 Found 586 hits with Last Name = 'heim' and Initial = 'd'
Found 586 hits with Last Name = 'heim' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
E3 ubiquitin-protein ligase XIAP [241-356]
(Homo sapiens (Human)) | BDBM620168
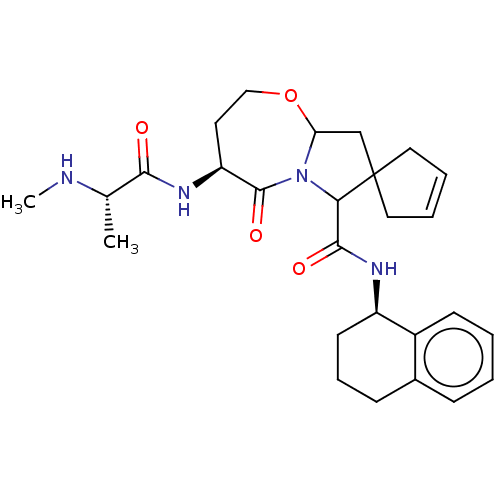
(US20230295181, Compound 81)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CCOC2CC3(CC=CC3)C(N2C1=O)C(=O)N[C@@H]1CCCc2ccccc12 |r,c:15| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP [241-356]
(Homo sapiens (Human)) | BDBM620165

(US20230295181, Compound 80)Show SMILES [H][C@]12CC3(CCCC3)[C@H](N1C(=O)[C@H](CCS2)NC(=O)[C@H](C)NC)C(=O)N[C@@H]1CCCc2ccccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP [241-356]
(Homo sapiens (Human)) | BDBM620153

(US20230295181, Compound 65)Show SMILES [H][C@]12CC(C)(C)[C@H](N1C(=O)[C@@H](NC(=O)[C@H](C)NC)C1CCCCC1O2)C(=O)N[C@@H]1CCCc2ccccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP [241-356]
(Homo sapiens (Human)) | BDBM391840

(US10300074, Compd 35 | US20230295181, Compound A)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CCS[C@H]2CC(C)(C)C(N2C1=O)C(=O)N[C@@H]1CCCc2ccccc12 |r,w:20.21| Show InChI InChI=1S/C25H36N4O3S/c1-15(26-4)22(30)28-19-12-13-33-20-14-25(2,3)21(29(20)24(19)32)23(31)27-18-11-7-9-16-8-5-6-10-17(16)18/h5-6,8,10,15,18-21,26H,7,9,11-14H2,1-4H3,(H,27,31)(H,28,30)/t15-,18+,19-,20-,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP [241-356]
(Homo sapiens (Human)) | BDBM620169

(US20230295181, Compound 82)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CCOC2CC3(CCCC3)C(N2C1=O)C(=O)N[C@@H]1CCCc2ccccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP [241-356]
(Homo sapiens (Human)) | BDBM620148

(US20230295181, Compound 38)Show SMILES [H][C@]12CC(CC)(CC)[C@H](N1C(=O)[C@H](CCS2)NC(=O)[C@H](C)NC)C(=O)N[C@@H]1CCCc2ccccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201193
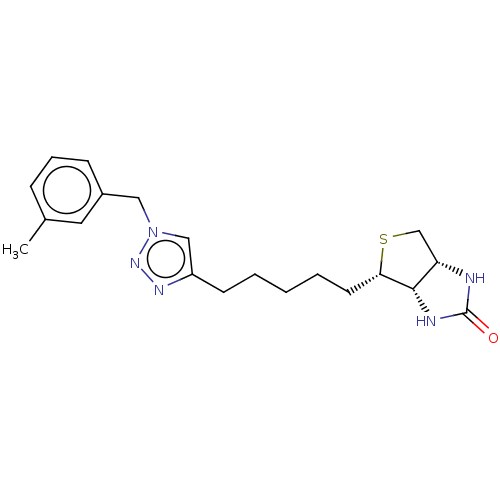
(CHEMBL3974372)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4cccc(C)c4)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C20H27N5OS/c1-14-6-5-7-15(10-14)11-25-12-16(23-24-25)8-3-2-4-9-18-19-17(13-27-18)21-20(26)22-19/h5-7,10,12,17-19H,2-4,8-9,11,13H2,1H3,(H2,21,22,26)/t17-,18-,19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201194
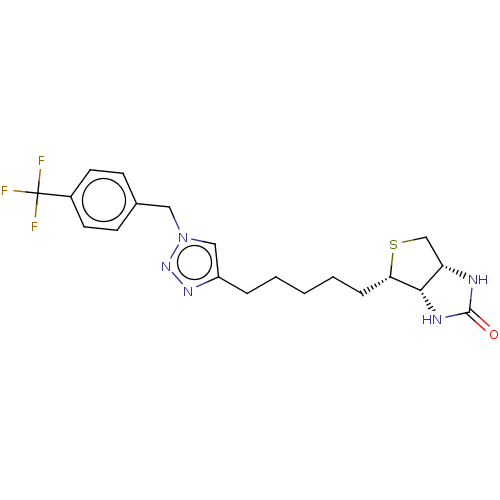
(CHEMBL3932600)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4ccc(cc4)C(F)(F)F)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C20H24F3N5OS/c21-20(22,23)14-8-6-13(7-9-14)10-28-11-15(26-27-28)4-2-1-3-5-17-18-16(12-30-17)24-19(29)25-18/h6-9,11,16-18H,1-5,10,12H2,(H2,24,25,29)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201195
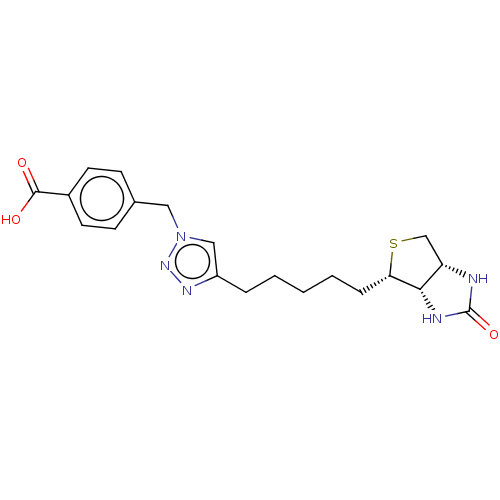
(CHEMBL3972792)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4ccc(cc4)C(O)=O)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C20H25N5O3S/c26-19(27)14-8-6-13(7-9-14)10-25-11-15(23-24-25)4-2-1-3-5-17-18-16(12-29-17)21-20(28)22-18/h6-9,11,16-18H,1-5,10,12H2,(H,26,27)(H2,21,22,28)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201196
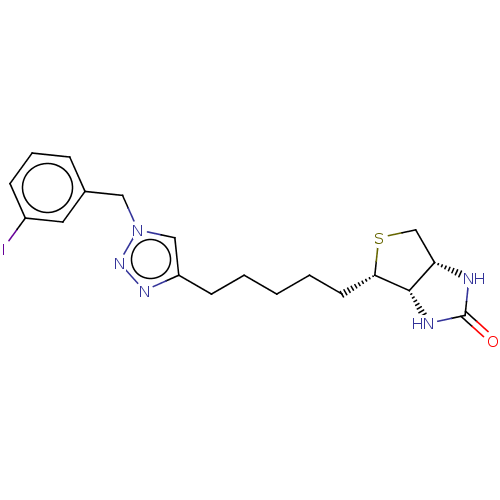
(CHEMBL3961751)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4cccc(I)c4)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C19H24IN5OS/c20-14-6-4-5-13(9-14)10-25-11-15(23-24-25)7-2-1-3-8-17-18-16(12-27-17)21-19(26)22-18/h4-6,9,11,16-18H,1-3,7-8,10,12H2,(H2,21,22,26)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201197
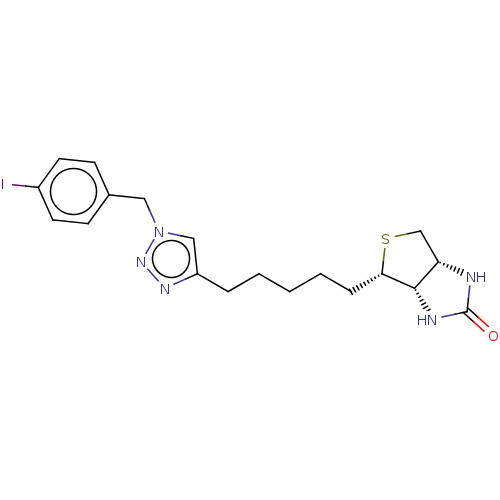
(CHEMBL3895686)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4ccc(I)cc4)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C19H24IN5OS/c20-14-8-6-13(7-9-14)10-25-11-15(23-24-25)4-2-1-3-5-17-18-16(12-27-17)21-19(26)22-18/h6-9,11,16-18H,1-5,10,12H2,(H2,21,22,26)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201198

