

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM381166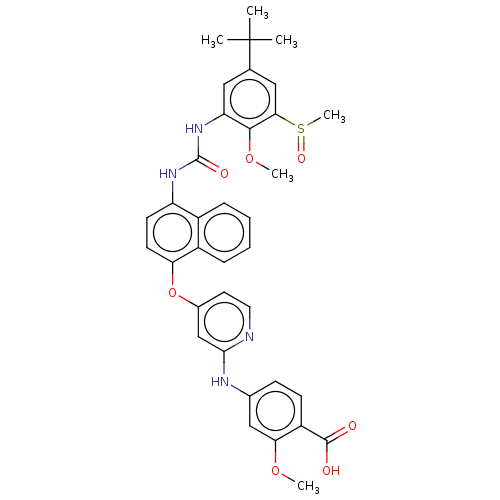 (US9890185, Example 79(g)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 2: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to t... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381082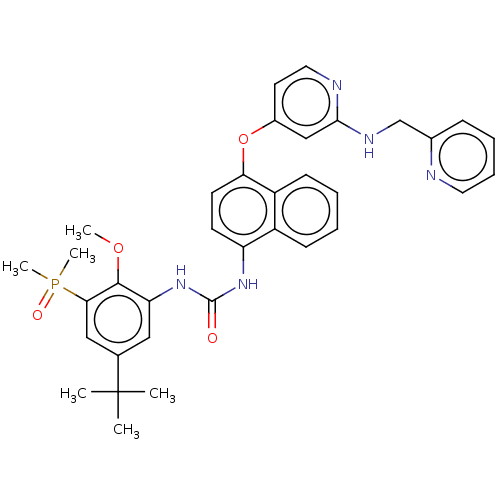 (US9890185, Example 38(e)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381094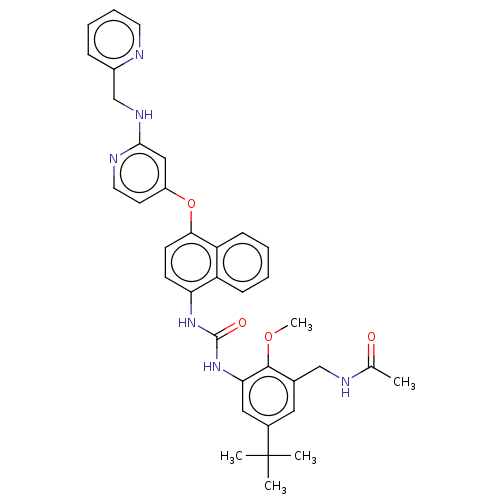 (US9890185, Example 38(q)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256521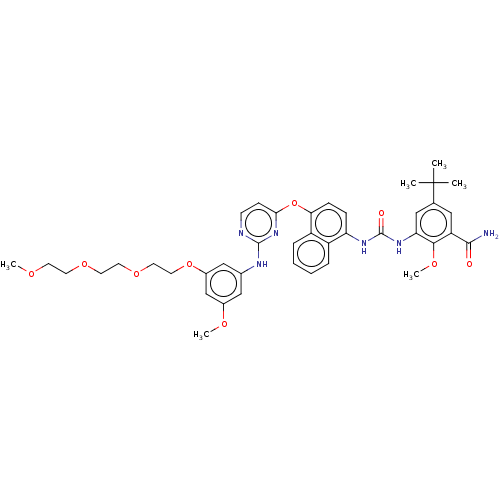 (US10435361, Example 20 | US9481648, 20 | US9790174...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381071 (1-(5-(tert-Butyl)-3-(dimethylphosphoryl)-2-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381167 (US9890185, Example 79(m)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM381167 (US9890185, Example 79(m)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 2: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to t... