5-14-31-22-20(30-2)11-8-17-15-19(24(29)27-21(17)22)23(28)26-13-12-16-6-9-18(25)10-7-16/h6-11,15H,3-5,12-14H2,1-2H3,(H,26,28)(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50308541

(7-Methoxy-2-oxo-8-pentyloxy-1,2-dihydroquinoline-3...)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)[nH]c12 Show InChI InChI=1S/C24H27FN2O4/c1-3-4-5-14-31-22-20(30-2)11-8-17-15-19(24(29)27-21(17)22)23(28)26-13-12-16-6-9-18(25)10-7-16/h6-11,15H,3-5,12-14H2,1-2H3,(H,26,28)(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50353091

(CHEMBL1822944)Show SMILES CCOc1c(OC)ccc2cc(C(=O)NCCc3ccncc3)c(=O)[nH]c12 Show InChI InChI=1S/C20H21N3O4/c1-3-27-18-16(26-2)5-4-14-12-15(20(25)23-17(14)18)19(24)22-11-8-13-6-9-21-10-7-13/h4-7,9-10,12H,3,8,11H2,1-2H3,(H,22,24)(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50082839
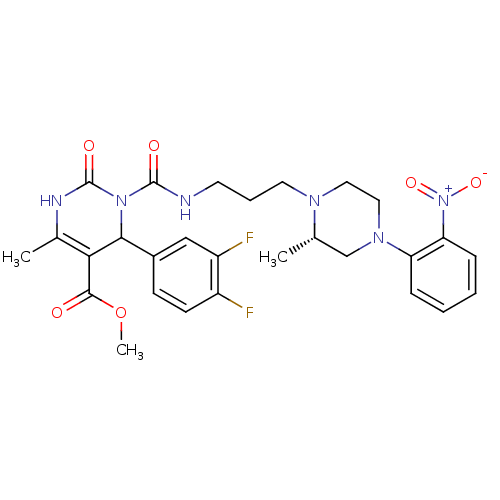
(4-(3,4-Difluoro-phenyl)-6-methyl-3-{3-[(S)-2-methy...)Show SMILES COC(=O)C1=C(C)NC(=O)N(C1c1ccc(F)c(F)c1)C(=O)NCCCN1CCN(C[C@@H]1C)c1ccccc1[N+]([O-])=O |c:4| Show InChI InChI=1S/C28H32F2N6O6/c1-17-16-34(22-7-4-5-8-23(22)36(40)41)14-13-33(17)12-6-11-31-27(38)35-25(19-9-10-20(29)21(30)15-19)24(26(37)42-3)18(2)32-28(35)39/h4-5,7-10,15,17,25H,6,11-14,16H2,1-3H3,(H,31,38)(H,32,39)/t17-,25?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. |
J Med Chem 42: 4794-803 (1999)
BindingDB Entry DOI: 10.7270/Q25B01P0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50353091

(CHEMBL1822944)Show SMILES CCOc1c(OC)ccc2cc(C(=O)NCCc3ccncc3)c(=O)[nH]c12 Show InChI InChI=1S/C20H21N3O4/c1-3-27-18-16(26-2)5-4-14-12-15(20(25)23-17(14)18)19(24)22-11-8-13-6-9-21-10-7-13/h4-7,9-10,12H,3,8,11H2,1-2H3,(H,22,24)(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123058
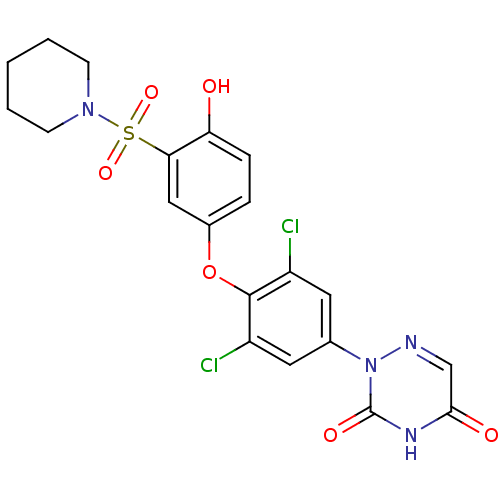
(2-(3,5-dichloro-4-(4-hydroxy-3-(piperidin-1-ylsulf...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1S(=O)(=O)N1CCCCC1 Show InChI InChI=1S/C20H18Cl2N4O6S/c21-14-8-12(26-20(29)24-18(28)11-23-26)9-15(22)19(14)32-13-4-5-16(27)17(10-13)33(30,31)25-6-2-1-3-7-25/h4-5,8-11,27H,1-3,6-7H2,(H,24,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073061
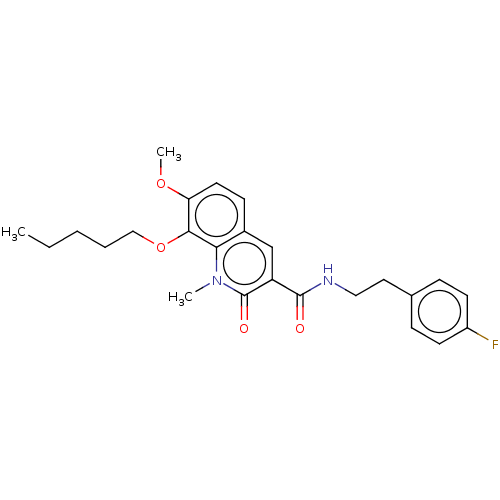
(CHEMBL3410832)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)n(C)c12 Show InChI InChI=1S/C25H29FN2O4/c1-4-5-6-15-32-23-21(31-3)12-9-18-16-20(25(30)28(2)22(18)23)24(29)27-14-13-17-7-10-19(26)11-8-17/h7-12,16H,4-6,13-15H2,1-3H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073061