(CHEMBL3968104)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4cccc(F)c4)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C19H24FN5OS/c20-14-6-4-5-13(9-14)10-25-11-15(23-24-25)7-2-1-3-8-17-18-16(12-27-17)21-19(26)22-18/h4-6,9,11,16-18H,1-3,7-8,10,12H2,(H2,21,22,26)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201199
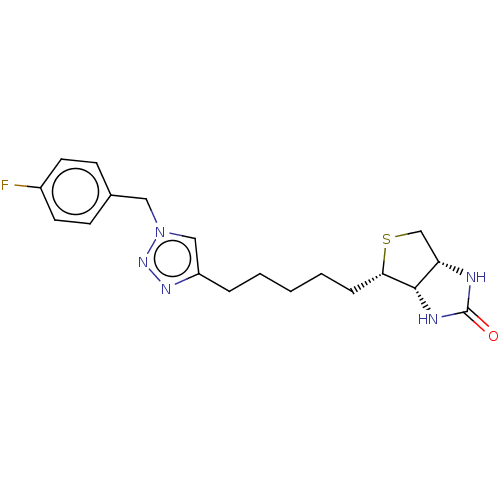
(CHEMBL3934430)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4ccc(F)cc4)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C19H24FN5OS/c20-14-8-6-13(7-9-14)10-25-11-15(23-24-25)4-2-1-3-5-17-18-16(12-27-17)21-19(26)22-18/h6-9,11,16-18H,1-5,10,12H2,(H2,21,22,26)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201200
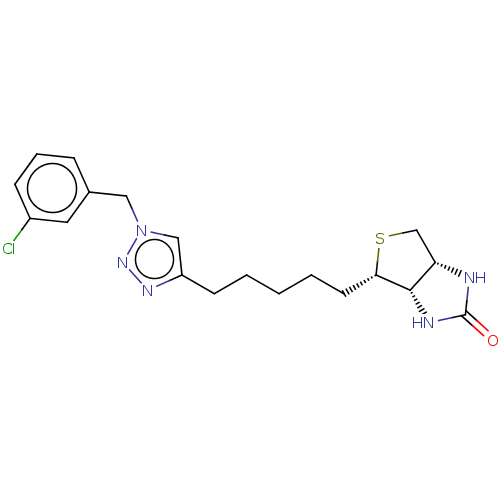
(CHEMBL3905875)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4cccc(Cl)c4)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C19H24ClN5OS/c20-14-6-4-5-13(9-14)10-25-11-15(23-24-25)7-2-1-3-8-17-18-16(12-27-17)21-19(26)22-18/h4-6,9,11,16-18H,1-3,7-8,10,12H2,(H2,21,22,26)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201201
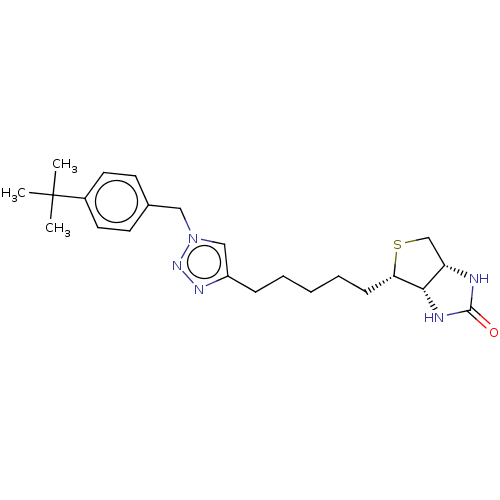
(CHEMBL3901708)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4ccc(cc4)C(C)(C)C)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C23H33N5OS/c1-23(2,3)17-11-9-16(10-12-17)13-28-14-18(26-27-28)7-5-4-6-8-20-21-19(15-30-20)24-22(29)25-21/h9-12,14,19-21H,4-8,13,15H2,1-3H3,(H2,24,25,29)/t19-,20-,21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201202
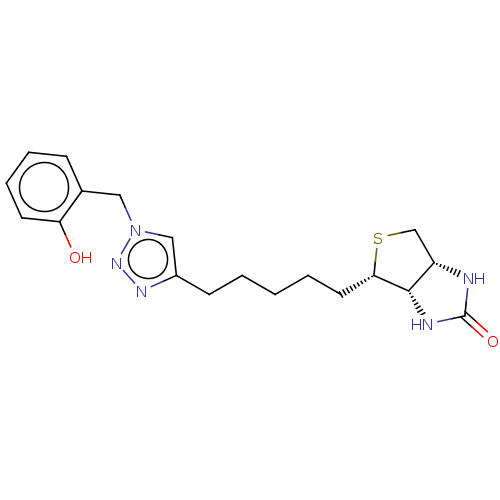
(CHEMBL3971239)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4ccccc4O)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C19H25N5O2S/c25-16-8-5-4-6-13(16)10-24-11-14(22-23-24)7-2-1-3-9-17-18-15(12-27-17)20-19(26)21-18/h4-6,8,11,15,17-18,25H,1-3,7,9-10,12H2,(H2,20,21,26)/t15-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201203
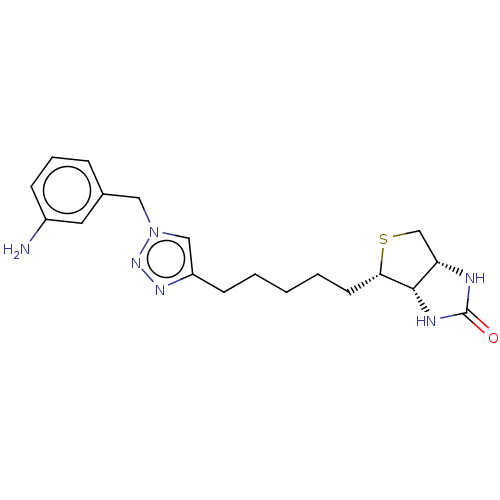
(CHEMBL3905724)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4cccc(N)c4)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C19H26N6OS/c20-14-6-4-5-13(9-14)10-25-11-15(23-24-25)7-2-1-3-8-17-18-16(12-27-17)21-19(26)22-18/h4-6,9,11,16-18H,1-3,7-8,10,12,20H2,(H2,21,22,26)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201204
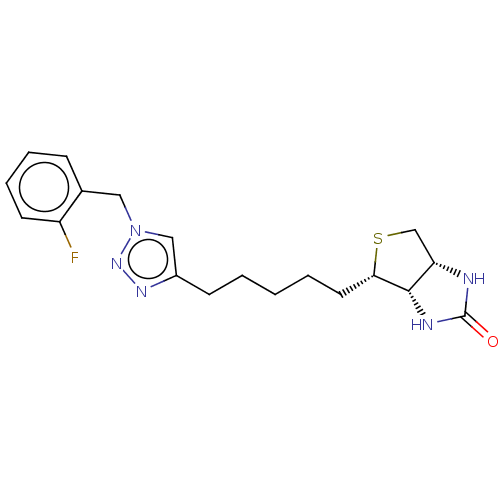
(CHEMBL3927537)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4ccccc4F)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C19H24FN5OS/c20-15-8-5-4-6-13(15)10-25-11-14(23-24-25)7-2-1-3-9-17-18-16(12-27-17)21-19(26)22-18/h4-6,8,11,16-18H,1-3,7,9-10,12H2,(H2,21,22,26)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201205
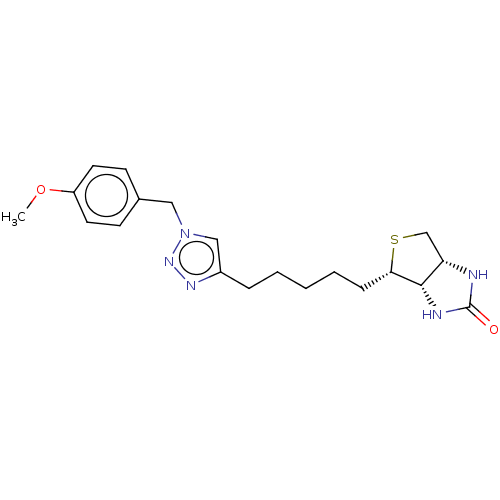
(CHEMBL3914706)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4ccc(OC)cc4)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C20H27N5O2S/c1-27-16-9-7-14(8-10-16)11-25-12-15(23-24-25)5-3-2-4-6-18-19-17(13-28-18)21-20(26)22-19/h7-10,12,17-19H,2-6,11,13H2,1H3,(H2,21,22,26)/t17-,18-,19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201206
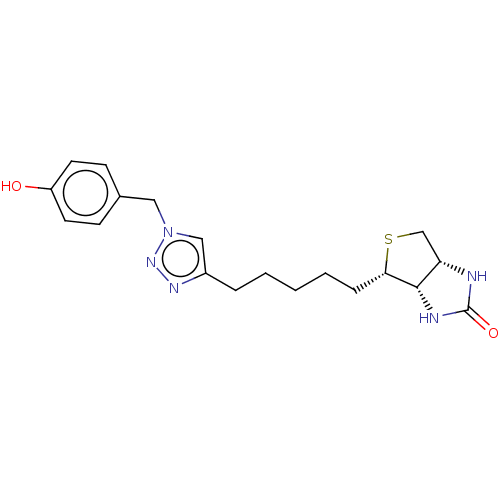