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256511 (US10435361, Example 9 | US9481648, 9 | US9790174, ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381123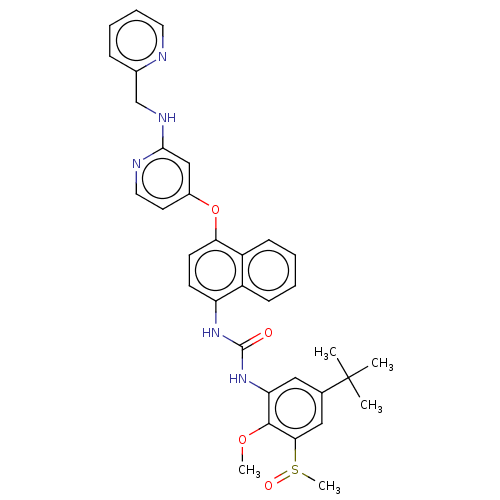 (1-(5-(tert-Butyl)-2-methoxy-3-(methylsulfinyl)phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM347466 (3-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description TBD | US Patent US10435361 (2019) BindingDB Entry DOI: 10.7270/Q25M6839 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381084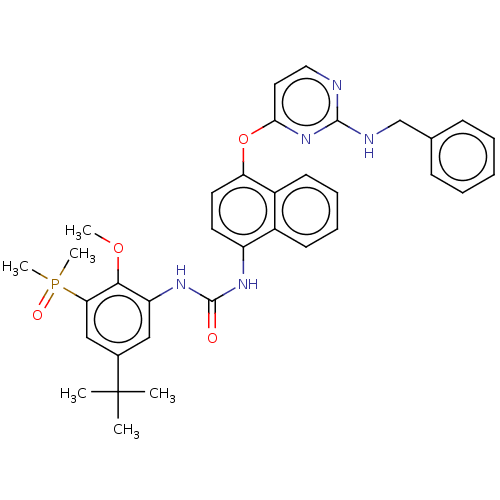 (US9890185, Example 38(g)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256515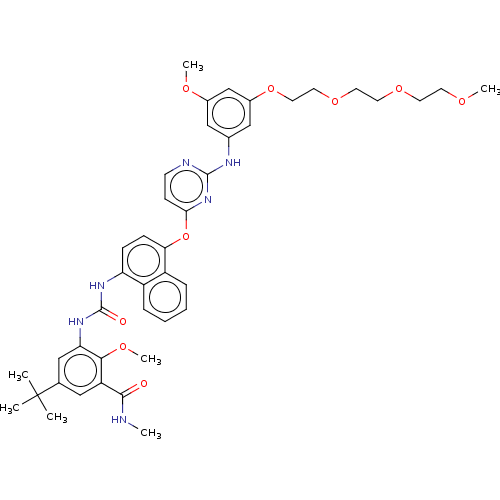 (US10435361, Example 13 | US9481648, 13 | US9790174...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM380997 (US9890185, Example 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256509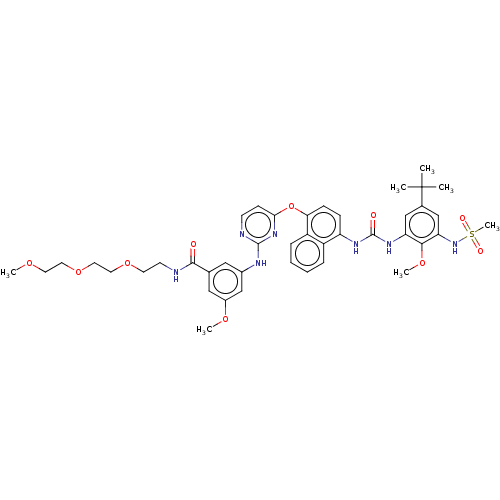 (US9481648, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381092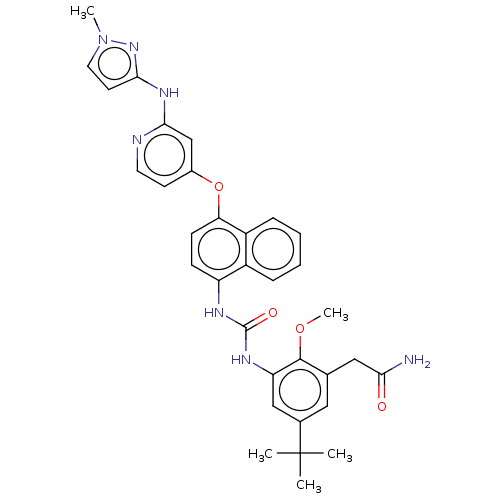 (US9890185, Example 38(o)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256519 (US10435361, Example 18 | US9481648, 18 | US9790174...