(CHEMBL3410832)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)n(C)c12 Show InChI InChI=1S/C25H29FN2O4/c1-4-5-6-15-32-23-21(31-3)12-9-18-16-20(25(30)28(2)22(18)23)24(29)27-14-13-17-7-10-19(26)11-8-17/h7-12,16H,4-6,13-15H2,1-3H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073068

(CHEMBL3410813)Show SMILES CCCCCOc1ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)[nH]c2c1OCCCCC Show InChI InChI=1S/C28H35FN2O4/c1-3-5-7-17-34-24-14-11-21-19-23(27(32)30-16-15-20-9-12-22(29)13-10-20)28(33)31-25(21)26(24)35-18-8-6-4-2/h9-14,19H,3-8,15-18H2,1-2H3,(H,30,32)(H,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073068

(CHEMBL3410813)Show SMILES CCCCCOc1ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)[nH]c2c1OCCCCC Show InChI InChI=1S/C28H35FN2O4/c1-3-5-7-17-34-24-14-11-21-19-23(27(32)30-16-15-20-9-12-22(29)13-10-20)28(33)31-25(21)26(24)35-18-8-6-4-2/h9-14,19H,3-8,15-18H2,1-2H3,(H,30,32)(H,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123046
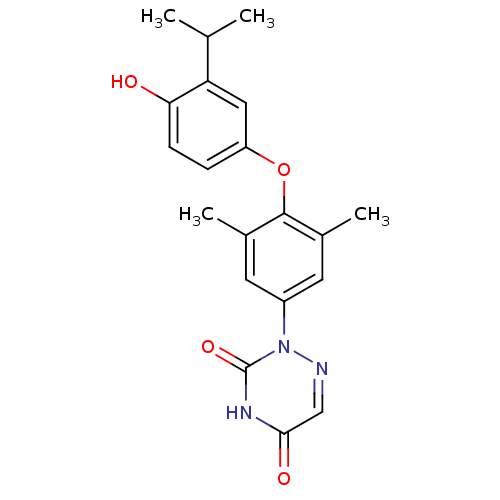
(2-[4-(4-Hydroxy-3-isopropyl-phenoxy)-3,5-dimethyl-...)Show SMILES CC(C)c1cc(Oc2c(C)cc(cc2C)-n2ncc(=O)[nH]c2=O)ccc1O Show InChI InChI=1S/C20H21N3O4/c1-11(2)16-9-15(5-6-17(16)24)27-19-12(3)7-14(8-13(19)4)23-20(26)22-18(25)10-21-23/h5-11,24H,1-4H3,(H,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123044
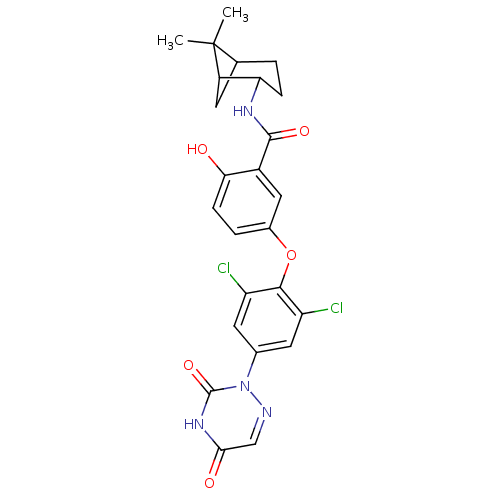
(5-(2,6-dichloro-4-(3,5-dioxo-4,5-dihydro-1,2,4-tri...)Show SMILES CC1(C)C2CC1C(CC2)NC(=O)c1cc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)ccc1O |THB:9:6:1:4| Show InChI InChI=1S/C25H24Cl2N4O5/c1-25(2)12-3-5-19(16(25)7-12)29-23(34)15-10-14(4-6-20(15)32)36-22-17(26)8-13(9-18(22)27)31-24(35)30-21(33)11-28-31/h4,6,8-12,16,19,32H,3,5,7H2,1-2H3,(H,29,34)(H,30,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50254445
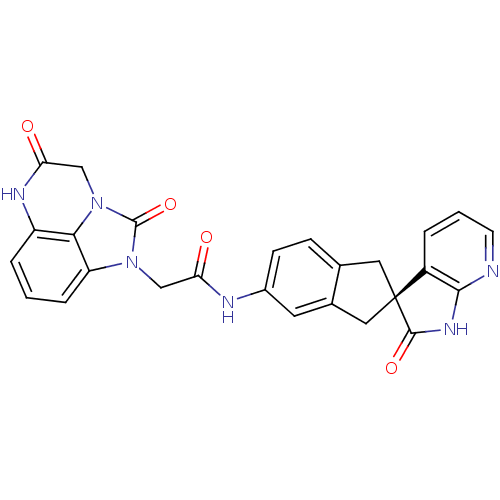
((S)-2-(2,5-dioxo-2,4,5,6-tetrahydro-1H-imidazo[1,5...)Show SMILES O=C(Cn1c2cccc3NC(=O)Cn(c23)c1=O)Nc1ccc2C[C@@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C26H20N6O4/c33-20(12-31-19-5-1-4-18-22(19)32(25(31)36)13-21(34)29-18)28-16-7-6-14-10-26(11-15(14)9-16)17-3-2-8-27-23(17)30-24(26)35/h1-9H,10-13H2,(H,28,33)(H,29,34)(H,27,30,35)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co
Curated by ChEMBL
| Assay Description
Displacement of [125I]hCGRP from human CGRP receptor expressed in HEK293 cells coexpressing RAMP1 |
Bioorg Med Chem Lett 19: 214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.106
BindingDB Entry DOI: 10.7270/Q21C1XSK |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123044

