 Found 421 hits with Last Name = 'hanson' and Initial = 'l'
Found 421 hits with Last Name = 'hanson' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239606

(CHEMBL4080667)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(C2)[C@H]3O)c1ccccc1 |r,wD:17.21,TLB:16:15:8.9.10:12,7:8:14.16.15:10.11.12,7:8:12:14.15.17,THB:16:9:12:14.15.17,18:17:8.9.10:12,17:15:8:10.11.12,17:11:8:14.16.15,19:8:14.16.15:10.11.12,19:8:12:14.15.17,(43.24,-21.55,;43.72,-20.08,;43.03,-18.71,;44.4,-18.01,;45.1,-19.38,;44.88,-16.54,;43.63,-15.64,;46.35,-16.07,;47.49,-17.11,;48.99,-16.68,;48.98,-15.1,;50.02,-13.87,;48.67,-14.34,;48.68,-15.83,;50,-16.32,;51.4,-15.97,;50.4,-17.25,;51.42,-14.44,;52.71,-13.59,;47.48,-18.64,;46.13,-19.39,;46.12,-20.93,;47.44,-21.71,;48.79,-20.95,;48.79,-19.41,)| Show InChI InChI=1S/C21H27NO3/c23-18-11-22(12-18)19(24)10-21(15-4-2-1-3-5-15)16-6-13-7-17(21)9-14(8-16)20(13)25/h1-5,13-14,16-18,20,23,25H,6-12H2/t13?,14?,16?,17?,20-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50539763
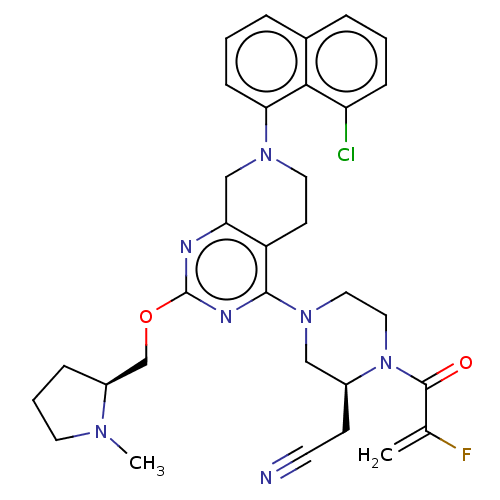
(Adagrasib | Mrtx-849 | Mrtx849)Show SMILES CN1CCC[C@H]1COc1nc2CN(CCc2c(n1)N1CCN([C@@H](CC#N)C1)C(=O)C(F)=C)c1cccc2cccc(Cl)c12 Show InChI InChI=1S/C32H35ClFN7O2/c1-21(34)31(42)41-17-16-40(18-23(41)11-13-35)30-25-12-15-39(28-10-4-7-22-6-3-9-26(33)29(22)28)19-27(25)36-32(37-30)43-20-24-8-5-14-38(24)2/h3-4,6-7,9-10,23-24H,1,5,8,11-12,14-20H2,2H3/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant KRAS G12C mutant (unknown origin) assessed as rate of inactivation by LC-MS analysis |
J Med Chem 63: 6679-6693 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02052
BindingDB Entry DOI: 10.7270/Q2G164C5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505991

(CHEMBL4470113)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3cccc(Cl)c3)CCn2c1 Show InChI InChI=1S/C23H22ClN7O/c1-15-12-25-23(27-20-6-7-26-29(20)2)28-21(15)17-11-19-22(32)31(9-8-30(19)14-17)13-16-4-3-5-18(24)10-16/h3-7,10-12,14H,8-9,13H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265958
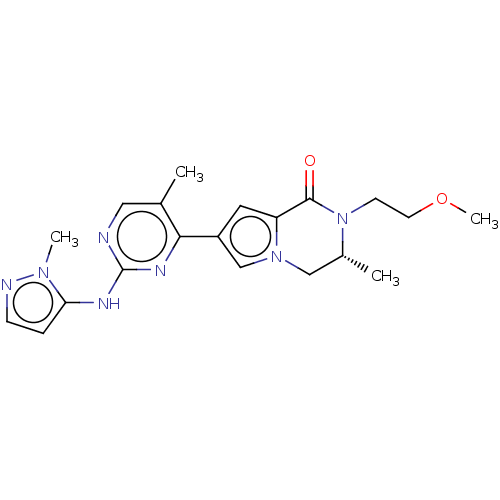
(CHEMBL4086507)Show SMILES COCCN1[C@H](C)Cn2cc(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-13-10-21-20(23-17-5-6-22-25(17)3)24-18(13)15-9-16-19(28)27(7-8-29-4)14(2)11-26(16)12-15/h5-6,9-10,12,14H,7-8,11H2,1-4H3,(H,21,23,24)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265968