(CHEMBL3960176)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4ccc(O)cc4)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C19H25N5O2S/c25-15-8-6-13(7-9-15)10-24-11-14(22-23-24)4-2-1-3-5-17-18-16(12-27-17)20-19(26)21-18/h6-9,11,16-18,25H,1-5,10,12H2,(H2,20,21,26)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201207
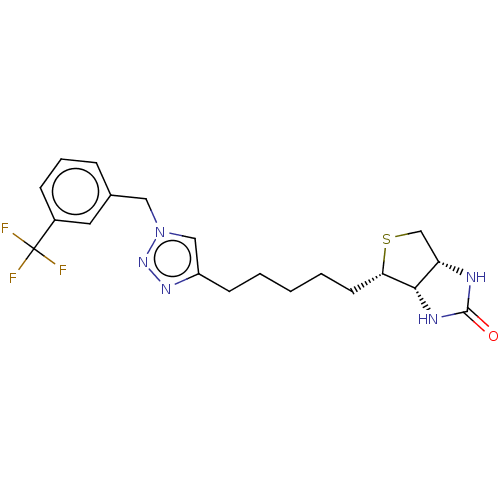
(CHEMBL3910734)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4cccc(c4)C(F)(F)F)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C20H24F3N5OS/c21-20(22,23)14-6-4-5-13(9-14)10-28-11-15(26-27-28)7-2-1-3-8-17-18-16(12-30-17)24-19(29)25-18/h4-6,9,11,16-18H,1-3,7-8,10,12H2,(H2,24,25,29)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201208
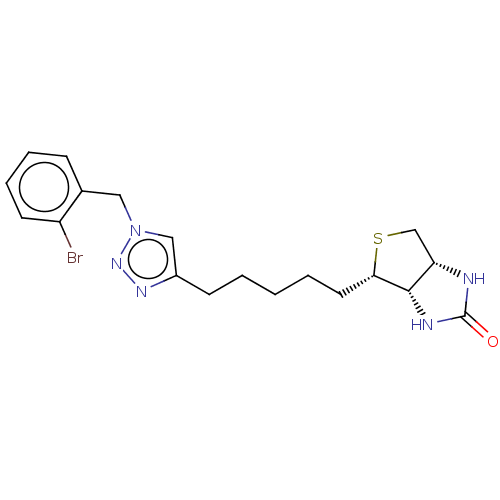
(CHEMBL3892750)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4ccccc4Br)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C19H24BrN5OS/c20-15-8-5-4-6-13(15)10-25-11-14(23-24-25)7-2-1-3-9-17-18-16(12-27-17)21-19(26)22-18/h4-6,8,11,16-18H,1-3,7,9-10,12H2,(H2,21,22,26)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201209
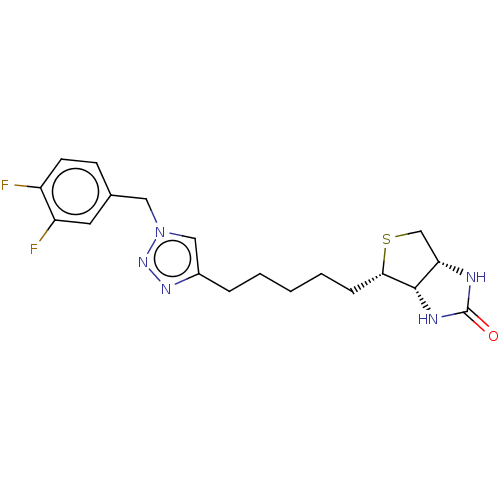
(CHEMBL3914868)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4ccc(F)c(F)c4)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C19H23F2N5OS/c20-14-7-6-12(8-15(14)21)9-26-10-13(24-25-26)4-2-1-3-5-17-18-16(11-28-17)22-19(27)23-18/h6-8,10,16-18H,1-5,9,11H2,(H2,22,23,27)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201210
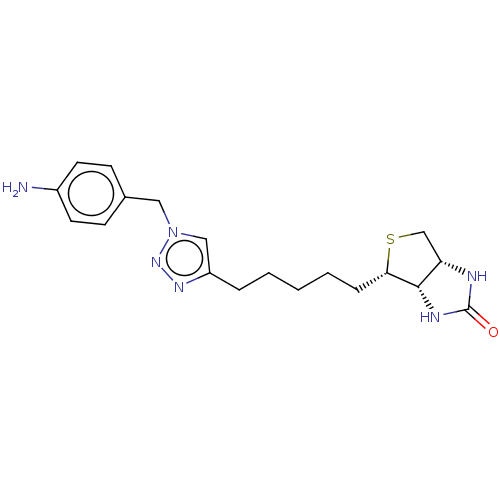
(CHEMBL3933745)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4ccc(N)cc4)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C19H26N6OS/c20-14-8-6-13(7-9-14)10-25-11-15(23-24-25)4-2-1-3-5-17-18-16(12-27-17)21-19(26)22-18/h6-9,11,16-18H,1-5,10,12,20H2,(H2,21,22,26)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201192
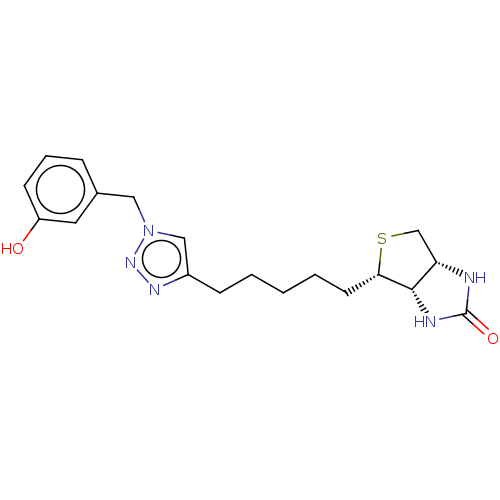
(CHEMBL3932681)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4cccc(O)c4)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C19H25N5O2S/c25-15-7-4-5-13(9-15)10-24-11-14(22-23-24)6-2-1-3-8-17-18-16(12-27-17)20-19(26)21-18/h4-5,7,9,11,16-18,25H,1-3,6,8,10,12H2,(H2,20,21,26)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201191
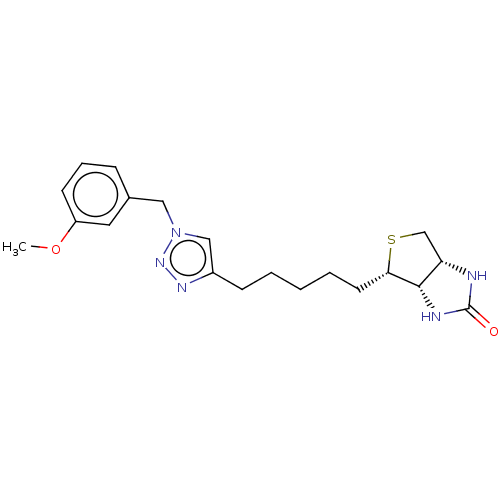
(CHEMBL3951442)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4cccc(OC)c4)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C20H27N5O2S/c1-27-16-8-5-6-14(10-16)11-25-12-15(23-24-25)7-3-2-4-9-18-19-17(13-28-18)21-20(26)22-19/h5-6,8,10,12,17-19H,2-4,7,9,11,13H2,1H3,(H2,21,22,26)/t17-,18-,19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201190
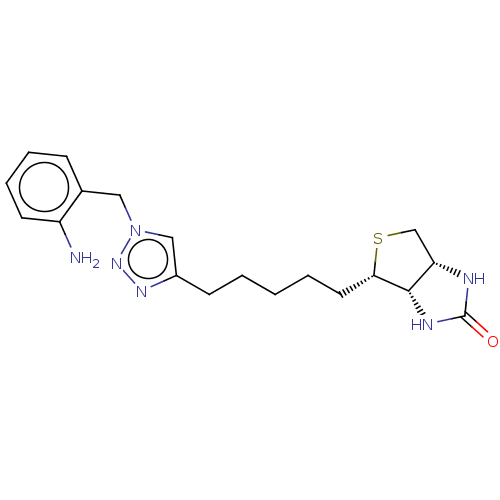
(CHEMBL3942705)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4ccccc4N)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C19H26N6OS/c20-15-8-5-4-6-13(15)10-25-11-14(23-24-25)7-2-1-3-9-17-18-16(12-27-17)21-19(26)22-18/h4-6,8,11,16-18H,1-3,7,9-10,12,20H2,(H2,21,22,26)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201189
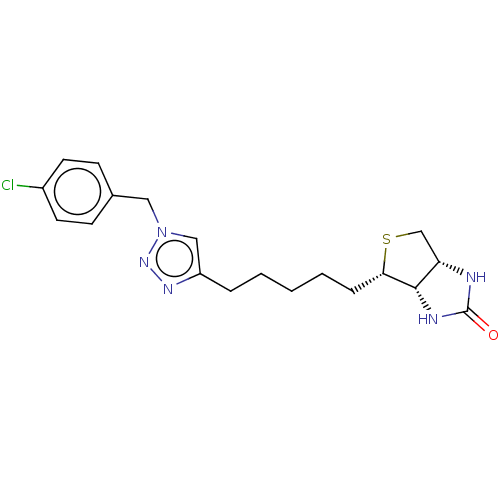
(CHEMBL3984675)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4ccc(Cl)cc4)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C19H24ClN5OS/c20-14-8-6-13(7-9-14)10-25-11-15(23-24-25)4-2-1-3-5-17-18-16(12-27-17)21-19(26)22-18/h6-9,11,16-18H,1-5,10,12H2,(H2,21,22,26)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201188