(5-(2,6-dichloro-4-(3,5-dioxo-4,5-dihydro-1,2,4-tri...)Show SMILES CC1(C)C2CC1C(CC2)NC(=O)c1cc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)ccc1O |THB:9:6:1:4| Show InChI InChI=1S/C25H24Cl2N4O5/c1-25(2)12-3-5-19(16(25)7-12)29-23(34)15-10-14(4-6-20(15)32)36-22-17(26)8-13(9-18(22)27)31-24(35)30-21(33)11-28-31/h4,6,8-12,16,19,32H,3,5,7H2,1-2H3,(H,29,34)(H,30,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16318
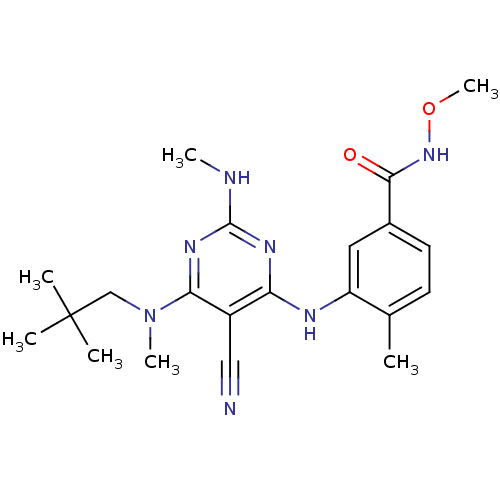
(3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]-...)Show SMILES CNc1nc(Nc2cc(ccc2C)C(=O)NOC)c(C#N)c(n1)N(C)CC(C)(C)C Show InChI InChI=1S/C21H29N7O2/c1-13-8-9-14(19(29)27-30-7)10-16(13)24-17-15(11-22)18(26-20(23-5)25-17)28(6)12-21(2,3)4/h8-10H,12H2,1-7H3,(H,27,29)(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0470 | -58.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50082851
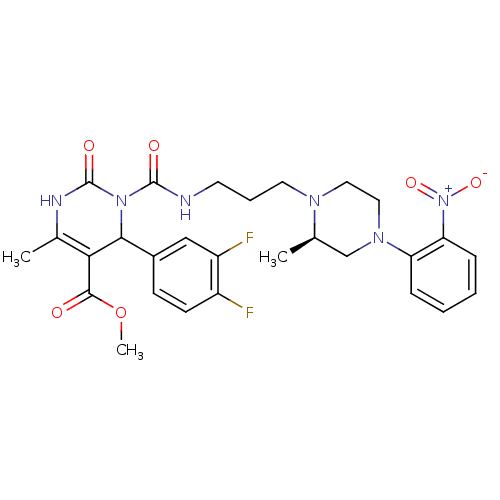
(4-(3,4-Difluoro-phenyl)-6-methyl-3-{3-[(R)-2-methy...)Show SMILES COC(=O)C1=C(C)NC(=O)N(C1c1ccc(F)c(F)c1)C(=O)NCCCN1CCN(C[C@H]1C)c1ccccc1[N+]([O-])=O |c:4| Show InChI InChI=1S/C28H32F2N6O6/c1-17-16-34(22-7-4-5-8-23(22)36(40)41)14-13-33(17)12-6-11-31-27(38)35-25(19-9-10-20(29)21(30)15-19)24(26(37)42-3)18(2)32-28(35)39/h4-5,7-10,15,17,25H,6,11-14,16H2,1-3H3,(H,31,38)(H,32,39)/t17-,25?