(CHEMBL4065992)Show SMILES COCCN1[C@H](C)Cc2[nH]c(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-12-11-21-20(24-17-5-6-22-26(17)3)25-18(12)16-10-14-15(23-16)9-13(2)27(19(14)28)7-8-29-4/h5-6,10-11,13,23H,7-9H2,1-4H3,(H,21,24,25)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265971

(CHEMBL4087393)Show SMILES COCCN1[C@@H](C)Cc2[nH]c(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-12-11-21-20(24-17-5-6-22-26(17)3)25-18(12)16-10-14-15(23-16)9-13(2)27(19(14)28)7-8-29-4/h5-6,10-11,13,23H,7-9H2,1-4H3,(H,21,24,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265961

(CHEMBL4090886)Show InChI InChI=1S/C19H23N7O2/c1-13-11-20-19(22-16-4-5-21-24(16)2)23-17(13)14-10-15-18(27)25(8-9-28-3)6-7-26(15)12-14/h4-5,10-12H,6-9H2,1-3H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265968

(CHEMBL4065992)Show SMILES COCCN1[C@H](C)Cc2[nH]c(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-12-11-21-20(24-17-5-6-22-26(17)3)25-18(12)16-10-14-15(23-16)9-13(2)27(19(14)28)7-8-29-4/h5-6,10-11,13,23H,7-9H2,1-4H3,(H,21,24,25)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265960

(CHEMBL4098608)Show SMILES COCCN1[C@@H](C)Cn2cc(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-13-10-21-20(23-17-5-6-22-25(17)3)24-18(13)15-9-16-19(28)27(7-8-29-4)14(2)11-26(16)12-15/h5-6,9-10,12,14H,7-8,11H2,1-4H3,(H,21,23,24)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265962
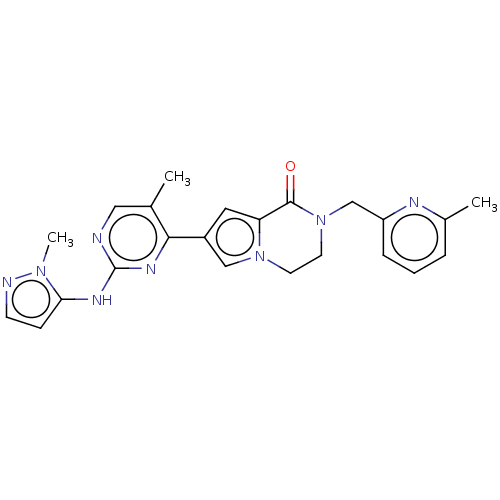
(CHEMBL4101072)Show SMILES Cc1cccc(CN2CCn3cc(cc3C2=O)-c2nc(Nc3ccnn3C)ncc2C)n1 Show InChI InChI=1S/C23H24N8O/c1-15-12-24-23(27-20-7-8-25-29(20)3)28-21(15)17-11-19-22(32)31(10-9-30(19)13-17)14-18-6-4-5-16(2)26-18/h4-8,11-13H,9-10,14H2,1-3H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265961

(CHEMBL4090886)Show InChI InChI=1S/C19H23N7O2/c1-13-11-20-19(22-16-4-5-21-24(16)2)23-17(13)14-10-15-18(27)25(8-9-28-3)6-7-26(15)12-14/h4-5,10-12H,6-9H2,1-3H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265960