(CHEMBL3891635)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4ccc(C)cc4)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C20H27N5OS/c1-14-7-9-15(10-8-14)11-25-12-16(23-24-25)5-3-2-4-6-18-19-17(13-27-18)21-20(26)22-19/h7-10,12,17-19H,2-6,11,13H2,1H3,(H2,21,22,26)/t17-,18-,19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201186
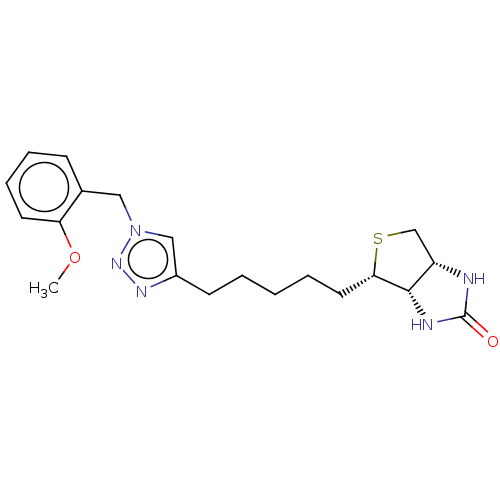
(CHEMBL3923732)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4ccccc4OC)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C20H27N5O2S/c1-27-17-9-6-5-7-14(17)11-25-12-15(23-24-25)8-3-2-4-10-18-19-16(13-28-18)21-20(26)22-19/h5-7,9,12,16,18-19H,2-4,8,10-11,13H2,1H3,(H2,21,22,26)/t16-,18-,19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Biotin--protein ligase
(Homo sapiens (Human)) | BDBM50201187
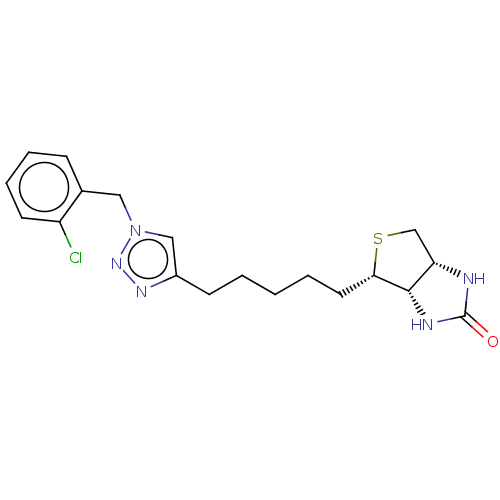
(CHEMBL3976601)Show SMILES [H][C@]12CS[C@@H](CCCCCc3cn(Cc4ccccc4Cl)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C19H24ClN5OS/c20-15-8-5-4-6-13(15)10-25-11-14(23-24-25)7-2-1-3-9-17-18-16(12-27-17)21-19(26)22-18/h4-6,8,11,16-18H,1-3,7,9-10,12H2,(H2,21,22,26)/t16-,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide
Curated by ChEMBL
| Assay Description
Inhibition of human biotin protein ligase assessed as reduction in 3H-biotin incorporation into pyruvate carboxylase biotin domain after 10 mins by l... |
ACS Med Chem Lett 7: 1068-1072 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00248
BindingDB Entry DOI: 10.7270/Q2QV3PGF |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50181139
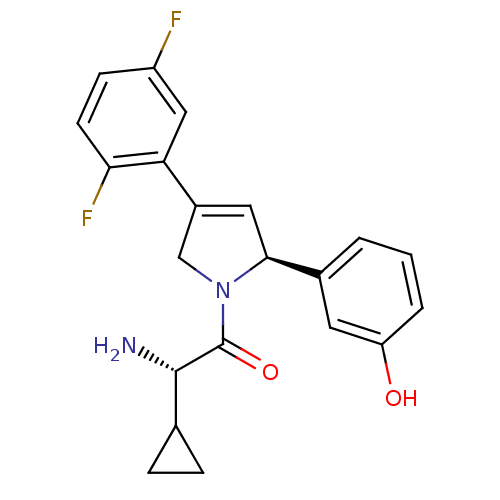
((S)-2-amino-2-cyclopropyl-1-((S)-4-(2,5-difluoroph...)Show SMILES N[C@@H](C1CC1)C(=O)N1CC(=C[C@H]1c1cccc(O)c1)c1cc(F)ccc1F |c:10| Show InChI InChI=1S/C21H20F2N2O2/c22-15-6-7-18(23)17(10-15)14-9-19(13-2-1-3-16(26)8-13)25(11-14)21(27)20(24)12-4-5-12/h1-3,6-10,12,19-20,26H,4-5,11,24H2/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KSP by ATPase assay |
Bioorg Med Chem Lett 16: 1780-3 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.094
BindingDB Entry DOI: 10.7270/Q2PZ58D2 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM16179
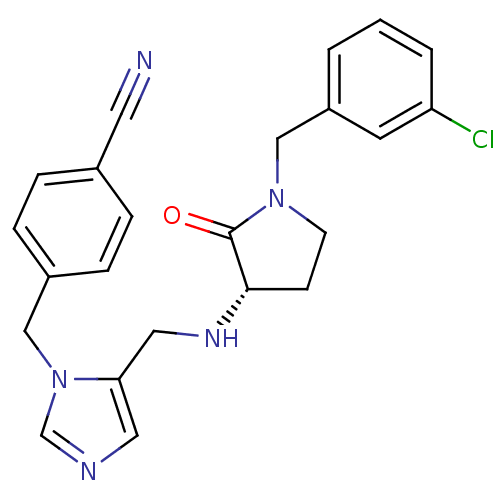
(4-{[5-({[(3S)-1-(3-chlorobenzyl)-2-oxopyrrolidin-3...)Show SMILES Clc1cccc(CN2CC[C@H](NCc3cncn3Cc3ccc(cc3)C#N)C2=O)c1 |r| Show InChI InChI=1S/C23H22ClN5O/c24-20-3-1-2-19(10-20)15-28-9-8-22(23(28)30)27-13-21-12-26-16-29(21)14-18-6-4-17(11-25)5-7-18/h1-7,10,12,16,22,27H,8-9,13-15H2/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to displace 50% of a potent radiolabeled FTI from farnesyl transferase in cultured H-ras-transformed Rat1 cells |
J Med Chem 44: 2933-49 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RNW |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM50012304

