 Found 47 hits with Last Name = 'heinrikson' and Initial = 'rl'
Found 47 hits with Last Name = 'heinrikson' and Initial = 'rl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50212827
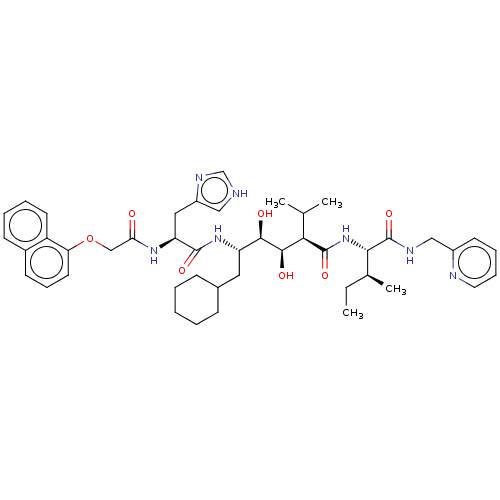
(CHEMBL3350189)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1cccc2ccccc12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O7/c1-5-29(4)40(45(58)48-25-32-18-11-12-21-47-32)52-44(57)39(28(2)3)42(55)41(54)35(22-30-14-7-6-8-15-30)51-43(56)36(23-33-24-46-27-49-33)50-38(53)26-59-37-20-13-17-31-16-9-10-19-34(31)37/h9-13,16-21,24,27-30,35-36,39-42,54-55H,5-8,14-15,22-23,25-26H2,1-4H3,(H,46,49)(H,48,58)(H,50,53)(H,51,56)(H,52,57)/t29-,35-,36-,39+,40-,41+,42+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 34: 2344-56 (1991)
BindingDB Entry DOI: 10.7270/Q20P10ND |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50229416
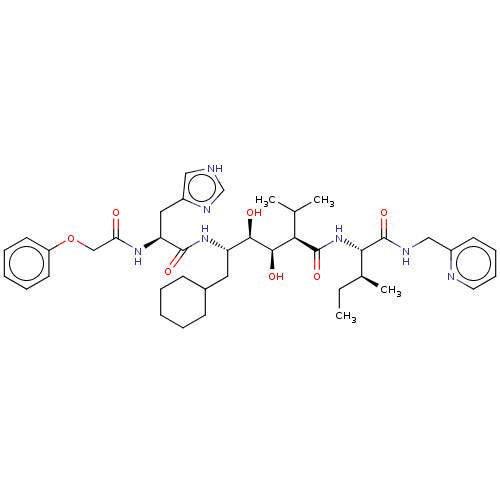
(CHEMBL3350192)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1ccccc1)C(=O)NCc1ccccn1 Show InChI InChI=1S/C41H59N7O7/c1-5-27(4)36(41(54)44-23-29-16-12-13-19-43-29)48-40(53)35(26(2)3)38(51)37(50)32(20-28-14-8-6-9-15-28)47-39(52)33(21-30-22-42-25-45-30)46-34(49)24-55-31-17-10-7-11-18-31/h7,10-13,16-19,22,25-28,32-33,35-38,50-51H,5-6,8-9,14-15,20-21,23-24H2,1-4H3,(H,42,45)(H,44,54)(H,46,49)(H,47,52)(H,48,53)/t27-,32-,33-,35+,36-,37+,38+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 34: 2344-56 (1991)
BindingDB Entry DOI: 10.7270/Q20P10ND |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046938

(CHEMBL366392 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)CCOCCOc1ccccc1)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C40H59N5O7/c1-5-27(4)36(40(50)41-25-33-42-30-18-12-13-19-31(30)43-33)45-39(49)35(26(2)3)38(48)37(47)32(24-28-14-8-6-9-15-28)44-34(46)20-21-51-22-23-52-29-16-10-7-11-17-29/h7,10-13,16-19,26-28,32,35-38,47-48H,5-6,8-9,14-15,20-25H2,1-4H3,(H,41,50)(H,42,43)(H,44,46)(H,45,49)/t27?,32-,35+,36-,37+,38+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50229415
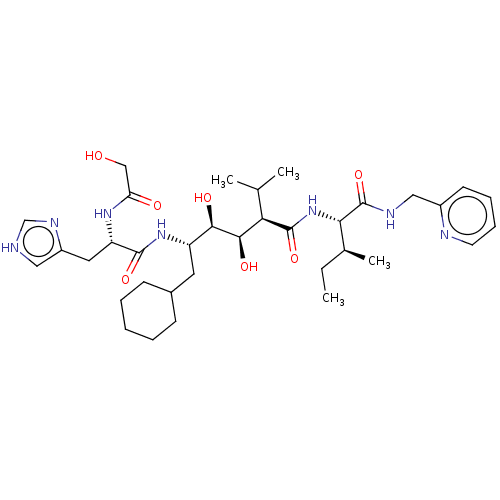
(CHEMBL3350190)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CO)C(=O)NCc1ccccn1 Show InChI InChI=1S/C35H55N7O7/c1-5-22(4)30(35(49)38-18-24-13-9-10-14-37-24)42-34(48)29(21(2)3)32(46)31(45)26(15-23-11-7-6-8-12-23)41-33(47)27(40-28(44)19-43)16-25-17-36-20-39-25/h9-10,13-14,17,20-23,26-27,29-32,43,45-46H,5-8,11-12,15-16,18-19H2,1-4H3,(H,36,39)(H,38,49)(H,40,44)(H,41,47)(H,42,48)/t22-,26-,27-,29+,30-,31+,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 34: 2344-56 (1991)
BindingDB Entry DOI: 10.7270/Q20P10ND |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046932

(CHEMBL173255 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)C(CC1CCCCC1)NC(=O)c1ccccc1OCSc1ccccc1)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C43H57N5O6S/c1-5-28(4)38(43(53)44-25-36-45-32-21-13-14-22-33(32)46-36)48-42(52)37(27(2)3)40(50)39(49)34(24-29-16-8-6-9-17-29)47-41(51)31-20-12-15-23-35(31)54-26-55-30-18-10-7-11-19-30/h7,10-15,18-23,27-29,34,37-40,49-50H,5-6,8-9,16-17,24-26H2,1-4H3,(H,44,53)(H,45,46)(H,47,51)(H,48,52)/t28?,34?,37-,38+,39-,40-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50368288

(CHEMBL1790564 | CHEMBL3350194)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1ccccc1)C(=O)NCc1ccccn1 Show InChI InChI=1S/C38H55N7O7/c1-7-25(6)33(38(51)41-20-26-13-11-12-16-40-26)45-37(50)32(24(4)5)35(48)34(47)29(17-23(2)3)44-36(49)30(18-27-19-39-22-42-27)43-31(46)21-52-28-14-9-8-10-15-28/h8-16,19,22-25,29-30,32-35,47-48H,7,17-18,20-21H2,1-6H3,(H,39,42)(H,41,51)(H,43,46)(H,44,49)(H,45,50)/t25-,29+,30+,32-,33+,34-,35-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 34: 2344-56 (1991)