(CHEMBL4098608)Show SMILES COCCN1[C@@H](C)Cn2cc(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-13-10-21-20(23-17-5-6-22-25(17)3)24-18(13)15-9-16-19(28)27(7-8-29-4)14(2)11-26(16)12-15/h5-6,9-10,12,14H,7-8,11H2,1-4H3,(H,21,23,24)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265959
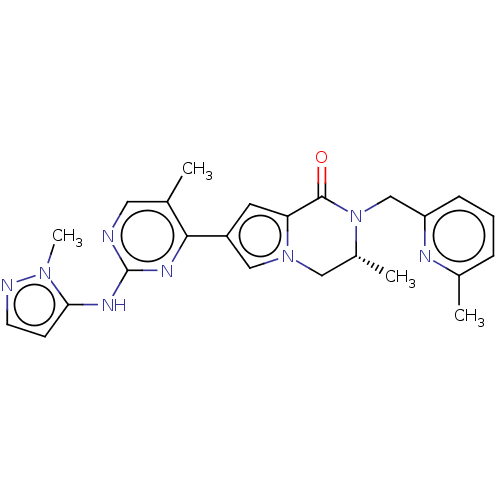
(CHEMBL4071576)Show SMILES C[C@@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265958

(CHEMBL4086507)Show SMILES COCCN1[C@H](C)Cn2cc(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-13-10-21-20(23-17-5-6-22-25(17)3)24-18(13)15-9-16-19(28)27(7-8-29-4)14(2)11-26(16)12-15/h5-6,9-10,12,14H,7-8,11H2,1-4H3,(H,21,23,24)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094465
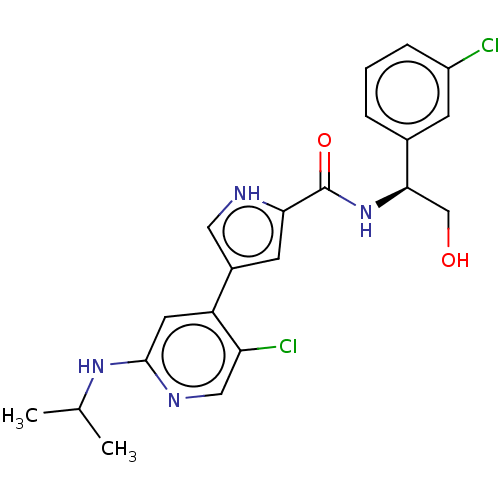
(CHEMBL3590106 | US10525036, Example BVD-523 | US10...)Show SMILES CC(C)Nc1cc(-c2c[nH]c(c2)C(=O)N[C@H](CO)c2cccc(Cl)c2)c(Cl)cn1 |r| Show InChI InChI=1S/C19H21NO3/c21-18(23-17-11-13-20-14-12-17)19(22,15-7-3-1-4-8-15)16-9-5-2-6-10-16/h1-10,17,20,22H,11-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265934

(CHEMBL4060050)Show SMILES COCCN1[C@@H](C)Cc2[nH]c(cc2C1=O)-c1ccnc(Nc2ccnn2C)n1 |r| Show InChI InChI=1S/C19H23N7O2/c1-12-10-15-13(18(27)26(12)8-9-28-3)11-16(22-15)14-4-6-20-19(23-14)24-17-5-7-21-25(17)2/h4-7,11-12,22H,8-10H2,1-3H3,(H,20,23,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265962

(CHEMBL4101072)Show SMILES Cc1cccc(CN2CCn3cc(cc3C2=O)-c2nc(Nc3ccnn3C)ncc2C)n1 Show InChI InChI=1S/C23H24N8O/c1-15-12-24-23(27-20-7-8-25-29(20)3)28-21(15)17-11-19-22(32)31(10-9-30(19)13-17)14-18-6-4-5-16(2)26-18/h4-8,11-13H,9-10,14H2,1-3H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265971

(CHEMBL4087393)Show SMILES COCCN1[C@@H](C)Cc2[nH]c(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-12-11-21-20(24-17-5-6-22-26(17)3)25-18(12)16-10-14-15(23-16)9-13(2)27(19(14)28)7-8-29-4/h5-6,10-11,13,23H,7-9H2,1-4H3,(H,21,24,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265973