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. |
J Med Chem 42: 4794-803 (1999)
BindingDB Entry DOI: 10.7270/Q25B01P0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50580080
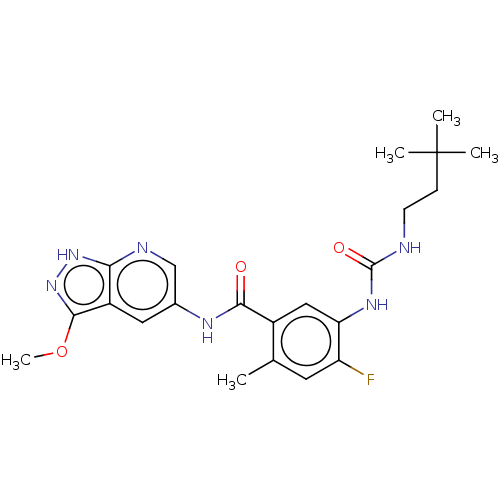
(CHEMBL5090624)Show SMILES COc1n[nH]c2ncc(NC(=O)c3cc(NC(=O)NCCC(C)(C)C)c(F)cc3C)cc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557773
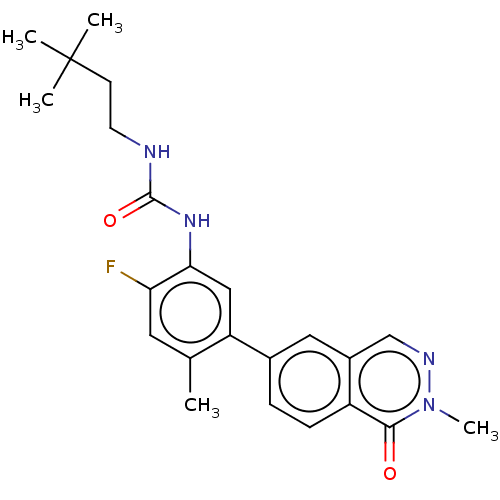
(CHEMBL4778772)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1-c1ccc2c(cnn(C)c2=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557772
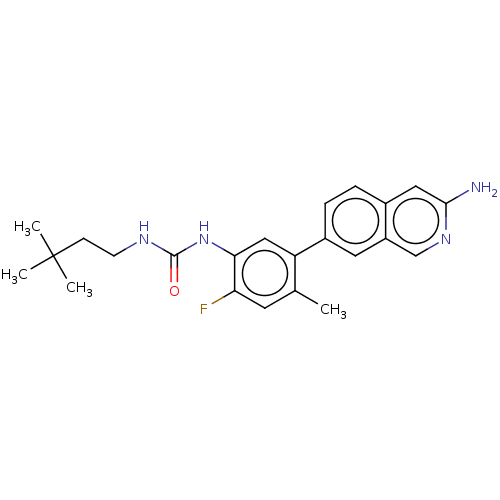
(CHEMBL4775998)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1-c1ccc2cc(N)ncc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50580084
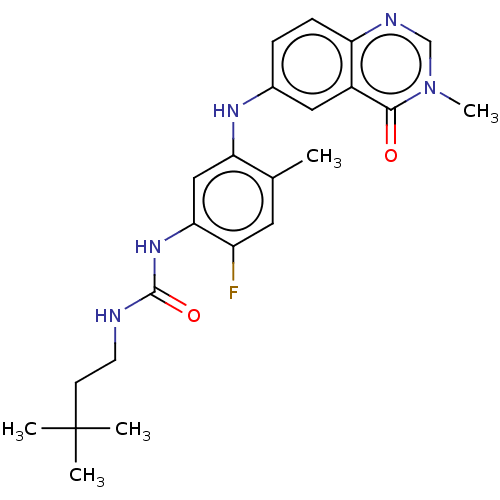
(CHEMBL5075174)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16319
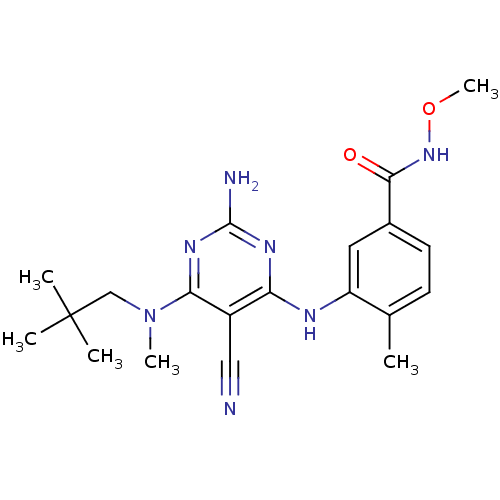