(2-{2-[2-[2-(2,2-Dimethyl-propionylamino)-3-(3H-imi...)Show SMILES CCCCC[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(C)(C)C)C(C)C Show InChI InChI=1S/C48H72N12O7/c1-10-11-12-15-32(18-28(2)3)56-44(64)38(20-33-23-49-26-53-33)57-40(61)25-52-46(66)41(29(4)5)60-42(62)30(6)55-43(63)37(19-31-22-51-36-17-14-13-16-35(31)36)58-45(65)39(21-34-24-50-27-54-34)59-47(67)48(7,8)9/h13-14,16-17,22-24,26-30,32,37-39,41,51H,10-12,15,18-21,25H2,1-9H3,(H,49,53)(H,50,54)(H,52,66)(H,55,63)(H,56,64)(H,57,61)(H,58,65)(H,59,67)(H,60,62)/t30-,32+,37-,38-,39-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Swiss 3T3 murine fibroblast cells. |
J Med Chem 34: 2102-7 (1991)
BindingDB Entry DOI: 10.7270/Q2X63KXB |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50082053
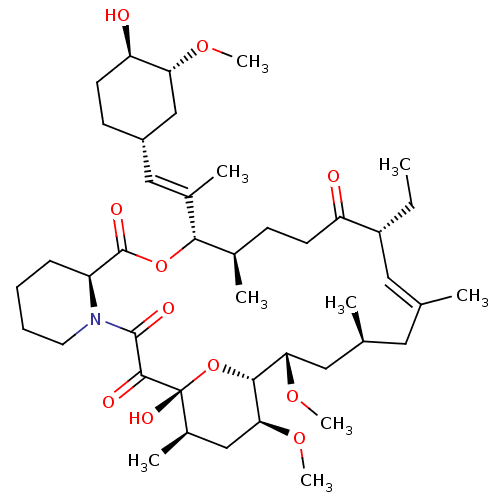
((E)-(1R,9S,12S,13R,17R,21S,23S,24R,25S,27R)-17-Eth...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)CCC1=O)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C43H69NO11/c1-10-31-20-25(2)19-26(3)21-36(52-8)39-37(53-9)23-29(6)43(50,55-39)40(47)41(48)44-18-12-11-13-32(44)42(49)54-38(27(4)14-16-33(31)45)28(5)22-30-15-17-34(46)35(24-30)51-7/h20,22,26-27,29-32,34-39,46,50H,10-19,21,23-24H2,1-9H3/b25-20+,28-22+/t26-,27+,29+,30-,31+,32-,34+,35+,36-,37-,38-,39+,43+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity against human FK506 binding protein 12 |
J Med Chem 42: 4456-61 (1999)
BindingDB Entry DOI: 10.7270/Q24J0DBT |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14018
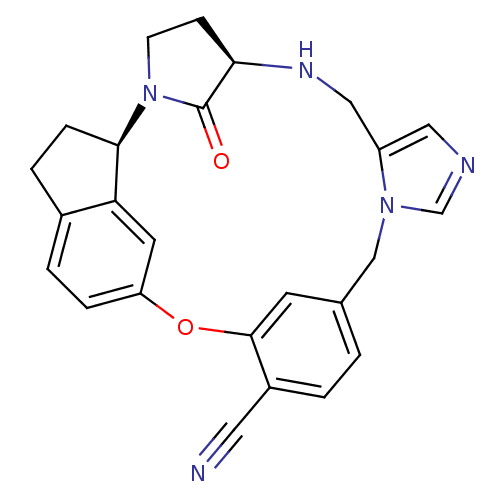
((17R, 20R)-19,20,21,22-Tetrahydro-19-oxo-17H-15,-1...)Show SMILES O=C1[C@H]2CCN1[C@@H]1CCc3ccc(Oc4cc(Cn5cncc5CN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C25H23N5O2/c26-11-18-2-1-16-9-24(18)32-20-5-3-17-4-6-23(21(17)10-20)30-8-7-22(25(30)31)28-13-19-12-27-15-29(19)14-16/h1-3,5,9-10,12,15,22-23,28H,4,6-8,13-14H2/t22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14023