(CHEMBL4075638)Show SMILES C[C@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239610
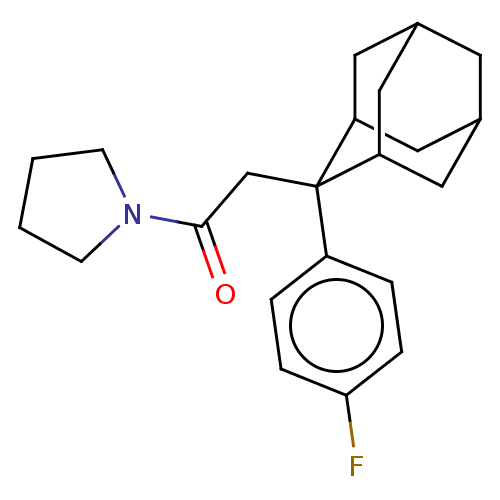
(CHEMBL4073961)Show SMILES Fc1ccc(cc1)C1(CC(=O)N2CCCC2)C2CC3CC(C2)CC1C3 |TLB:24:23:21:17.18.19,8:7:17.24.18:22.20.21,4:7:21:17.18.19,THB:24:18:7.23.22:21,19:18:7:22.20.21,19:20:7:17.24.18,8:7:21:17.18.19,4:7:17.24.18:22.20.21,(23.94,-24.95,;23.96,-23.41,;22.63,-22.62,;22.65,-21.09,;23.99,-20.34,;25.31,-21.11,;25.3,-22.64,;24.01,-18.8,;22.86,-17.77,;21.4,-18.25,;20.15,-17.34,;20.92,-19.71,;19.45,-20.18,;19.45,-21.72,;20.91,-22.2,;21.82,-20.95,;25.21,-17.53,;26.53,-18.01,;27.92,-17.67,;27.94,-16.14,;26.54,-15.56,;25.2,-16.04,;25.5,-16.8,;25.5,-18.39,;26.91,-18.95,)| Show InChI InChI=1S/C22H28FNO/c23-20-5-3-17(4-6-20)22(14-21(25)24-7-1-2-8-24)18-10-15-9-16(12-18)13-19(22)11-15/h3-6,15-16,18-19H,1-2,7-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505993

(CHEMBL4587118)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3ccc(F)c(F)c3)CCn2c1 Show InChI InChI=1S/C23H21F2N7O/c1-14-11-26-23(28-20-5-6-27-30(20)2)29-21(14)16-10-19-22(33)32(8-7-31(19)13-16)12-15-3-4-17(24)18(25)9-15/h3-6,9-11,13H,7-8,12H2,1-2H3,(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505988
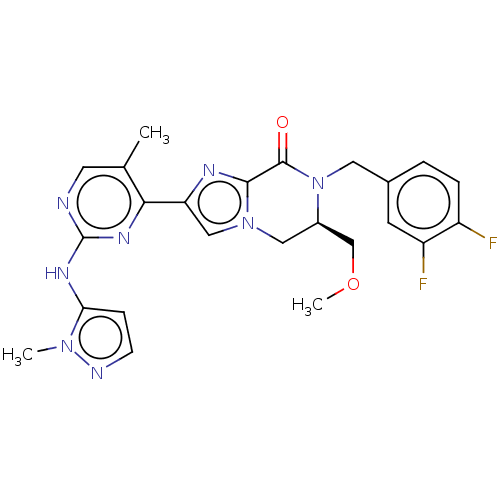
(CHEMBL4482864)Show SMILES COC[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H24F2N8O2/c1-14-9-27-24(30-20-6-7-28-32(20)2)31-21(14)19-12-33-11-16(13-36-3)34(23(35)22(33)29-19)10-15-4-5-17(25)18(26)8-15/h4-9,12,16H,10-11,13H2,1-3H3,(H,27,30,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505994

(CHEMBL4440109)Show SMILES C[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C23H22F2N8O/c1-13-9-26-23(29-19-6-7-27-31(19)3)30-20(13)18-12-32-10-14(2)33(22(34)21(32)28-18)11-15-4-5-16(24)17(25)8-15/h4-9,12,14H,10-11H2,1-3H3,(H,26,29,30)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505989

(CHEMBL4551714)Show SMILES COC[C@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C25H28N8O2/c1-16-11-26-25(29-22-8-9-27-31(22)3)30-23(16)18-10-21-24(34)33(13-19-7-5-6-17(2)28-19)20(15-35-4)14-32(21)12-18/h5-12,20H,13-15H2,1-4H3,(H,26,29,30)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505992

(CHEMBL4452853)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cn2CCN(Cc3cccc(Cl)c3)C(=O)c2n1 Show InChI InChI=1S/C22H21ClN8O/c1-14-11-24-22(27-18-6-7-25-29(18)2)28-19(14)17-13-30-8-9-31(21(32)20(30)26-17)12-15-4-3-5-16(23)10-15/h3-7,10-11,13H,8-9,12H2,1-2H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505991