(3-({2-amino-5-cyano-6-[(2,2-dimethylpropyl)(methyl...)Show SMILES CONC(=O)c1ccc(C)c(Nc2nc(N)nc(N(C)CC(C)(C)C)c2C#N)c1 Show InChI InChI=1S/C20H27N7O2/c1-12-7-8-13(18(28)26-29-6)9-15(12)23-16-14(10-21)17(25-19(22)24-16)27(5)11-20(2,3)4/h7-9H,11H2,1-6H3,(H,26,28)(H3,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | -58.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557775
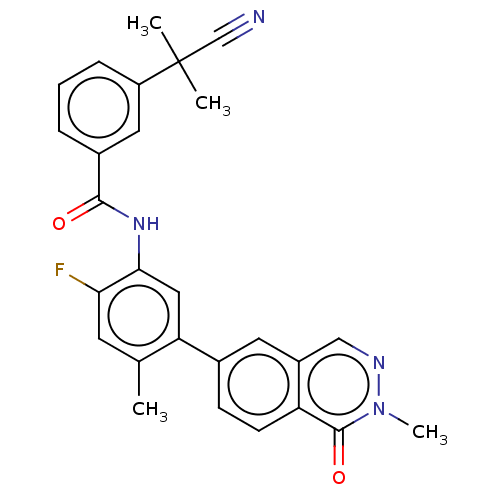
(CHEMBL4758903)Show SMILES Cc1cc(F)c(NC(=O)c2cccc(c2)C(C)(C)C#N)cc1-c1ccc2c(cnn(C)c2=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50580082
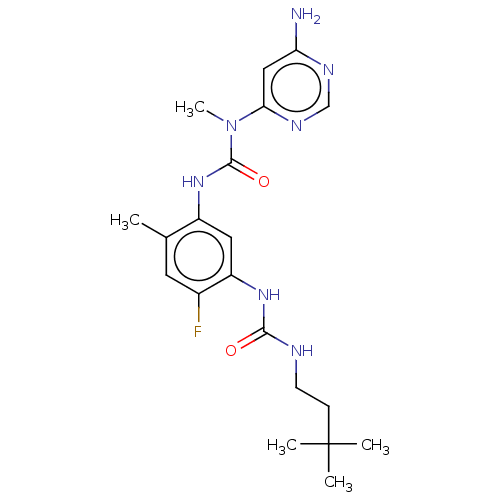
(CHEMBL5079215)Show SMILES CN(C(=O)Nc1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C)c1cc(N)ncn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50580083
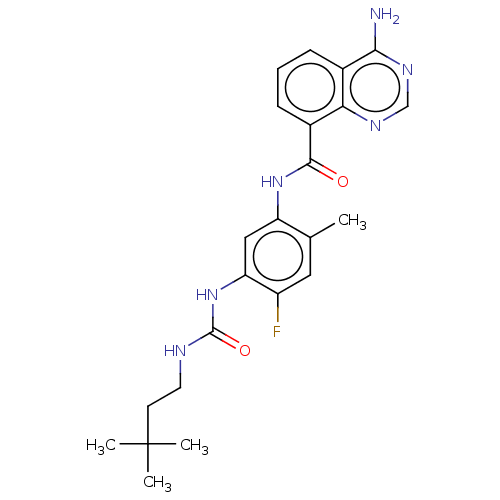
(CHEMBL5094268)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1NC(=O)c1cccc2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072968
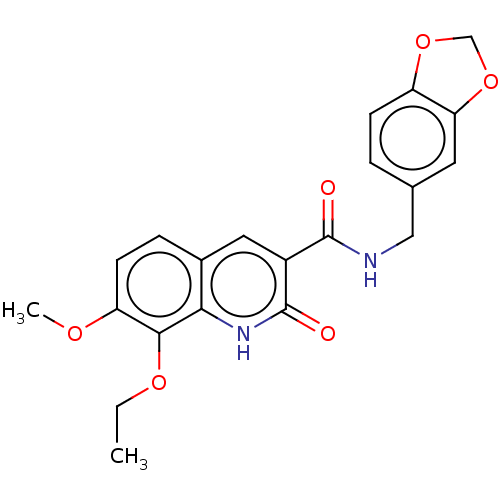
(CHEMBL3410818)Show SMILES CCOc1c(OC)ccc2cc(C(=O)NCc3ccc4OCOc4c3)c(=O)[nH]c12 Show InChI InChI=1S/C21H20N2O6/c1-3-27-19-16(26-2)7-5-13-9-14(21(25)23-18(13)19)20(24)22-10-12-4-6-15-17(8-12)29-11-28-15/h4-9H,3,10-11H2,1-2H3,(H,22,24)(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072968