((1R,2R,5R)-30-oxo-19-oxa-2,6,10,12-tetraazahexacyc...)Show SMILES O=C1[C@H]2CCN1[C@@H]1CCCc3ccc(Oc4cc(Cn5cncc5CN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C26H25N5O2/c27-12-19-5-4-17-10-25(19)33-21-7-6-18-2-1-3-24(22(18)11-21)31-9-8-23(26(31)32)29-14-20-13-28-16-30(20)15-17/h4-7,10-11,13,16,23-24,29H,1-3,8-9,14-15H2/t23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14025

((1R,2R,5R)-30-oxo-19,24-dioxa-2,6,10,12-tetraazahe...)Show SMILES O=C1[C@H]2CCN1[C@@H]1CCOc3ccc(Oc4cc(Cn5cncc5CN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C25H23N5O3/c26-11-17-2-1-16-9-24(17)33-19-3-4-23-20(10-19)22(6-8-32-23)30-7-5-21(25(30)31)28-13-18-12-27-15-29(18)14-16/h1-4,9-10,12,15,21-22,28H,5-8,13-14H2/t21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14014
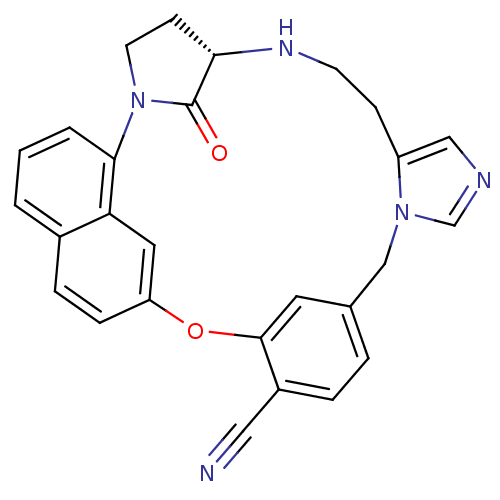
((5S)-31-oxo-20-oxa-2,6,11,13-tetraazahexacyclo[19....)Show SMILES O=C1[C@@H]2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5CCN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C27H23N5O2/c28-14-20-5-4-18-12-26(20)34-22-7-6-19-2-1-3-25(23(19)13-22)32-11-9-24(27(32)33)30-10-8-21-15-29-17-31(21)16-18/h1-7,12-13,15,17,24,30H,8-11,16H2/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50103360
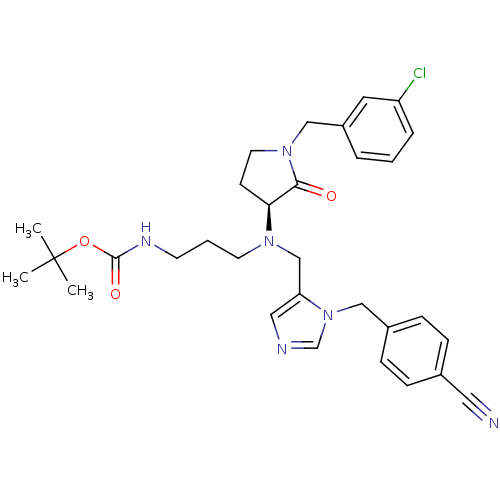
((3-{[1-(3-Chloro-benzyl)-2-oxo-pyrrolidin-3-yl]-[3...)Show SMILES CC(C)(C)OC(=O)NCCCN(Cc1cncn1Cc1ccc(cc1)C#N)[C@H]1CCN(Cc2cccc(Cl)c2)C1=O Show InChI InChI=1S/C31H37ClN6O3/c1-31(2,3)41-30(40)35-13-5-14-36(28-12-15-37(29(28)39)20-25-6-4-7-26(32)16-25)21-27-18-34-22-38(27)19-24-10-8-23(17-33)9-11-24/h4,6-11,16,18,22,28H,5,12-15,19-21H2,1-3H3,(H,35,40)/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to displace 50% of a potent radiolabeled FTI from farnesyl transferase in cultured H-ras-transformed Rat1 cells |
J Med Chem 44: 2933-49 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RNW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14017
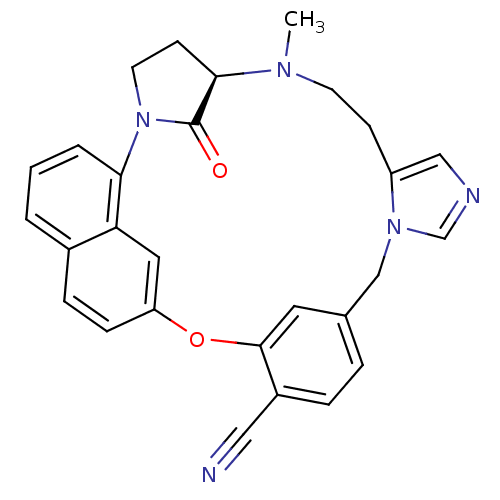
((5R)-6-methyl-31-oxo-20-oxa-2,6,11,13-tetraazahexa...)Show SMILES CN1CCc2cncn2Cc2ccc(C#N)c(Oc3ccc4cccc(N5CC[C@@H]1C5=O)c4c3)c2 |r| Show InChI InChI=1S/C28H25N5O2/c1-31-11-9-22-16-30-18-32(22)17-19-5-6-21(15-29)27(13-19)35-23-8-7-20-3-2-4-25(24(20)14-23)33-12-10-26(31)28(33)34/h2-8,13-14,16,18,26H,9-12,17H2,1H3/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM50012313