(CHEMBL4470113)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3cccc(Cl)c3)CCn2c1 Show InChI InChI=1S/C23H22ClN7O/c1-15-12-25-23(27-20-6-7-26-29(20)2)28-21(15)17-11-19-22(32)31(9-8-30(19)14-17)13-16-4-3-5-18(24)10-16/h3-7,10-12,14H,8-9,13H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265959

(CHEMBL4071576)Show SMILES C[C@@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265970

(CHEMBL4097186)Show SMILES O=C1N(Cc2ccccc2)CCc2[nH]c(cc12)-c1ccnc(NC2CCOCC2)n1 Show InChI InChI=1S/C23H25N5O2/c29-22-18-14-21(20-6-10-24-23(27-20)25-17-8-12-30-13-9-17)26-19(18)7-11-28(22)15-16-4-2-1-3-5-16/h1-6,10,14,17,26H,7-9,11-13,15H2,(H,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265938
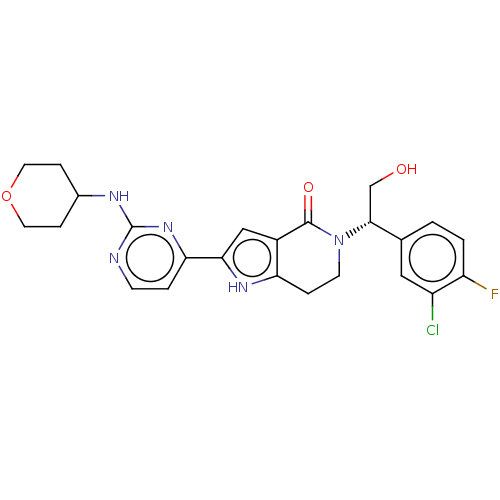
(CHEMBL4096522)Show SMILES OC[C@@H](N1CCc2[nH]c(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c25-17-11-14(1-2-18(17)26)22(13-32)31-8-4-19-16(23(31)33)12-21(29-19)20-3-7-27-24(30-20)28-15-5-9-34-10-6-15/h1-3,7,11-12,15,22,29,32H,4-6,8-10,13H2,(H,27,28,30)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505994

(CHEMBL4440109)Show SMILES C[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C23H22F2N8O/c1-13-9-26-23(29-19-6-7-27-31(19)3)30-20(13)18-12-32-10-14(2)33(22(34)21(32)28-18)11-15-4-5-16(24)17(25)8-15/h4-9,12,14H,10-11H2,1-3H3,(H,26,29,30)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265970

(CHEMBL4097186)Show SMILES O=C1N(Cc2ccccc2)CCc2[nH]c(cc12)-c1ccnc(NC2CCOCC2)n1 Show InChI InChI=1S/C23H25N5O2/c29-22-18-14-21(20-6-10-24-23(27-20)25-17-8-12-30-13-9-17)26-19(18)7-11-28(22)15-16-4-2-1-3-5-16/h1-6,10,14,17,26H,7-9,11-13,15H2,(H,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265934

(CHEMBL4060050)Show SMILES COCCN1[C@@H](C)Cc2[nH]c(cc2C1=O)-c1ccnc(Nc2ccnn2C)n1 |r| Show InChI InChI=1S/C19H23N7O2/c1-12-10-15-13(18(27)26(12)8-9-28-3)11-16(22-15)14-4-6-20-19(23-14)24-17-5-7-21-25(17)2/h4-7,11-12,22H,8-10H2,1-3H3,(H,20,23,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265939

(CHEMBL4078489)Show SMILES OC[C@@H](N1CCc2[nH]c(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1ccccc1 |r| Show InChI InChI=1S/C24H27N5O3/c30-15-22(16-4-2-1-3-5-16)29-11-7-19-18(23(29)31)14-21(27-19)20-6-10-25-24(28-20)26-17-8-12-32-13-9-17/h1-6,10,14,17,22,27,30H,7-9,11-13,15H2,(H,25,26,28)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505992

(CHEMBL4452853)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cn2CCN(Cc3cccc(Cl)c3)C(=O)c2n1 Show InChI InChI=1S/C22H21ClN8O/c1-14-11-24-22(27-18-6-7-25-29(18)2)28-19(14)17-13-30-8-9-31(21(32)20(30)26-17)12-15-4-3-5-16(23)10-15/h3-7,10-11,13H,8-9,12H2,1-2H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505993