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM347465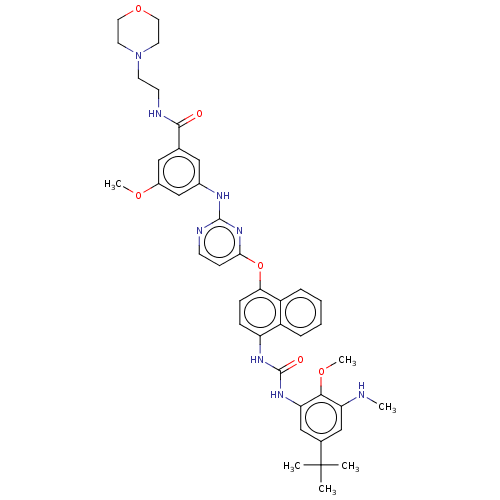 (3-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description TBD | US Patent US10435361 (2019) BindingDB Entry DOI: 10.7270/Q25M6839 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM380998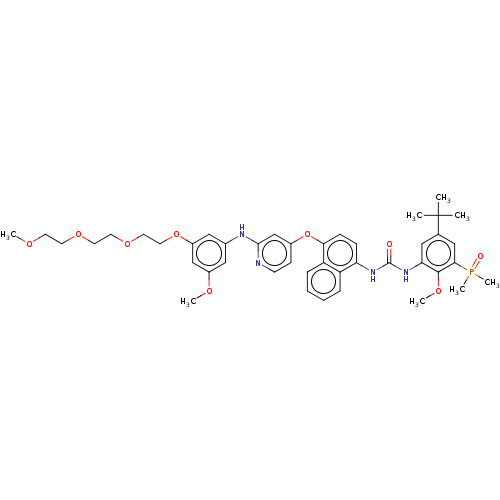 (1-(5-(tert-Butyl)-3-(dimethylphosphoryl)-2-methoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM256524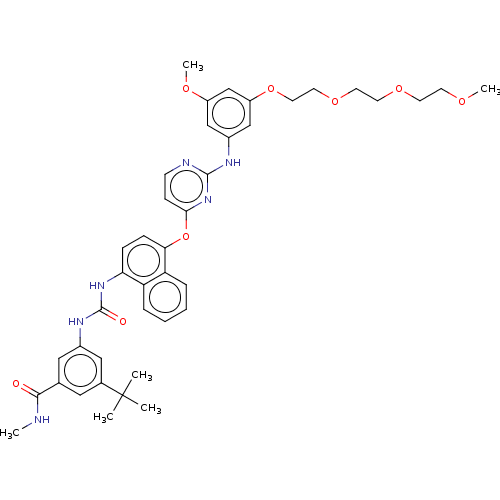 (US10435361, Example 23 | US9481648, 23 | US9790174...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description Method 2: This method follows the same steps as Method 1 above, but utilises a higher concentration of the p38 MAPKα protein (2.5 uL of 200 ng/... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256534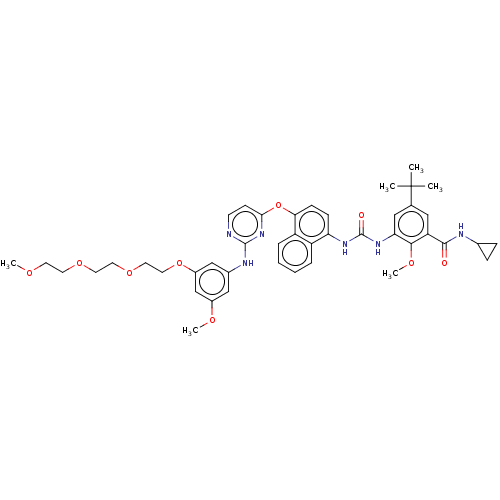 (US10435361, Example 33 | US9481648, 33 | US9790174...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256516 (US10435361, Example 14 | US9481648, 14 | US9790174...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256524 (US10435361, Example 23 | US9481648, 23 | US9790174...