(CHEMBL3410818)Show SMILES CCOc1c(OC)ccc2cc(C(=O)NCc3ccc4OCOc4c3)c(=O)[nH]c12 Show InChI InChI=1S/C21H20N2O6/c1-3-27-19-16(26-2)7-5-13-9-14(21(25)23-18(13)19)20(24)22-10-12-4-6-15-17(8-12)29-11-28-15/h4-9H,3,10-11H2,1-2H3,(H,22,24)(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16329
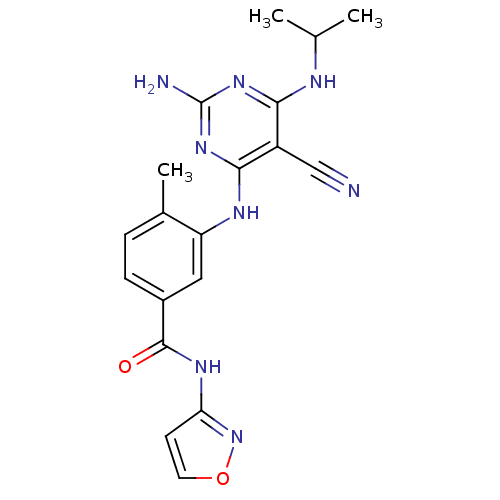
(3-({2-amino-5-cyano-6-[(1-methylethyl)amino]pyrimi...)Show SMILES CC(C)Nc1nc(N)nc(Nc2cc(ccc2C)C(=O)Nc2ccon2)c1C#N Show InChI InChI=1S/C19H20N8O2/c1-10(2)22-16-13(9-20)17(26-19(21)25-16)23-14-8-12(5-4-11(14)3)18(28)24-15-6-7-29-27-15/h4-8,10H,1-3H3,(H,24,27,28)(H4,21,22,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0570 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16317
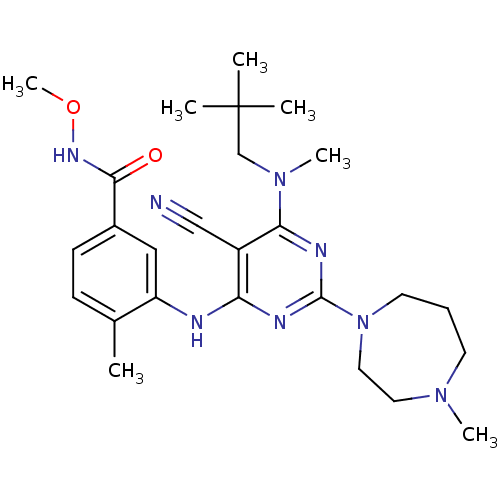
(3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]-...)Show SMILES CONC(=O)c1ccc(C)c(Nc2nc(nc(N(C)CC(C)(C)C)c2C#N)N2CCCN(C)CC2)c1 Show InChI InChI=1S/C26H38N8O2/c1-18-9-10-19(24(35)31-36-7)15-21(18)28-22-20(16-27)23(33(6)17-26(2,3)4)30-25(29-22)34-12-8-11-32(5)13-14-34/h9-10,15H,8,11-14,17H2,1-7H3,(H,31,35)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0570 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50082811
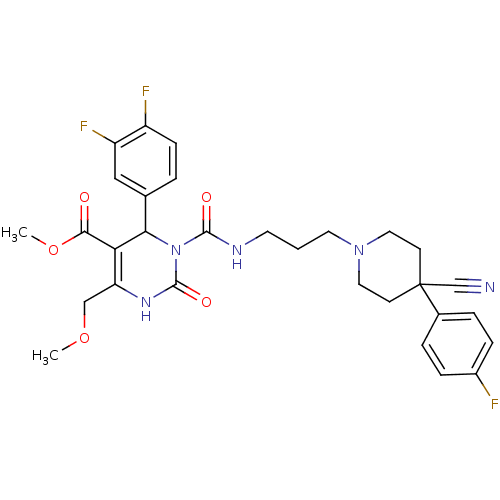
(3-{3-[4-Cyano-4-(4-fluoro-phenyl)-piperidin-1-yl]-...)Show SMILES COCC1=C(C(N(C(=O)NCCCN2CCC(CC2)(C#N)c2ccc(F)cc2)C(=O)N1)c1ccc(F)c(F)c1)C(=O)OC |t:3| Show InChI InChI=1S/C30H32F3N5O5/c1-42-17-24-25(27(39)43-2)26(19-4-9-22(32)23(33)16-19)38(29(41)36-24)28(40)35-12-3-13-37-14-10-30(18-34,11-15-37)20-5-7-21(31)8-6-20/h4-9,16,26H,3,10-15,17H2,1-2H3,(H,35,40)(H,36,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes |
J Med Chem 42: 4778-93 (1999)
BindingDB Entry DOI: 10.7270/Q2930SCW |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50082827
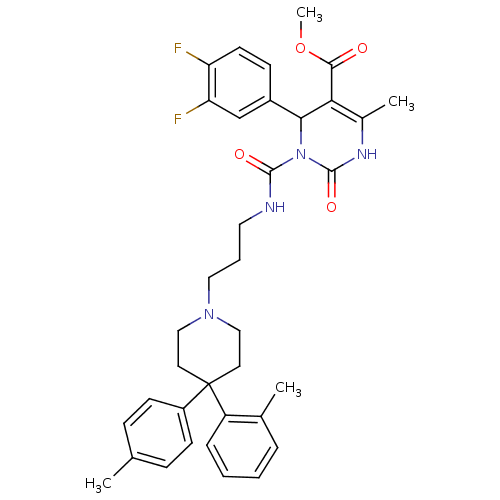
(4-(3,4-Difluoro-phenyl)-6-methyl-2-oxo-3-[3-(4-o-t...)Show SMILES COC(=O)C1=C(C)NC(=O)N(C1c1ccc(F)c(F)c1)C(=O)NCCCN1CCC(CC1)(c1ccc(C)cc1)c1ccccc1C |c:4| Show InChI InChI=1S/C36H40F2N4O4/c1-23-10-13-27(14-11-23)36(28-9-6-5-8-24(28)2)16-20-41(21-17-36)19-7-18-39-34(44)42-32(26-12-15-29(37)30(38)22-26)31(33(43)46-4)25(3)40-35(42)45/h5-6,8-15,22,32H,7,16-21H2,1-4H3,(H,39,44)(H,40,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes |
J Med Chem 42: 4778-93 (1999)
BindingDB Entry DOI: 10.7270/Q2930SCW |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557774
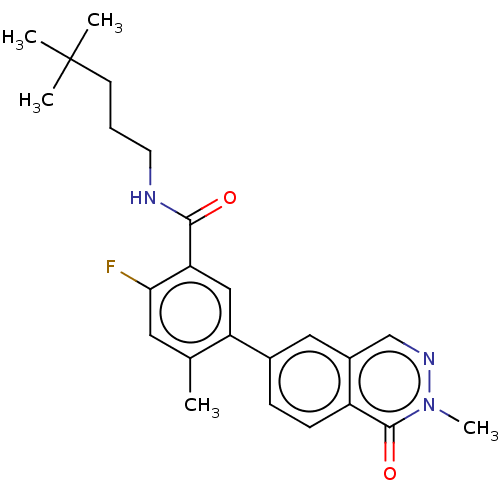
(CHEMBL4776565)Show SMILES Cc1cc(F)c(cc1-c1ccc2c(cnn(C)c2=O)c1)C(=O)NCCCC(C)(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50580081
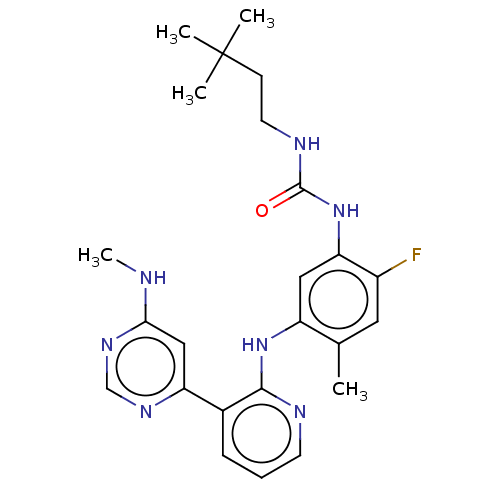
(CHEMBL5094514)Show SMILES CNc1cc(ncn1)-c1cccnc1Nc1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50096279
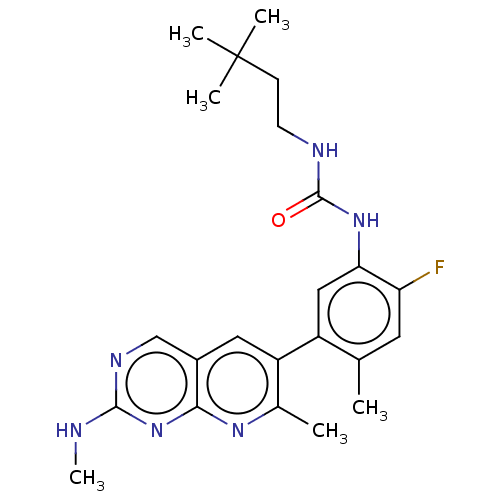
(CHEMBL3577124)Show SMILES CNc1ncc2cc(c(C)nc2n1)-c1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C Show InChI InChI=1S/C18H13N3O5/c22-14(23)5-4-10-8-21-13-7-12-9(6-15(24)25)2-1-3-11(12)16(13)20-18(26)17(21)19-10/h1-5,8H,6-7H2,(H,20,26)(H,22,23)(H,24,25)/b5-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557770