(CHEMBL4587118)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3ccc(F)c(F)c3)CCn2c1 Show InChI InChI=1S/C23H21F2N7O/c1-14-11-26-23(28-20-5-6-27-30(20)2)29-21(14)16-10-19-22(33)32(8-7-31(19)13-16)12-15-3-4-17(24)18(25)9-15/h3-6,9-11,13H,7-8,12H2,1-2H3,(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265963
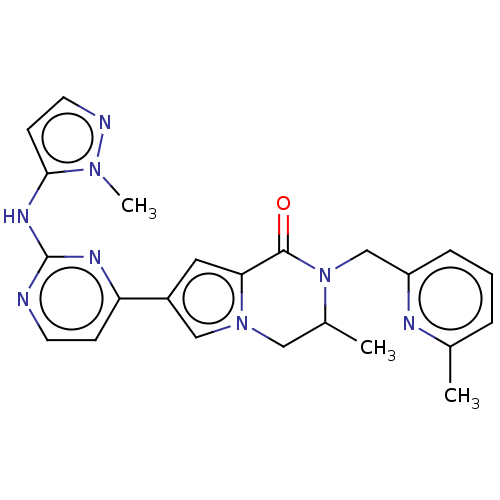
(CHEMBL4083098)Show SMILES CC1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1ccnc(Nc2ccnn2C)n1 Show InChI InChI=1S/C23H24N8O/c1-15-5-4-6-18(26-15)14-31-16(2)12-30-13-17(11-20(30)22(31)32)19-7-9-24-23(27-19)28-21-8-10-25-29(21)3/h4-11,13,16H,12,14H2,1-3H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265939

(CHEMBL4078489)Show SMILES OC[C@@H](N1CCc2[nH]c(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1ccccc1 |r| Show InChI InChI=1S/C24H27N5O3/c30-15-22(16-4-2-1-3-5-16)29-11-7-19-18(23(29)31)14-21(27-19)20-6-10-25-24(28-20)26-17-8-12-32-13-9-17/h1-6,10,14,17,22,27,30H,7-9,11-13,15H2,(H,25,26,28)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265936

(CHEMBL4068705)Show SMILES Cc1cccc(CN2CCc3[nH]c(cc3C2=O)-c2ccnc(NC3CCOCC3)n2)n1 Show InChI InChI=1S/C23H26N6O2/c1-15-3-2-4-17(25-15)14-29-10-6-19-18(22(29)30)13-21(27-19)20-5-9-24-23(28-20)26-16-7-11-31-12-8-16/h2-5,9,13,16,27H,6-8,10-12,14H2,1H3,(H,24,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265966

(CHEMBL4103768)Show SMILES Cn1c2CCN(Cc3ccccc3)C(=O)c2cc1-c1ccnc(NC2CCOCC2)n1 Show InChI InChI=1S/C24H27N5O2/c1-28-21-8-12-29(16-17-5-3-2-4-6-17)23(30)19(21)15-22(28)20-7-11-25-24(27-20)26-18-9-13-31-14-10-18/h2-7,11,15,18H,8-10,12-14,16H2,1H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265959

(CHEMBL4071576)Show SMILES C[C@@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265959

(CHEMBL4071576)Show SMILES C[C@@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239632
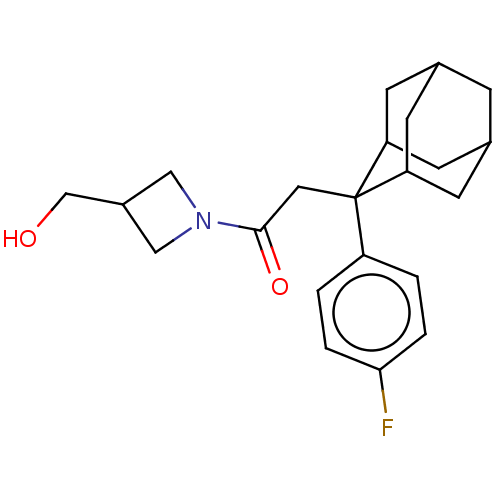
(CHEMBL4071232)Show SMILES OCC1CN(C1)C(=O)CC1(C2CC3CC(C2)CC1C3)c1ccc(F)cc1 |TLB:18:17:15:11.12.13,8:9:11.18.12:16.14.15,19:9:15:11.12.13,THB:18:12:9.17.16:15,13:12:9:16.14.15,13:14:9:11.18.12,8:9:15:11.12.13,19:9:11.18.12:16.14.15,(48.51,-12.14,;50.02,-11.82,;50.49,-10.36,;49.8,-8.98,;51.18,-8.28,;51.87,-9.66,;51.66,-6.82,;50.41,-5.91,;53.13,-6.34,;54.27,-7.37,;55.47,-6.1,;56.79,-6.59,;58.18,-6.24,;58.2,-4.71,;56.8,-4.13,;55.46,-4.61,;55.76,-5.37,;55.77,-6.96,;57.18,-7.52,;54.26,-8.91,;52.91,-9.66,;52.9,-11.19,;54.22,-11.98,;54.21,-13.52,;55.57,-11.21,;55.58,-9.68,)| Show InChI InChI=1S/C22H28FNO2/c23-20-3-1-17(2-4-20)22(10-21(26)24-11-16(12-24)13-25)18-6-14-5-15(8-18)9-19(22)7-14/h1-4,14-16,18-19,25H,5-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505989