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256508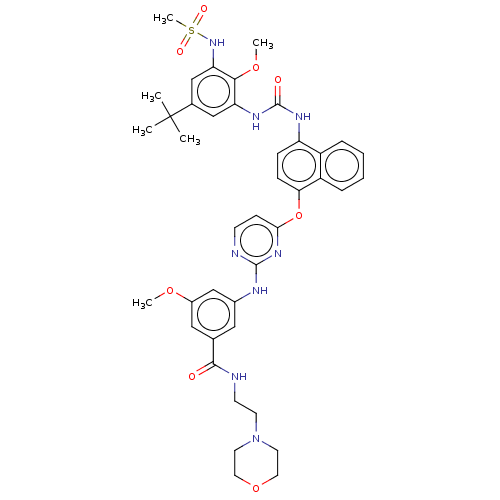 (US9481648, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381080 (US9890185, Example 38(c)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381126 (3-((4-((4-(3-(3-(2-Amino-2-oxoethyl)-5-(tert-butyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381134 (3-((4-((4-(3-(3-(2-Amino-2-oxoethyl)-5-(tert-butyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256538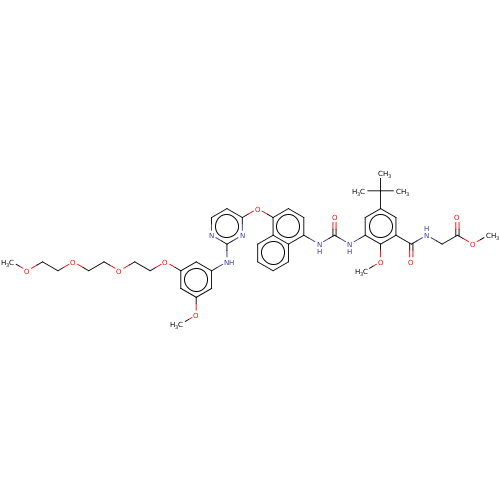 (US10435361, Example 37 | US9481648, 37 | US9790174...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381150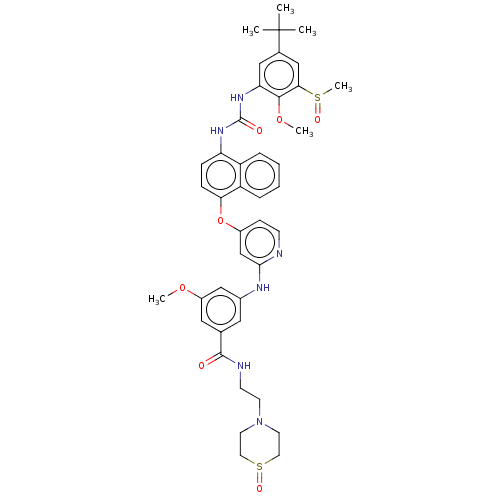 (3-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256503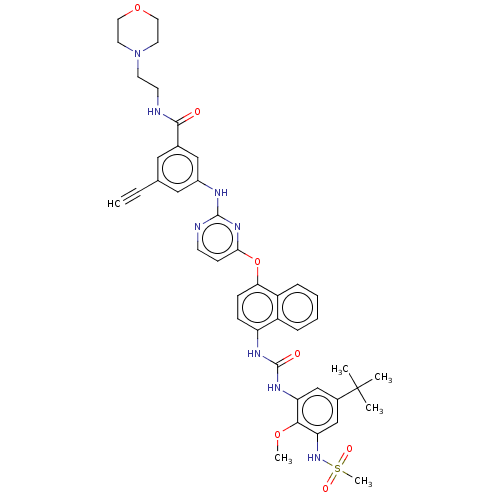 (US9481648, 1 | US9790174, Example 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM414113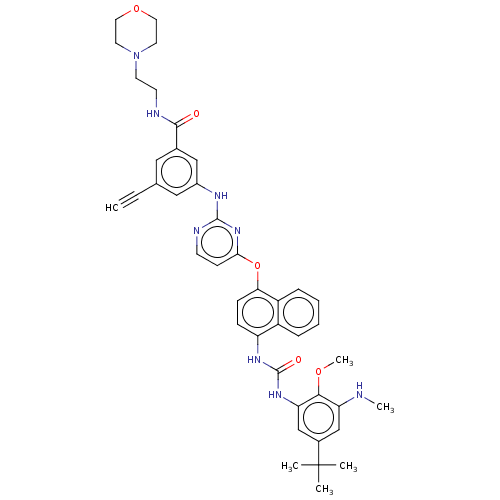 (US10435361, Example 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description TBD | US Patent US10435361 (2019) BindingDB Entry DOI: 10.7270/Q25M6839 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381081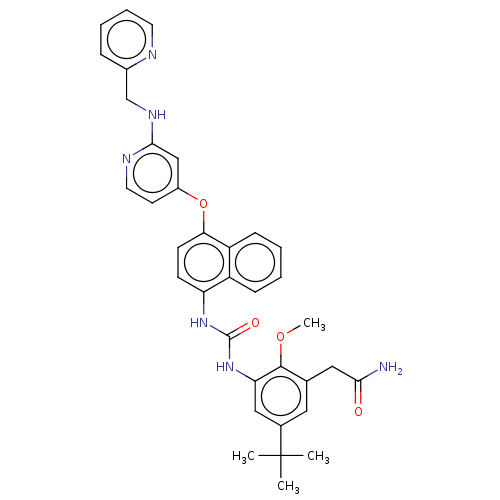 (US9890185, Example 38(d)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381098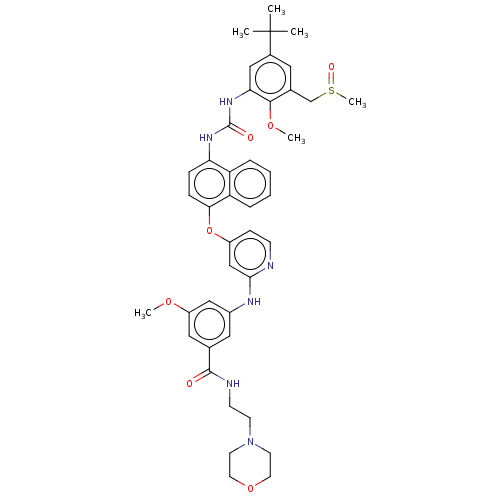 (US9890185, Example 38(u)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256536 (US10435361, Example 35 | US9481648, 35 | US9790174...