(2-{2-[2-[2-Acetylamino-3-(3H-imidazol-4-yl)-propio...)Show SMILES CC(C)CC(CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C Show InChI InChI=1S/C44H64N12O7/c1-24(2)13-30(14-25(3)4)53-42(61)37(17-32-20-46-23-50-32)54-38(58)21-48-44(63)39(26(5)6)56-40(59)27(7)51-41(60)35(15-29-18-47-34-12-10-9-11-33(29)34)55-43(62)36(52-28(8)57)16-31-19-45-22-49-31/h9-12,18-20,22-27,30,35-37,39,47H,13-17,21H2,1-8H3,(H,45,49)(H,46,50)(H,48,63)(H,51,60)(H,52,57)(H,53,61)(H,54,58)(H,55,62)(H,56,59)/t27-,35-,36-,37-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for mitogenic inhibition against Swiss 3T3 murine fibroblast cells. |
J Med Chem 34: 2102-7 (1991)
BindingDB Entry DOI: 10.7270/Q2X63KXB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14015

((5R)-31-oxo-20-oxa-2,6,11,13-tetraazahexacyclo[19....)Show SMILES O=C1[C@H]2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5CCN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C27H23N5O2/c28-14-20-5-4-18-12-26(20)34-22-7-6-19-2-1-3-25(23(19)13-22)32-11-9-24(27(32)33)30-10-8-21-15-29-17-31(21)16-18/h1-7,12-13,15,17,24,30H,8-11,16H2/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50181138