BindingDB Entry DOI: 10.7270/Q20P10ND |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50000794

(CHEMBL299376)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1cccc2ccccc12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C46H63N7O7/c1-5-30(4)41(46(59)49-26-33-18-11-12-21-48-33)53-44(57)36(22-29(2)3)42(55)43(56)37(23-31-14-7-6-8-15-31)52-45(58)38(24-34-25-47-28-50-34)51-40(54)27-60-39-20-13-17-32-16-9-10-19-35(32)39/h9-13,16-21,25,28-31,36-38,41-43,55-56H,5-8,14-15,22-24,26-27H2,1-4H3,(H,47,50)(H,49,59)(H,51,54)(H,52,58)(H,53,57)/t30-,36+,37-,38-,41-,42+,43+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 34: 2344-56 (1991)
BindingDB Entry DOI: 10.7270/Q20P10ND |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046922

(CHEMBL428804 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1ccccc1OCCOCCOC)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C41H61N5O8/c1-6-27(4)36(41(51)42-25-34-43-30-17-11-12-18-31(30)44-34)46-40(50)35(26(2)3)38(48)37(47)32(24-28-14-8-7-9-15-28)45-39(49)29-16-10-13-19-33(29)54-23-22-53-21-20-52-5/h10-13,16-19,26-28,32,35-38,47-48H,6-9,14-15,20-25H2,1-5H3,(H,42,51)(H,43,44)(H,45,49)(H,46,50)/t27?,32-,35+,36-,37+,38+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50000696
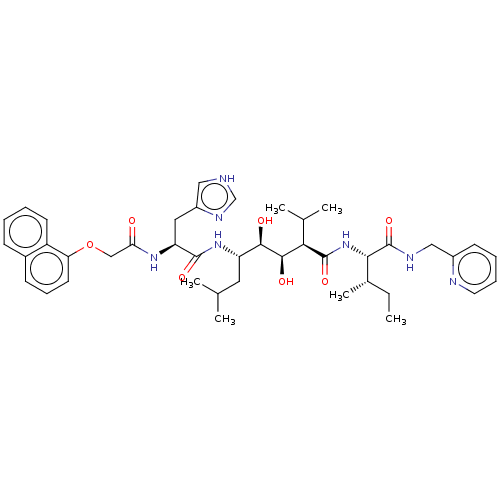
(CHEMBL301833)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1cccc2ccccc12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C42H57N7O7/c1-7-27(6)37(42(55)45-22-29-15-10-11-18-44-29)49-41(54)36(26(4)5)39(52)38(51)32(19-25(2)3)48-40(53)33(20-30-21-43-24-46-30)47-35(50)23-56-34-17-12-14-28-13-8-9-16-31(28)34/h8-18,21,24-27,32-33,36-39,51-52H,7,19-20,22-23H2,1-6H3,(H,43,46)(H,45,55)(H,47,50)(H,48,53)(H,49,54)/t27-,32-,33-,36+,37-,38+,39+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 34: 2344-56 (1991)
BindingDB Entry DOI: 10.7270/Q20P10ND |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50000758

(CHEMBL48770)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@@H](O)[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1cccc2ccccc12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C43H59N7O7/c1-7-28(6)38(43(56)46-23-30-15-10-11-18-45-30)50-41(54)33(19-26(2)3)39(52)40(53)34(20-27(4)5)49-42(55)35(21-31-22-44-25-47-31)48-37(51)24-57-36-17-12-14-29-13-8-9-16-32(29)36/h8-18,22,25-28,33-35,38-40,52-53H,7,19-21,23-24H2,1-6H3,(H,44,47)(H,46,56)(H,48,51)(H,49,55)(H,50,54)/t28-,33+,34-,35-,38-,39+,40+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 34: 2344-56 (1991)
BindingDB Entry DOI: 10.7270/Q20P10ND |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046940

(CHEMBL176353 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1ccccc1OCCOc1ccccc1)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C44H59N5O7/c1-5-29(4)39(44(54)45-27-37-46-33-21-13-14-22-34(33)47-37)49-43(53)38(28(2)3)41(51)40(50)35(26-30-16-8-6-9-17-30)48-42(52)32-20-12-15-23-36(32)56-25-24-55-31-18-10-7-11-19-31/h7,10-15,18-23,28-30,35,38-41,50-51H,5-6,8-9,16-17,24-27H2,1-4H3,(H,45,54)(H,46,47)(H,48,52)(H,49,53)/t29?,35-,38+,39-,40+,41+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046941