(CHEMBL4551714)Show SMILES COC[C@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C25H28N8O2/c1-16-11-26-25(29-22-8-9-27-31(22)3)30-23(16)18-10-21-24(34)33(13-19-7-5-6-17(2)28-19)20(15-35-4)14-32(21)12-18/h5-12,20H,13-15H2,1-4H3,(H,26,29,30)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505988

(CHEMBL4482864)Show SMILES COC[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H24F2N8O2/c1-14-9-27-24(30-20-6-7-28-32(20)2)31-21(14)19-12-33-11-16(13-36-3)34(23(35)22(33)29-19)10-15-4-5-17(25)18(26)8-15/h4-9,12,16H,10-11,13H2,1-3H3,(H,27,30,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239628

(CHEMBL4102283)Show SMILES OC1CN(C1)C(=O)CC1(C2CC3CC1CC(C2)C3O)c1ccc(cc1)-c1ccc(F)cc1 |TLB:12:11:8.13.14:16,7:8:16:10.11.17,19:8:10.12.11:14.15.16,THB:18:17:8.13.14:16,12:13:16:10.11.17,17:11:8:14.15.16,17:15:8:10.12.11,7:8:10.12.11:14.15.16,19:8:16:10.11.17,(5.77,-1.2,;6.92,-2.24,;8.46,-2.16,;8.53,-3.7,;7,-3.78,;9.68,-4.73,;9.36,-6.24,;11.15,-4.26,;12.29,-5.3,;13.49,-4.02,;14.82,-4.5,;16.22,-4.16,;15.2,-5.44,;13.79,-4.87,;13.78,-3.28,;14.83,-2.05,;13.48,-2.53,;16.23,-2.63,;17.52,-1.78,;12.27,-6.84,;10.94,-7.58,;10.92,-9.12,;12.24,-9.91,;13.59,-9.14,;13.6,-7.61,;12.23,-11.45,;10.89,-12.21,;10.87,-13.75,;12.2,-14.54,;12.19,-16.08,;13.55,-13.77,;13.56,-12.23,)| Show InChI InChI=1S/C27H30FNO3/c28-23-7-3-17(4-8-23)16-1-5-20(6-2-16)27(13-25(31)29-14-24(30)15-29)21-9-18-10-22(27)12-19(11-21)26(18)32/h1-8,18-19,21-22,24,26,30,32H,9-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265938