((S)-2-amino-1-((S)-4-(2,5-difluorophenyl)-2-(3-hyd...)Show SMILES CC(C)[C@H](N)C(=O)N1CC(=C[C@H]1c1cccc(O)c1)c1cc(F)ccc1F |c:9| Show InChI InChI=1S/C21H22F2N2O2/c1-12(2)20(24)21(27)25-11-14(17-10-15(22)6-7-18(17)23)9-19(25)13-4-3-5-16(26)8-13/h3-10,12,19-20,26H,11,24H2,1-2H3/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KSP by ATPase assay |
Bioorg Med Chem Lett 16: 1780-3 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.094
BindingDB Entry DOI: 10.7270/Q2PZ58D2 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50181137
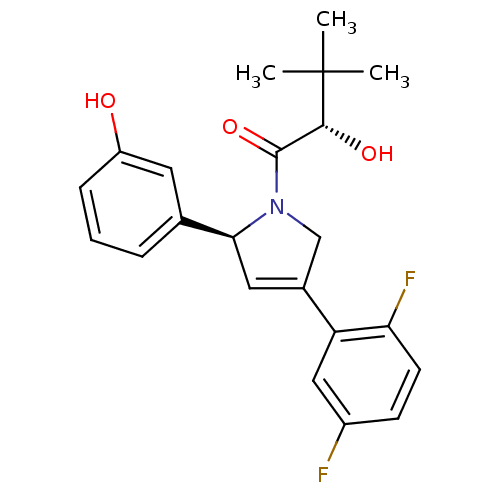
((S)-1-((S)-4-(2,5-difluorophenyl)-2-(3-hydroxyphen...)Show SMILES CC(C)(C)[C@H](O)C(=O)N1CC(=C[C@H]1c1cccc(O)c1)c1cc(F)ccc1F |c:10| Show InChI InChI=1S/C22H23F2NO3/c1-22(2,3)20(27)21(28)25-12-14(17-11-15(23)7-8-18(17)24)10-19(25)13-5-4-6-16(26)9-13/h4-11,19-20,26-27H,12H2,1-3H3/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KSP by ATPase assay |
Bioorg Med Chem Lett 16: 1780-3 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.094
BindingDB Entry DOI: 10.7270/Q2PZ58D2 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14008
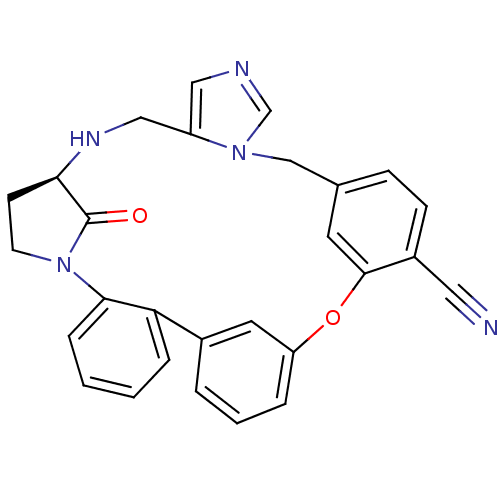
((11R)-32-oxo-25-oxa-8,12,16,18-tetraazahexacyclo[2...)Show SMILES O=C1[C@H]2CCN1c1ccccc1-c1cccc(Oc3cc(Cn4cncc4CN2)ccc3C#N)c1 |r| Show InChI InChI=1S/C28H23N5O2/c29-14-21-9-8-19-12-27(21)35-23-5-3-4-20(13-23)24-6-1-2-7-26(24)33-11-10-25(28(33)34)31-16-22-15-30-18-32(22)17-19/h1-9,12-13,15,18,25,31H,10-11,16-17H2/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50103360

((3-{[1-(3-Chloro-benzyl)-2-oxo-pyrrolidin-3-yl]-[3...)Show SMILES CC(C)(C)OC(=O)NCCCN(Cc1cncn1Cc1ccc(cc1)C#N)[C@H]1CCN(Cc2cccc(Cl)c2)C1=O Show InChI InChI=1S/C31H37ClN6O3/c1-31(2,3)41-30(40)35-13-5-14-36(28-12-15-37(29(28)39)20-25-6-4-7-26(32)16-25)21-27-18-34-22-38(27)19-24-10-8-23(17-33)9-11-24/h4,6-11,16,18,22,28H,5,12-15,19-21H2,1-3H3,(H,35,40)/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase using purified recombinant human enzyme |
J Med Chem 44: 2933-49 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RNW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14022
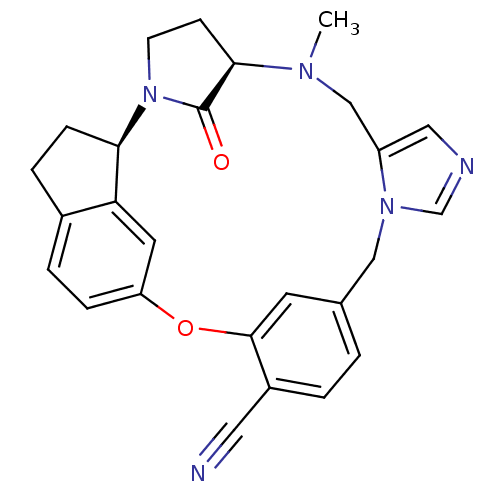
((1R,2R,5R)-6-methyl-29-oxo-19-oxa-2,6,10,12-tetraa...)Show SMILES CN1Cc2cncn2Cc2ccc(C#N)c(Oc3ccc4CC[C@@H](N5CC[C@@H]1C5=O)c4c3)c2 |r| Show InChI InChI=1S/C26H25N5O2/c1-29-15-20-13-28-16-30(20)14-17-2-3-19(12-27)25(10-17)33-21-6-4-18-5-7-23(22(18)11-21)31-9-8-24(29)26(31)32/h2-4,6,10-11,13,16,23-24H,5,7-9,14-15H2,1H3/t23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14007
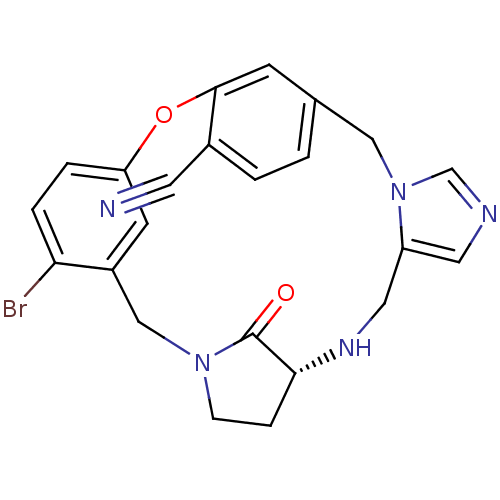
((6R)-24-bromo-27-oxo-20-oxa-3,7,11,13-tetraazapent...)Show SMILES Brc1ccc2Oc3cc(Cn4cncc4CN[C@@H]4CCN(Cc1c2)C4=O)ccc3C#N |r| Show InChI InChI=1S/C23H20BrN5O2/c24-20-4-3-19-8-17(20)13-28-6-5-21(23(28)30)27-11-18-10-26-14-29(18)12-15-1-2-16(9-25)22(7-15)31-19/h1-4,7-8,10,14,21,27H,5-6,11-13H2/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM50012318

(2-{2-[2-[2-Acetylamino-3-(3H-imidazol-4-yl)-propio...)Show SMILES CCCC[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C Show InChI InChI=1S/C44H64N12O7/c1-8-9-12-30(15-25(2)3)53-42(61)37(18-32-21-46-24-50-32)54-38(58)22-48-44(63)39(26(4)5)56-40(59)27(6)51-41(60)35(16-29-19-47-34-14-11-10-13-33(29)34)55-43(62)36(52-28(7)57)17-31-20-45-23-49-31/h10-11,13-14,19-21,23-27,30,35-37,39,47H,8-9,12,15-18,22H2,1-7H3,(H,45,49)(H,46,50)(H,48,63)(H,51,60)(H,52,57)(H,53,61)(H,54,58)(H,55,62)(H,56,59)/t27-,30+,35-,36-,37-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Swiss 3T3 murine fibroblast cells. |
J Med Chem 34: 2102-7 (1991)
BindingDB Entry DOI: 10.7270/Q2X63KXB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data