(CHEMBL175393 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)C(CC1CCCCC1)NC(=O)c1ccccc1OCCOCCOCCOC)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C43H65N5O9/c1-6-29(4)38(43(53)44-27-36-45-32-17-11-12-18-33(32)46-36)48-42(52)37(28(2)3)40(50)39(49)34(26-30-14-8-7-9-15-30)47-41(51)31-16-10-13-19-35(31)57-25-24-56-23-22-55-21-20-54-5/h10-13,16-19,28-30,34,37-40,49-50H,6-9,14-15,20-27H2,1-5H3,(H,44,53)(H,45,46)(H,47,51)(H,48,52)/t29?,34?,37-,38+,39-,40-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50229414
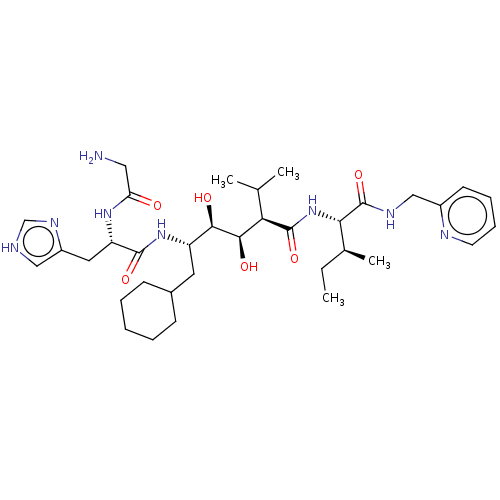
(CHEMBL3350191)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN)C(=O)NCc1ccccn1 Show InChI InChI=1S/C35H56N8O6/c1-5-22(4)30(35(49)39-19-24-13-9-10-14-38-24)43-34(48)29(21(2)3)32(46)31(45)26(15-23-11-7-6-8-12-23)42-33(47)27(41-28(44)17-36)16-25-18-37-20-40-25/h9-10,13-14,18,20-23,26-27,29-32,45-46H,5-8,11-12,15-17,19,36H2,1-4H3,(H,37,40)(H,39,49)(H,41,44)(H,42,47)(H,43,48)/t22-,26-,27-,29+,30-,31+,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 34: 2344-56 (1991)
BindingDB Entry DOI: 10.7270/Q20P10ND |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50229413

(CHEMBL3350218)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1cccc2ccccc12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C49H55N7O7/c1-3-32(2)44(49(62)52-29-36-21-12-13-24-51-36)56-47(60)39(25-33-15-6-4-7-16-33)45(58)46(59)40(26-34-17-8-5-9-18-34)55-48(61)41(27-37-28-50-31-53-37)54-43(57)30-63-42-23-14-20-35-19-10-11-22-38(35)42/h4-24,28,31-32,39-41,44-46,58-59H,3,25-27,29-30H2,1-2H3,(H,50,53)(H,52,62)(H,54,57)(H,55,61)(H,56,60)/t32-,39-,40-,41-,44-,45+,46+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 34: 2344-56 (1991)
BindingDB Entry DOI: 10.7270/Q20P10ND |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046920
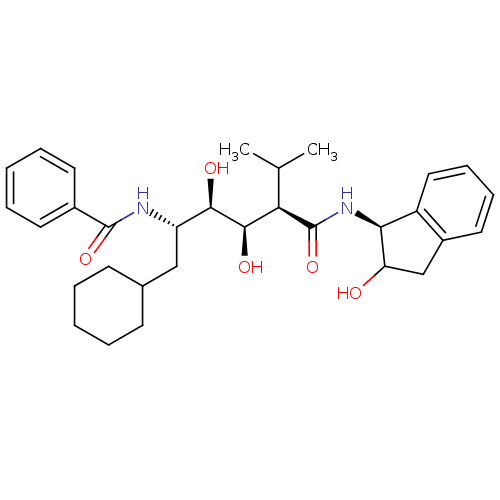
(CHEMBL172927 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES CC(C)[C@H]([C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1ccccc1)C(=O)N[C@@H]1C(O)Cc2ccccc12 Show InChI InChI=1S/C31H42N2O5/c1-19(2)26(31(38)33-27-23-16-10-9-15-22(23)18-25(27)34)29(36)28(35)24(17-20-11-5-3-6-12-20)32-30(37)21-13-7-4-8-14-21/h4,7-10,13-16,19-20,24-29,34-36H,3,5-6,11-12,17-18H2,1-2H3,(H,32,37)(H,33,38)/t24-,25?,26+,27-,28+,29+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50368830
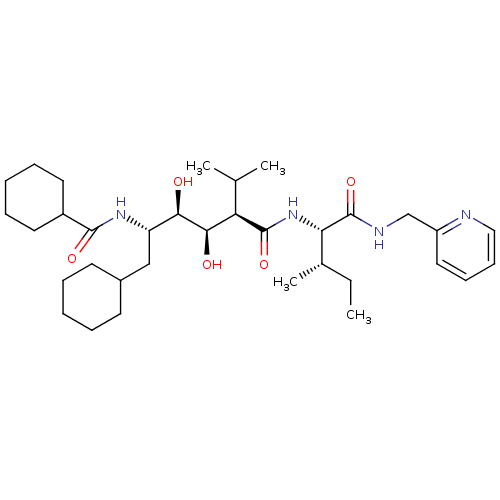
(CHEMBL1790518)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)C1CCCCC1)C(=O)NCc1ccccn1 Show InChI InChI=1S/C34H56N4O5/c1-5-23(4)29(34(43)36-21-26-18-12-13-19-35-26)38-33(42)28(22(2)3)31(40)30(39)27(20-24-14-8-6-9-15-24)37-32(41)25-16-10-7-11-17-25/h12-13,18-19,22-25,27-31,39-40H,5-11,14-17,20-21H2,1-4H3,(H,36,43)(H,37,41)(H,38,42)/t23-,27-,28+,29-,30+,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50453058
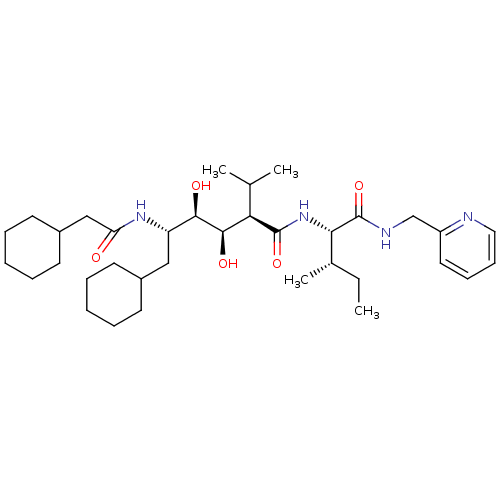
(CHEMBL3085447)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)CC1CCCCC1)C(=O)NCc1ccccn1 Show InChI InChI=1S/C35H58N4O5/c1-5-24(4)31(35(44)37-22-27-18-12-13-19-36-27)39-34(43)30(23(2)3)33(42)32(41)28(20-25-14-8-6-9-15-25)38-29(40)21-26-16-10-7-11-17-26/h12-13,18-19,23-26,28,30-33,41-42H,5-11,14-17,20-22H2,1-4H3,(H,37,44)(H,38,40)(H,39,43)/t24-,28-,30+,31-,32+,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 34: 2344-56 (1991)
BindingDB Entry DOI: 10.7270/Q20P10ND |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50000732
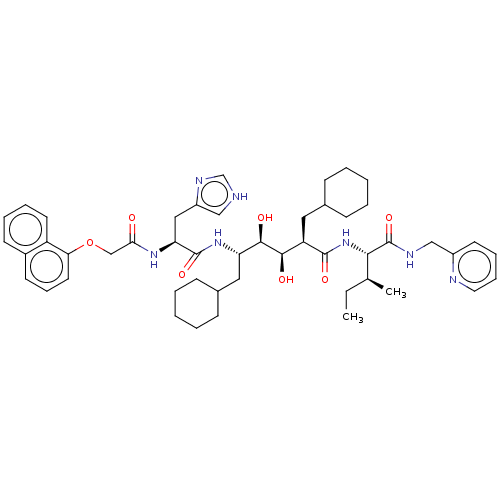