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381149 (3-((4-((4-(3-(3-(2-Amino-2-oxoethyl)-5-(tert-butyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256570 (US10435361, Example 69 | US9481648, 69 | US9790174...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM347463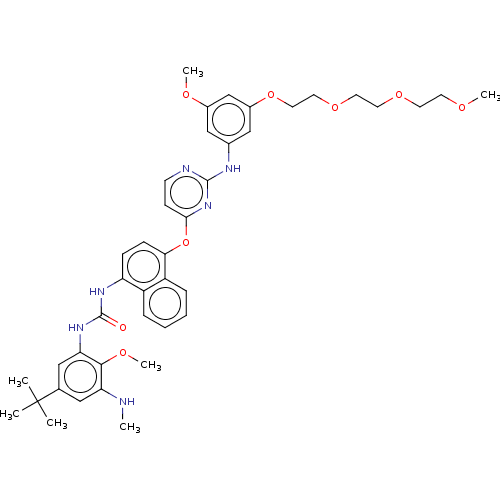 (N-(5-(tert-Butyl)-2-methoxy-3-(3-(4-((2-((3-methox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description TBD | US Patent US10435361 (2019) BindingDB Entry DOI: 10.7270/Q25M6839 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381152 (3-((4-((4-(3-(3-(Acetamidomethyl)-5-(tert-butyl)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256525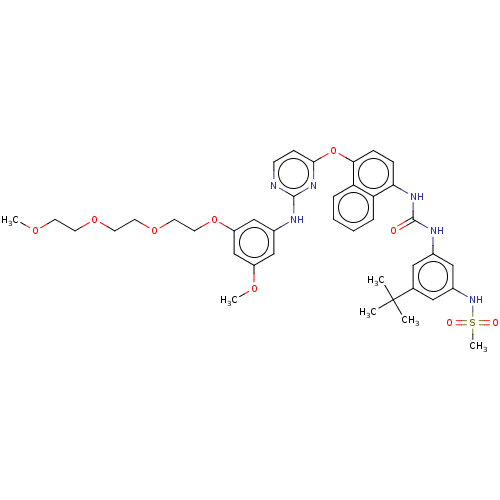 (US10435361, Example 24 | US9481648, 24 | US9790174...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381012 (US9890185, Example 15(b)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256506 (US9481648, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381004 (5-(tert-Butyl)-2-methoxy-3-(3-(4-((2-((3-methoxy-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381098 (US9890185, Example 38(u)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381102 (US9890185, Example 38(y)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381057 (1-(5-(tert-Butyl)-3-(dimethylphosphoryl)-2-methoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256552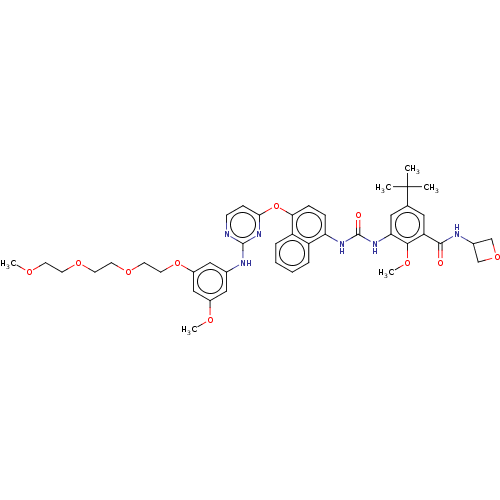 (US10435361, Example 51 | US9481648, 51 | US9790174...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256547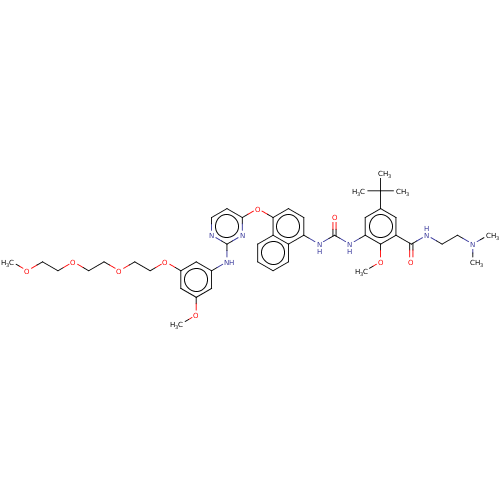 (US10435361, Example 46 | US9481648, 46 | US9790174...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256548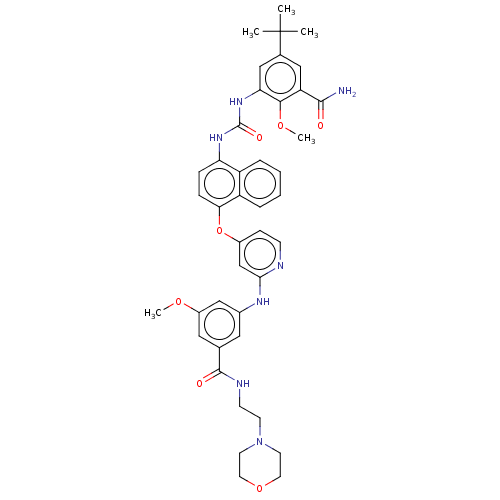 (US10435361, Example 47 | US9481648, 47 | US9790174...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381146 (3-((4-((4-(3-(3-(2-Amino-2-oxoethyl)-5-(tert-butyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381002 (1-(5-(tert-Butyl)-3-((dimethylphosphoryl)methoxy)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381006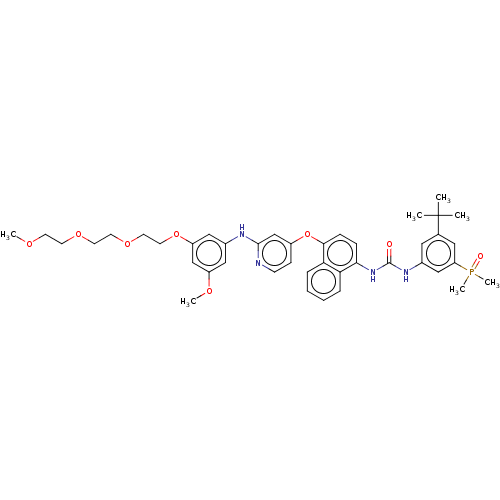 (1-(3-(tert-Butyl)-5-(dimethylphosphoryl)phenyl)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 852 total ) | Next | Last >> |