(CHEMBL4096522)Show SMILES OC[C@@H](N1CCc2[nH]c(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c25-17-11-14(1-2-18(17)26)22(13-32)31-8-4-19-16(23(31)33)12-21(29-19)20-3-7-27-24(30-20)28-15-5-9-34-10-6-15/h1-3,7,11-12,15,22,29,32H,4-6,8-10,13H2,(H,27,28,30)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239619
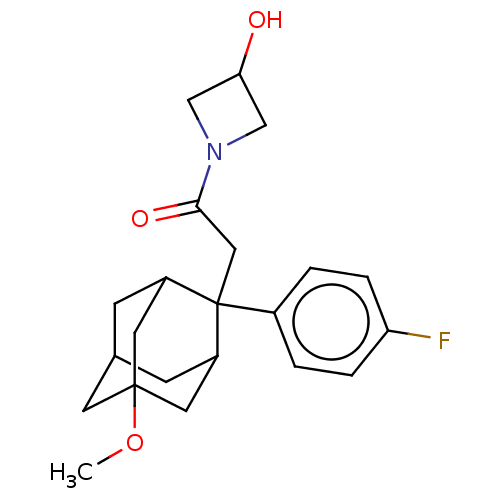
(CHEMBL4087497)Show SMILES COC12CC3CC(C1)C(CC(=O)N1CC(O)C1)(C(C3)C2)c1ccc(F)cc1 |TLB:18:17:7:5.4.3,20:8:5.18.4:19.2.7,20:8:7:5.4.3,THB:18:4:8.17.19:7,3:4:8:19.2.7,3:2:8:5.18.4,1:2:8:5.18.4,9:8:5.18.4:19.2.7,9:8:7:5.4.3,(28.8,-17.02,;27.27,-17.21,;26.66,-18.63,;28.07,-19.21,;28.05,-20.74,;26.65,-21.08,;25.33,-20.59,;25.32,-19.11,;24.13,-21.87,;22.99,-20.84,;21.52,-21.31,;20.27,-20.4,;21.04,-22.78,;19.66,-23.47,;20.36,-24.85,;19.88,-26.32,;21.74,-24.15,;25.63,-21.45,;27.04,-22.02,;25.62,-19.86,;24.12,-23.41,;22.77,-24.16,;22.76,-25.7,;24.08,-26.48,;24.07,-28.02,;25.43,-25.72,;25.44,-24.18,)| Show InChI InChI=1S/C22H28FNO3/c1-27-21-8-14-6-16(9-21)22(17(7-14)10-21,15-2-4-18(23)5-3-15)11-20(26)24-12-19(25)13-24/h2-5,14,16-17,19,25H,6-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505990
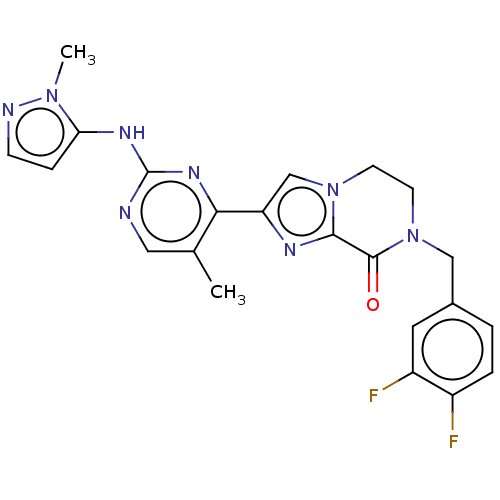
(CHEMBL4458562)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cn2CCN(Cc3ccc(F)c(F)c3)C(=O)c2n1 Show InChI InChI=1S/C22H20F2N8O/c1-13-10-25-22(28-18-5-6-26-30(18)2)29-19(13)17-12-31-7-8-32(21(33)20(31)27-17)11-14-3-4-15(23)16(24)9-14/h3-6,9-10,12H,7-8,11H2,1-2H3,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265973

(CHEMBL4075638)Show SMILES C[C@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50239609

(CHEMBL4093016)Show SMILES Fc1ccc(cc1)C1(CC(=O)N2CCC2)C2CC3CC(C2)CC1C3 |TLB:23:22:20:16.17.18,8:7:16.23.17:21.19.20,4:7:20:16.17.18,THB:23:17:7.22.21:20,18:17:7:21.19.20,18:19:7:16.23.17,8:7:20:16.17.18,4:7:16.23.17:21.19.20,(23.9,-12.85,;23.91,-11.3,;22.58,-10.52,;22.6,-8.98,;23.94,-8.23,;25.26,-9,;25.25,-10.54,;23.96,-6.69,;22.81,-5.65,;21.34,-6.13,;20.1,-5.22,;20.86,-7.6,;19.48,-8.3,;20.18,-9.68,;21.56,-8.98,;25.16,-5.41,;26.48,-5.9,;27.88,-5.55,;27.9,-4.02,;26.49,-3.44,;25.15,-3.92,;25.45,-4.68,;25.46,-6.27,;26.87,-6.84,)| Show InChI InChI=1S/C21H26FNO/c22-19-4-2-16(3-5-19)21(13-20(24)23-6-1-7-23)17-9-14-8-15(11-17)12-18(21)10-14/h2-5,14-15,17-18H,1,6-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 60: 4932-4948 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00211
BindingDB Entry DOI: 10.7270/Q2DV1N23 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data