(CHEMBL432309)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC1CCCCC1)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1cccc2ccccc12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C49H67N7O7/c1-3-32(2)44(49(62)52-29-36-21-12-13-24-51-36)56-47(60)39(25-33-15-6-4-7-16-33)45(58)46(59)40(26-34-17-8-5-9-18-34)55-48(61)41(27-37-28-50-31-53-37)54-43(57)30-63-42-23-14-20-35-19-10-11-22-38(35)42/h10-14,19-24,28,31-34,39-41,44-46,58-59H,3-9,15-18,25-27,29-30H2,1-2H3,(H,50,53)(H,52,62)(H,54,57)(H,55,61)(H,56,60)/t32-,39+,40-,41-,44-,45+,46+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 34: 2344-56 (1991)
BindingDB Entry DOI: 10.7270/Q20P10ND |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50368283
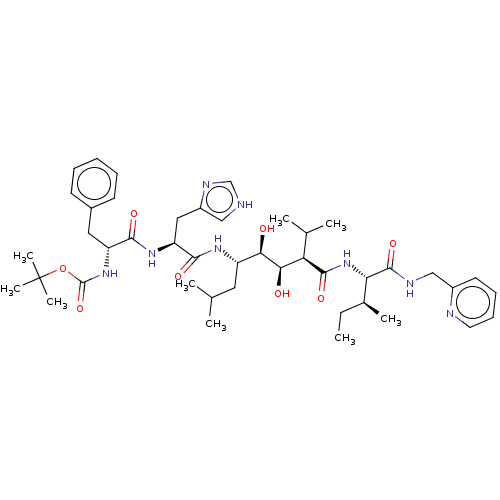
(CHEMBL1790565 | CHEMBL3350195)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C44H66N8O8/c1-10-28(6)36(42(58)47-24-30-18-14-15-19-46-30)52-41(57)35(27(4)5)38(54)37(53)32(20-26(2)3)49-40(56)34(22-31-23-45-25-48-31)50-39(55)33(21-29-16-12-11-13-17-29)51-43(59)60-44(7,8)9/h11-19,23,25-28,32-38,53-54H,10,20-22,24H2,1-9H3,(H,45,48)(H,47,58)(H,49,56)(H,50,55)(H,51,59)(H,52,57)/t28-,32+,33-,34+,35-,36+,37-,38-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 34: 2344-56 (1991)
BindingDB Entry DOI: 10.7270/Q20P10ND |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50368829
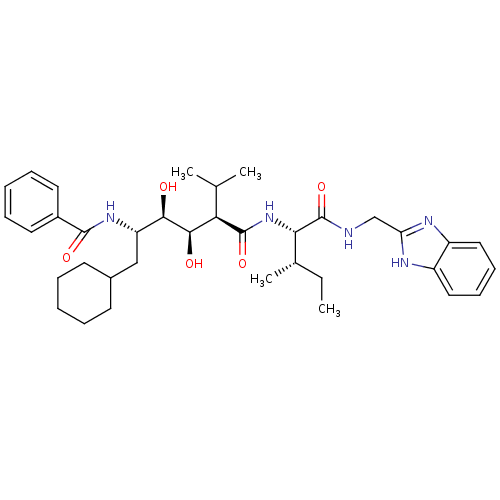
(CHEMBL1790517)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1ccccc1)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C36H51N5O5/c1-5-23(4)31(36(46)37-21-29-38-26-18-12-13-19-27(26)39-29)41-35(45)30(22(2)3)33(43)32(42)28(20-24-14-8-6-9-15-24)40-34(44)25-16-10-7-11-17-25/h7,10-13,16-19,22-24,28,30-33,42-43H,5-6,8-9,14-15,20-21H2,1-4H3,(H,37,46)(H,38,39)(H,40,44)(H,41,45)/t23-,28-,30+,31-,32+,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046929

(CHEMBL369428 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1ccc(C)cc1OCCOc1ccccc1)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C45H61N5O7/c1-6-30(5)40(45(55)46-27-38-47-34-19-13-14-20-35(34)48-38)50-44(54)39(28(2)3)42(52)41(51)36(26-31-15-9-7-10-16-31)49-43(53)33-22-21-29(4)25-37(33)57-24-23-56-32-17-11-8-12-18-32/h8,11-14,17-22,25,28,30-31,36,39-42,51-52H,6-7,9-10,15-16,23-24,26-27H2,1-5H3,(H,46,55)(H,47,48)(H,49,53)(H,50,54)/t30?,36-,39+,40-,41+,42+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046934
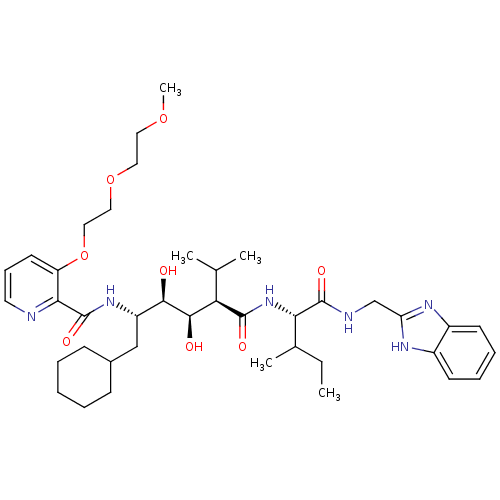
(CHEMBL174307 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1ncccc1OCCOCCOC)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C40H60N6O8/c1-6-26(4)34(39(50)42-24-32-43-28-15-10-11-16-29(28)44-32)46-38(49)33(25(2)3)37(48)36(47)30(23-27-13-8-7-9-14-27)45-40(51)35-31(17-12-18-41-35)54-22-21-53-20-19-52-5/h10-12,15-18,25-27,30,33-34,36-37,47-48H,6-9,13-14,19-24H2,1-5H3,(H,42,50)(H,43,44)(H,45,51)(H,46,49)/t26?,30-,33+,34-,36+,37+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046921
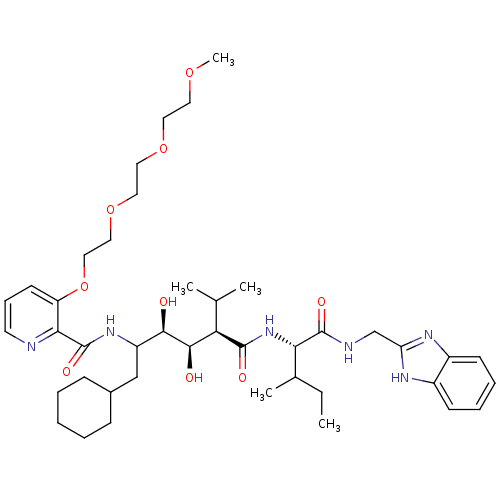
(CHEMBL176737 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)C(CC1CCCCC1)NC(=O)c1ncccc1OCCOCCOCCOC)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C42H64N6O9/c1-6-28(4)36(41(52)44-26-34-45-30-15-10-11-16-31(30)46-34)48-40(51)35(27(2)3)39(50)38(49)32(25-29-13-8-7-9-14-29)47-42(53)37-33(17-12-18-43-37)57-24-23-56-22-21-55-20-19-54-5/h10-12,15-18,27-29,32,35-36,38-39,49-50H,6-9,13-14,19-26H2,1-5H3,(H,44,52)(H,45,46)(H,47,53)(H,48,51)/t28?,32?,35-,36+,38-,39-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046935
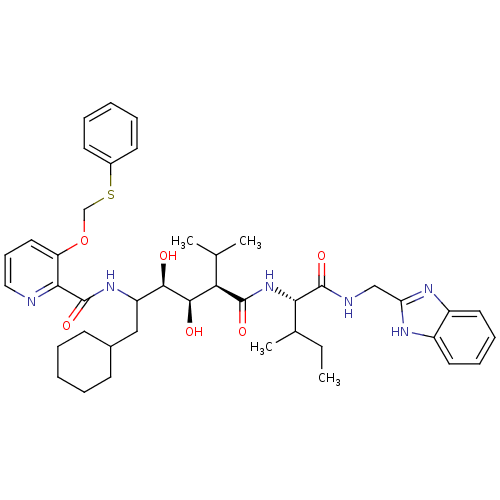