(CHEMBL4780060)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50096279

(CHEMBL3577124)Show SMILES CNc1ncc2cc(c(C)nc2n1)-c1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C Show InChI InChI=1S/C18H13N3O5/c22-14(23)5-4-10-8-21-13-7-12-9(6-15(24)25)2-1-3-11(12)16(13)20-18(26)17(21)19-10/h1-5,8H,6-7H2,(H,20,26)(H,22,23)(H,24,25)/b5-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557770

(CHEMBL4780060)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557776
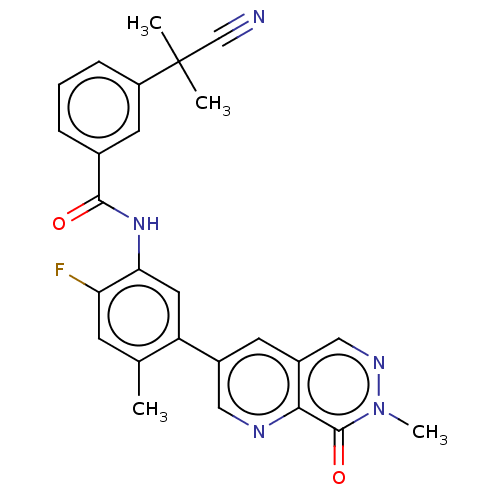
(CHEMBL4778419)Show SMILES Cc1cc(F)c(NC(=O)c2cccc(c2)C(C)(C)C#N)cc1-c1cnc2c(cnn(C)c2=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123045
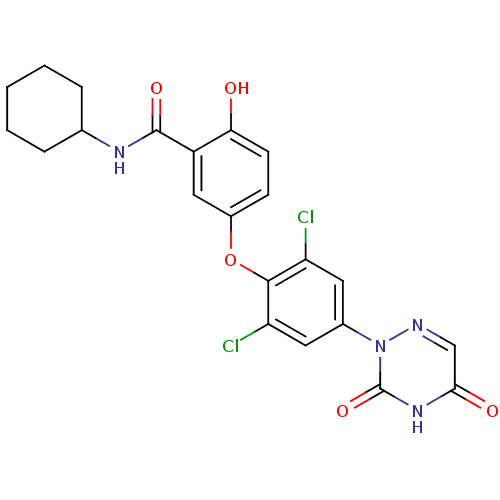
(CHEMBL413699 | N-Cyclohexyl-5-[2,6-dichloro-4-(3,5...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1C(=O)NC1CCCCC1 Show InChI InChI=1S/C22H20Cl2N4O5/c23-16-8-13(28-22(32)27-19(30)11-25-28)9-17(24)20(16)33-14-6-7-18(29)15(10-14)21(31)26-12-4-2-1-3-5-12/h6-12,29H,1-5H2,(H,26,31)(H,27,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557775

(CHEMBL4758903)Show SMILES Cc1cc(F)c(NC(=O)c2cccc(c2)C(C)(C)C#N)cc1-c1ccc2c(cnn(C)c2=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557773

(CHEMBL4778772)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1-c1ccc2c(cnn(C)c2=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557772

(CHEMBL4775998)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1-c1ccc2cc(N)ncc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50580083

(CHEMBL5094268)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1NC(=O)c1cccc2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557770

(CHEMBL4780060)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50580084

(CHEMBL5075174)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50580081

(CHEMBL5094514)Show SMILES CNc1cc(ncn1)-c1cccnc1Nc1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50580082

(CHEMBL5079215)Show SMILES CN(C(=O)Nc1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C)c1cc(N)ncn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557770

(CHEMBL4780060)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557771
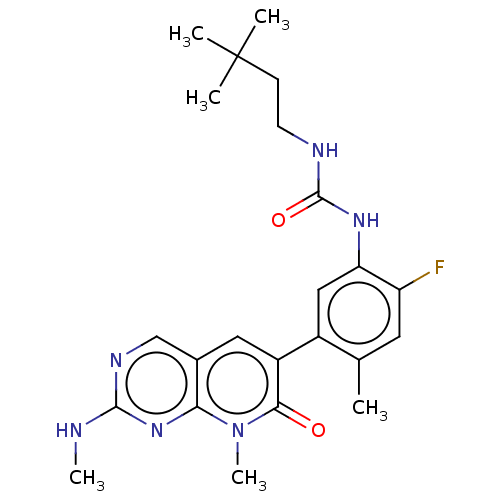
(CHEMBL4740241)Show SMILES CNc1ncc2cc(-c3cc(NC(=O)NCCC(C)(C)C)c(F)cc3C)c(=O)n(C)c2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data