(CHEMBL367066 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)C(CC1CCCCC1)NC(=O)c1ncccc1OCSc1ccccc1)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C42H56N6O6S/c1-5-27(4)36(41(52)44-24-34-45-30-19-12-13-20-31(30)46-34)48-40(51)35(26(2)3)39(50)38(49)32(23-28-15-8-6-9-16-28)47-42(53)37-33(21-14-22-43-37)54-25-55-29-17-10-7-11-18-29/h7,10-14,17-22,26-28,32,35-36,38-39,49-50H,5-6,8-9,15-16,23-25H2,1-4H3,(H,44,52)(H,45,46)(H,47,53)(H,48,51)/t27?,32?,35-,36+,38-,39-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046931
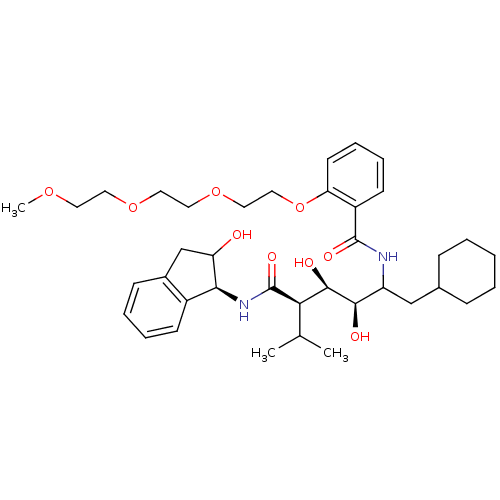
(CHEMBL176840 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES COCCOCCOCCOc1ccccc1C(=O)NC(CC1CCCCC1)[C@@H](O)[C@H](O)[C@@H](C(C)C)C(=O)N[C@@H]1C(O)Cc2ccccc12 Show InChI InChI=1S/C38H56N2O9/c1-25(2)33(38(45)40-34-28-14-8-7-13-27(28)24-31(34)41)36(43)35(42)30(23-26-11-5-4-6-12-26)39-37(44)29-15-9-10-16-32(29)49-22-21-48-20-19-47-18-17-46-3/h7-10,13-16,25-26,30-31,33-36,41-43H,4-6,11-12,17-24H2,1-3H3,(H,39,44)(H,40,45)/t30?,31?,33-,34+,35-,36-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046933
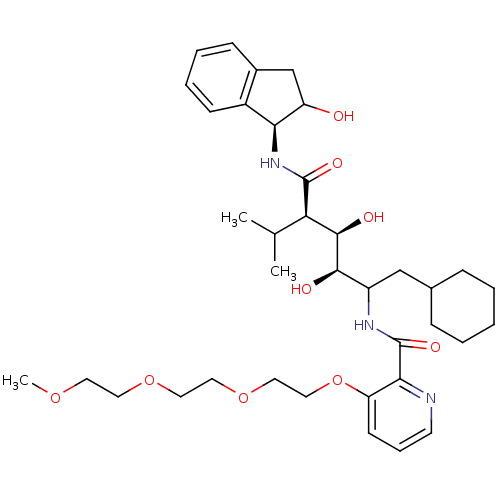
(CHEMBL176674 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES COCCOCCOCCOc1cccnc1C(=O)NC(CC1CCCCC1)[C@@H](O)[C@H](O)[C@@H](C(C)C)C(=O)N[C@@H]1C(O)Cc2ccccc12 Show InChI InChI=1S/C37H55N3O9/c1-24(2)31(36(44)40-32-27-13-8-7-12-26(27)23-29(32)41)35(43)34(42)28(22-25-10-5-4-6-11-25)39-37(45)33-30(14-9-15-38-33)49-21-20-48-19-18-47-17-16-46-3/h7-9,12-15,24-25,28-29,31-32,34-35,41-43H,4-6,10-11,16-23H2,1-3H3,(H,39,45)(H,40,44)/t28?,29?,31-,32+,34-,35-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046928
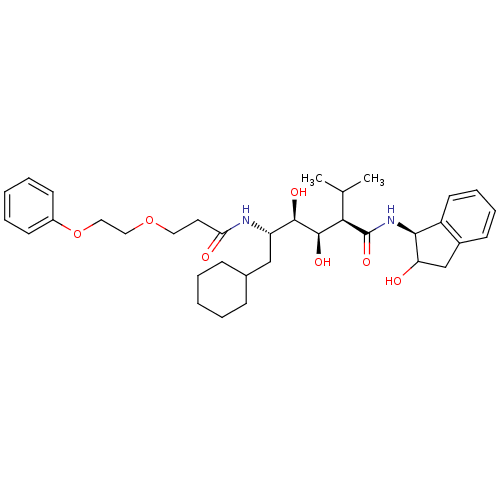
(CHEMBL176613 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES CC(C)[C@H]([C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)CCOCCOc1ccccc1)C(=O)N[C@@H]1C(O)Cc2ccccc12 Show InChI InChI=1S/C35H50N2O7/c1-23(2)31(35(42)37-32-27-16-10-9-13-25(27)22-29(32)38)34(41)33(40)28(21-24-11-5-3-6-12-24)36-30(39)17-18-43-19-20-44-26-14-7-4-8-15-26/h4,7-10,13-16,23-24,28-29,31-34,38,40-41H,3,5-6,11-12,17-22H2,1-2H3,(H,36,39)(H,37,42)/t28-,29?,31+,32-,33+,34+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046925
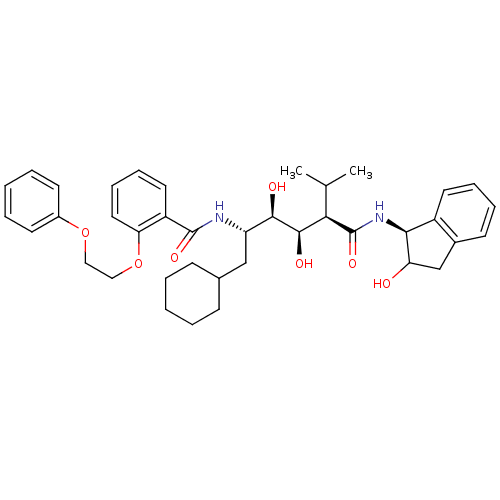
(CHEMBL367637 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES CC(C)[C@H]([C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1ccccc1OCCOc1ccccc1)C(=O)N[C@@H]1C(O)Cc2ccccc12 Show InChI InChI=1S/C39H50N2O7/c1-25(2)34(39(46)41-35-29-18-10-9-15-27(29)24-32(35)42)37(44)36(43)31(23-26-13-5-3-6-14-26)40-38(45)30-19-11-12-20-33(30)48-22-21-47-28-16-7-4-8-17-28/h4,7-12,15-20,25-26,31-32,34-37,42-44H,3,5-6,13-14,21-24H2,1-2H3,(H,40,45)(H,41,46)/t31-,32?,34+,35-,36+,37+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046923
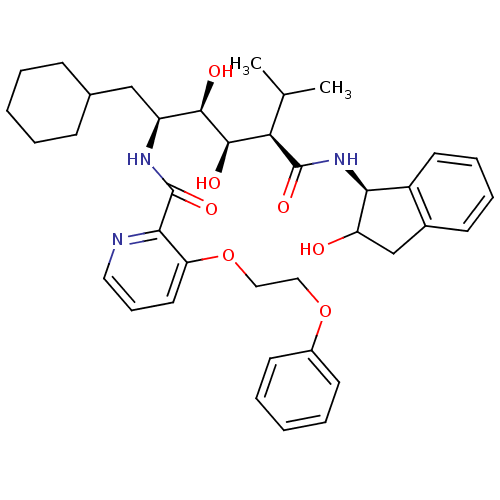
(CHEMBL173652 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES CC(C)[C@H]([C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1ncccc1OCCOc1ccccc1)C(=O)N[C@@H]1C(O)Cc2ccccc12 Show InChI InChI=1S/C38H49N3O7/c1-24(2)32(37(45)41-33-28-17-10-9-14-26(28)23-30(33)42)36(44)35(43)29(22-25-12-5-3-6-13-25)40-38(46)34-31(18-11-19-39-34)48-21-20-47-27-15-7-4-8-16-27/h4,7-11,14-19,24-25,29-30,32-33,35-36,42-44H,3,5-6,12-13,20-23H2,1-2H3,(H,40,46)(H,41,45)/t29-,30?,32+,33-,35+,36+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046924
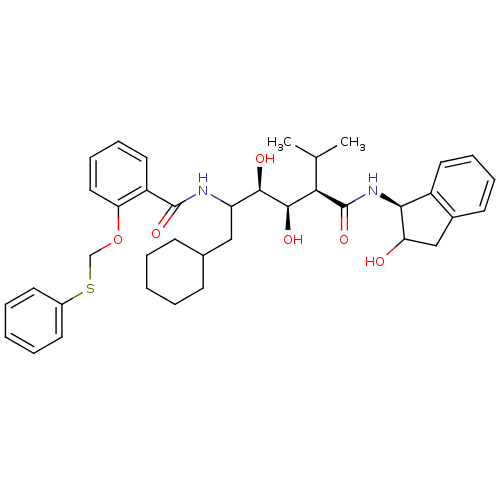
(CHEMBL366531 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES CC(C)[C@H]([C@@H](O)[C@H](O)C(CC1CCCCC1)NC(=O)c1ccccc1OCSc1ccccc1)C(=O)N[C@@H]1C(O)Cc2ccccc12 Show InChI InChI=1S/C38H48N2O6S/c1-24(2)33(38(45)40-34-28-18-10-9-15-26(28)22-31(34)41)36(43)35(42)30(21-25-13-5-3-6-14-25)39-37(44)29-19-11-12-20-32(29)46-23-47-27-16-7-4-8-17-27/h4,7-12,15-20,24-25,30-31,33-36,41-43H,3,5-6,13-14,21-23H2,1-2H3,(H,39,44)(H,40,45)/t30?,31?,33-,34+,35-,36-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046927
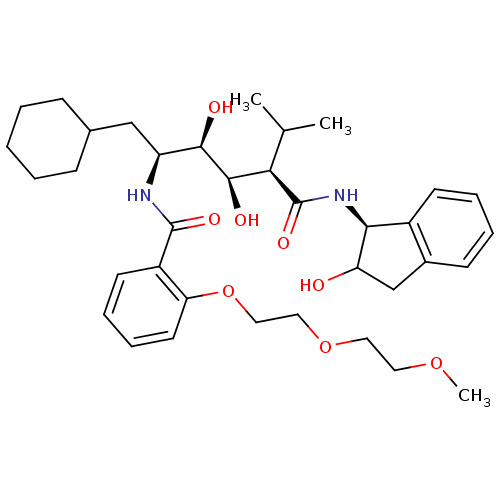
(CHEMBL355815 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES COCCOCCOc1ccccc1C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@H](O)[C@@H](C(C)C)C(=O)N[C@@H]1C(O)Cc2ccccc12 Show InChI InChI=1S/C36H52N2O8/c1-23(2)31(36(43)38-32-26-14-8-7-13-25(26)22-29(32)39)34(41)33(40)28(21-24-11-5-4-6-12-24)37-35(42)27-15-9-10-16-30(27)46-20-19-45-18-17-44-3/h7-10,13-16,23-24,28-29,31-34,39-41H,4-6,11-12,17-22H2,1-3H3,(H,37,42)(H,38,43)/t28-,29?,31+,32-,33+,34+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046939
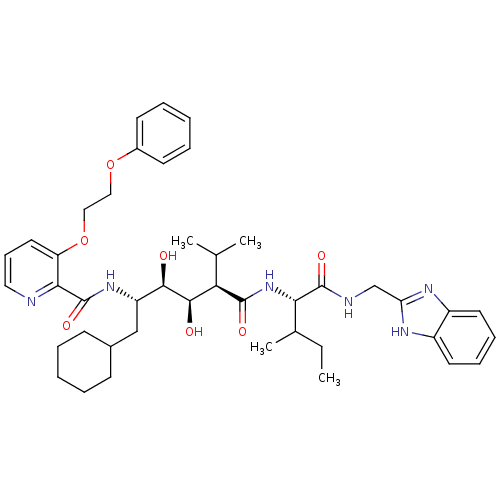
(CHEMBL367712 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1ncccc1OCCOc1ccccc1)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C43H58N6O7/c1-5-28(4)37(42(53)45-26-35-46-31-19-12-13-20-32(31)47-35)49-41(52)36(27(2)3)40(51)39(50)33(25-29-15-8-6-9-16-29)48-43(54)38-34(21-14-22-44-38)56-24-23-55-30-17-10-7-11-18-30/h7,10-14,17-22,27-29,33,36-37,39-40,50-51H,5-6,8-9,15-16,23-26H2,1-4H3,(H,45,53)(H,46,47)(H,48,54)(H,49,52)/t28?,33-,36+,37-,39+,40+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046936
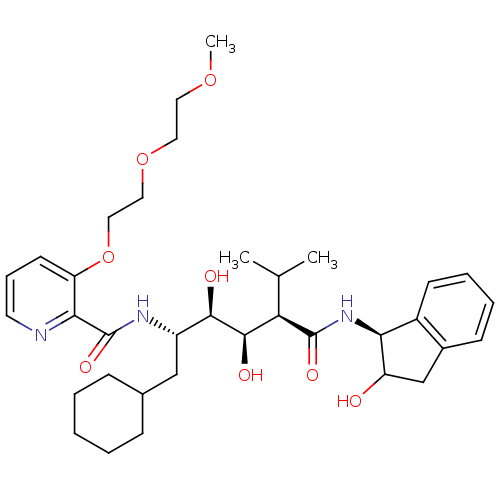
(CHEMBL355806 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES COCCOCCOc1cccnc1C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@H](O)[C@@H](C(C)C)C(=O)N[C@@H]1C(O)Cc2ccccc12 Show InChI InChI=1S/C35H51N3O8/c1-22(2)29(34(42)38-30-25-13-8-7-12-24(25)21-27(30)39)33(41)32(40)26(20-23-10-5-4-6-11-23)37-35(43)31-28(14-9-15-36-31)46-19-18-45-17-16-44-3/h7-9,12-15,22-23,26-27,29-30,32-33,39-41H,4-6,10-11,16-21H2,1-3H3,(H,37,43)(H,38,42)/t26-,27?,29+,30-,32+,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50000754
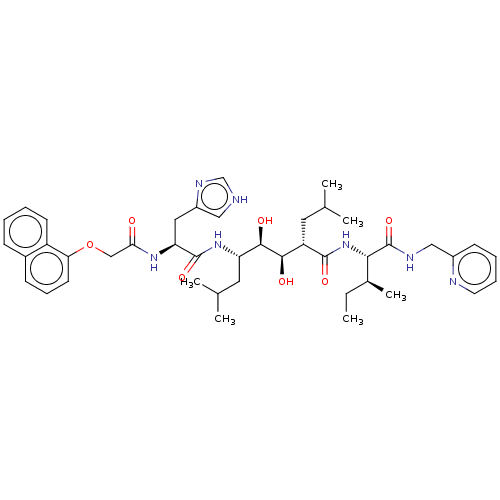
(CHEMBL300475)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(C)C)[C@@H](O)[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1cccc2ccccc12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C43H59N7O7/c1-7-28(6)38(43(56)46-23-30-15-10-11-18-45-30)50-41(54)33(19-26(2)3)39(52)40(53)34(20-27(4)5)49-42(55)35(21-31-22-44-25-47-31)48-37(51)24-57-36-17-12-14-29-13-8-9-16-32(29)36/h8-18,22,25-28,33-35,38-40,52-53H,7,19-21,23-24H2,1-6H3,(H,44,47)(H,46,56)(H,48,51)(H,49,55)(H,50,54)/t28-,33-,34-,35-,38-,39+,40+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 34: 2344-56 (1991)
BindingDB Entry DOI: 10.7270/Q20P10ND |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50009326
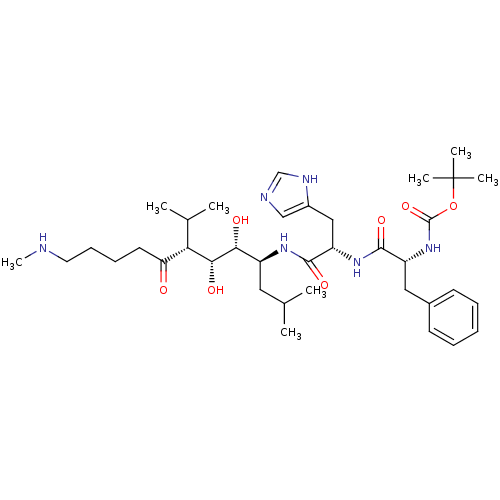
(CHEMBL76484 | {1-[1-(2,3-Dihydroxy-1-isobutyl-4-is...)Show SMILES CNCCCCC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C37H60N6O7/c1-23(2)18-27(32(45)33(46)31(24(3)4)30(44)16-12-13-17-38-8)41-35(48)29(20-26-21-39-22-40-26)42-34(47)28(19-25-14-10-9-11-15-25)43-36(49)50-37(5,6)7/h9-11,14-15,21-24,27-29,31-33,38,45-46H,12-13,16-20H2,1-8H3,(H,39,40)(H,41,48)(H,42,47)(H,43,49)/t27-,28+,29-,31-,32+,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 34: 2344-56 (1991)
BindingDB Entry DOI: 10.7270/Q20P10ND |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50009323
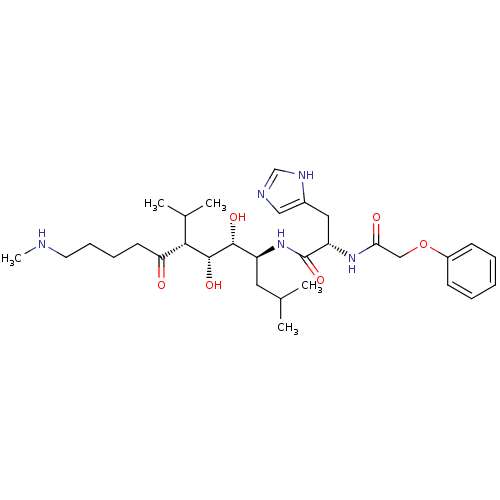
(CHEMBL307682 | N-(2,3-Dihydroxy-1-isobutyl-4-isopr...)Show SMILES CNCCCCC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)COc1ccccc1 Show InChI InChI=1S/C31H49N5O6/c1-20(2)15-24(29(39)30(40)28(21(3)4)26(37)13-9-10-14-32-5)36-31(41)25(16-22-17-33-19-34-22)35-27(38)18-42-23-11-7-6-8-12-23/h6-8,11-12,17,19-21,24-25,28-30,32,39-40H,9-10,13-16,18H2,1-5H3,(H,33,34)(H,35,38)(H,36,41)/t24-,25-,28-,29+,30+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 34: 2344-56 (1991)
BindingDB Entry DOI: 10.7270/Q20P10ND |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50368286
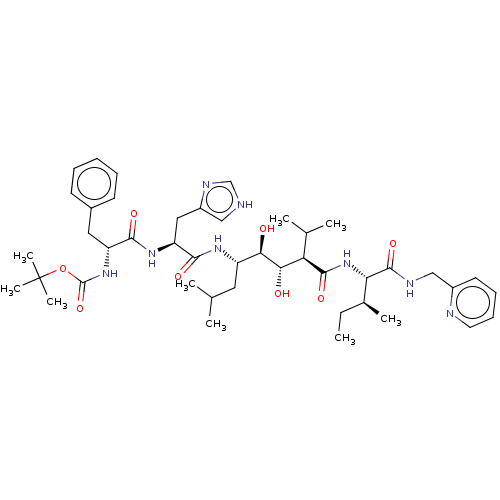
(CHEMBL1790561 | CHEMBL3350193)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@H](O)[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C44H66N8O8/c1-10-28(6)36(42(58)47-24-30-18-14-15-19-46-30)52-41(57)35(27(4)5)38(54)37(53)32(20-26(2)3)49-40(56)34(22-31-23-45-25-48-31)50-39(55)33(21-29-16-12-11-13-17-29)51-43(59)60-44(7,8)9/h11-19,23,25-28,32-38,53-54H,10,20-22,24H2,1-9H3,(H,45,48)(H,47,58)(H,49,56)(H,50,55)(H,51,59)(H,52,57)/t28-,32+,33-,34+,35-,36+,37-,38+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 34: 2344-56 (1991)
BindingDB Entry DOI: 10.7270/Q20P10ND |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50046926
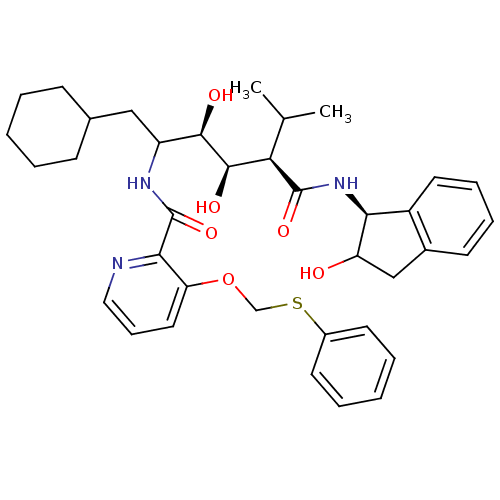
(CHEMBL176304 | N-(4-{1-[(1H-Benzoimidazol-2-ylmeth...)Show SMILES CC(C)[C@H]([C@@H](O)[C@H](O)C(CC1CCCCC1)NC(=O)c1ncccc1OCSc1ccccc1)C(=O)N[C@@H]1C(O)Cc2ccccc12 Show InChI InChI=1S/C37H47N3O6S/c1-23(2)31(36(44)40-32-27-17-10-9-14-25(27)21-29(32)41)35(43)34(42)28(20-24-12-5-3-6-13-24)39-37(45)33-30(18-11-19-38-33)46-22-47-26-15-7-4-8-16-26/h4,7-11,14-19,23-24,28-29,31-32,34-35,41-43H,3,5-6,12-13,20-22H2,1-2H3,(H,39,45)(H,40,44)/t28?,29?,31-,32+,34-,35-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in vitro. |
J Med Chem 36: 941-52 (1993)
BindingDB Entry DOI: 10.7270/Q2VX0H54 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16047

((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)C(C)C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C41H64N8O14/c1-20(2)16-27(46-39(60)28(19-31(43)51)47-40(61)34(21(3)4)49-37(58)25(42)12-14-32(52)53)30(50)17-22(5)35(56)44-23(6)36(57)45-26(13-15-33(54)55)38(59)48-29(41(62)63)18-24-10-8-7-9-11-24/h7-11,20-23,25-30,34,50H,12-19,42H2,1-6H3,(H2,43,51)(H,44,56)(H,45,57)(H,46,60)(H,47,61)(H,48,59)(H,49,58)(H,52,53)(H,54,55)(H,62,63)/t22-,23+,25+,26+,27+,28+,29+,30+,34+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human beta-Secretase (BACE) |
J Med Chem 46: 4625-30 (2003)
Article DOI: 10.1021/jm030247h
BindingDB Entry DOI: 10.7270/Q2PR7VCB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16250
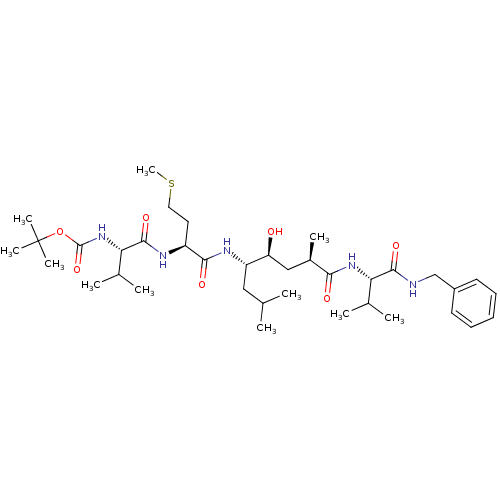
(CHEMBL290001 | N-(tert-butoxycarbonyl)-L-valyl-N-[...)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C37H63N5O7S/c1-22(2)19-28(29(43)20-25(7)32(44)41-30(23(3)4)34(46)38-21-26-15-13-12-14-16-26)40-33(45)27(17-18-50-11)39-35(47)31(24(5)6)42-36(48)49-37(8,9)10/h12-16,22-25,27-31,43H,17-21H2,1-11H3,(H,38,46)(H,39,47)(H,40,45)(H,41,44)(H,42,48)/t25-,27+,28+,29+,30+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human beta-Secretase (BACE) |
J Med Chem 46: 4625-30 (2003)
Article DOI: 10.1021/jm030247h
BindingDB Entry DOI: 10.7270/Q2PR7VCB |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50134706

(5-{2-[4-(2-Biphenyl-2-yl-2-hydroxy-acetylamino)-5-...)Show SMILES CC(C)[C@H](NC(=O)C[C@H](O)[C@H](Cc1cc(F)cc(F)c1)NC(=O)C(O)c1ccccc1-c1ccccc1)C(=O)Nc1cc(cc(c1)C(O)=O)C(O)=O Show InChI InChI=1S/C38H37F2N3O9/c1-20(2)33(35(47)41-27-16-23(37(49)50)15-24(17-27)38(51)52)43-32(45)19-31(44)30(14-21-12-25(39)18-26(40)13-21)42-36(48)34(46)29-11-7-6-10-28(29)22-8-4-3-5-9-22/h3-13,15-18,20,30-31,33-34,44,46H,14,19H2,1-2H3,(H,41,47)(H,42,48)(H,43,45)(H,49,50)(H,51,52)/t30-,31-,33-,34?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human beta-Secretase (BACE) |
J Med Chem 46: 4625-30 (2003)
Article DOI: 10.1021/jm030247h
BindingDB Entry DOI: 10.7270/Q2PR7VCB |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50134704

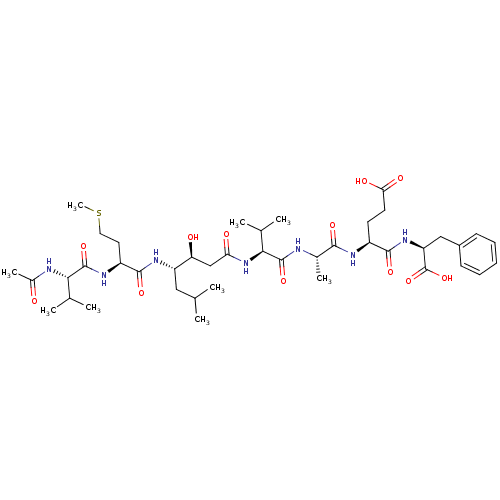
(CHEMBL2370886 | Peptide BACE derivative)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCN)C(O)CC)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C74H121N15O26S/c1-12-39(9)60(88-68(108)46(24-28-57(100)101)79-64(104)44(22-26-55(96)97)81-73(113)61(51(91)13-2)89-63(103)42(76)20-17-30-75)72(112)85-50(35-90)69(109)80-45(23-27-56(98)99)67(107)87-59(38(7)8)71(111)82-47(29-31-116-11)66(106)83-48(32-36(3)4)52(92)34-53(93)86-58(37(5)6)70(110)77-40(10)62(102)78-43(21-25-54(94)95)65(105)84-49(74(114)115)33-41-18-15-14-16-19-41/h14-16,18-19,36-40,42-52,58-61,90-92H,12-13,17,20-35,75-76H2,1-11H3,(H,77,110)(H,78,102)(H,79,104)(H,80,109)(H,81,113)(H,82,111)(H,83,106)(H,84,105)(H,85,112)(H,86,93)(H,87,107)(H,88,108)(H,89,103)(H,94,95)(H,96,97)(H,98,99)(H,100,101)(H,114,115)/t39-,40-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51?,52-,58-,59-,60-,61-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human beta-Secretase (BACE) |
J Med Chem 46: 4625-30 (2003)
Article DOI: 10.1021/jm030247h
BindingDB Entry DOI: 10.7270/Q2PR7VCB |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50134705
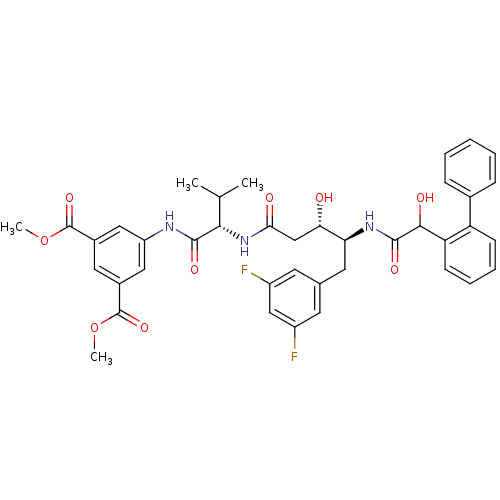
(5-{2-[4-(2-Biphenyl-2-yl-2-hydroxy-acetylamino)-5-...)Show SMILES COC(=O)c1cc(NC(=O)[C@@H](NC(=O)C[C@H](O)[C@H](Cc2cc(F)cc(F)c2)NC(=O)C(O)c2ccccc2-c2ccccc2)C(C)C)cc(c1)C(=O)OC Show InChI InChI=1S/C40H41F2N3O9/c1-22(2)35(37(49)43-29-18-25(39(51)53-3)17-26(19-29)40(52)54-4)45-34(47)21-33(46)32(16-23-14-27(41)20-28(42)15-23)44-38(50)36(48)31-13-9-8-12-30(31)24-10-6-5-7-11-24/h5-15,17-20,22,32-33,35-36,46,48H,16,21H2,1-4H3,(H,43,49)(H,44,50)(H,45,47)/t32-,33-,35-,36?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human beta-Secretase (BACE) |
J Med Chem 46: 4625-30 (2003)
Article DOI: 10.1021/jm030247h
BindingDB Entry DOI: 10.7270/Q2PR7VCB |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50127427
((S)-4-[(S)-2-((S)-2-{(S)-4-[2-((1S,2S)-2-Acetylami...)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(C)=O)C(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C42H67N7O12S/c1-22(2)19-30(47-39(57)29(17-18-62-9)46-41(59)35(23(3)4)44-26(8)50)32(51)21-33(52)49-36(24(5)6)40(58)43-25(7)37(55)45-28(15-16-34(53)54)38(56)48-31(42(60)61)20-27-13-11-10-12-14-27/h10-14,22-25,28-32,35-36,51H,15-21H2,1-9H3,(H,43,58)(H,44,50)(H,45,55)(H,46,59)(H,47,57)(H,48,56)(H,49,52)(H,53,54)(H,60,61)/t25-,28-,29-,30-,31-,32-,35-,36-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human beta-Secretase (BACE) |
J Med Chem 46: 4625-30 (2003)
Article DOI: 10.1021/jm030247h
BindingDB Entry DOI: 10.7270/Q2PR7VCB |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50134703
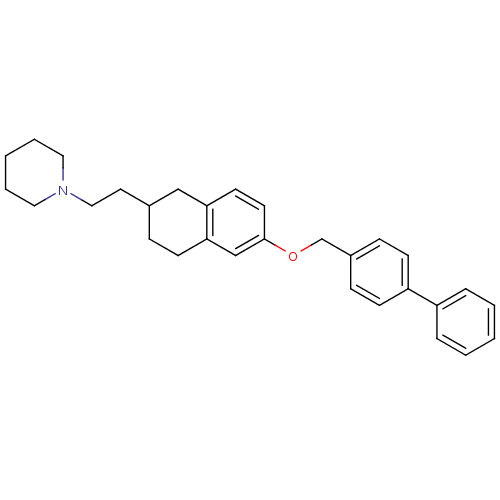
(1-{2-[6-(Biphenyl-4-ylmethoxy)-1,2,3,4-tetrahydro-...)Show SMILES C(CN1CCCCC1)C1CCc2cc(OCc3ccc(cc3)-c3ccccc3)ccc2C1 Show InChI InChI=1S/C30H35NO/c1-3-7-26(8-4-1)27-12-10-25(11-13-27)23-32-30-16-15-28-21-24(9-14-29(28)22-30)17-20-31-18-5-2-6-19-31/h1,3-4,7-8,10-13,15-16,22,24H,2,5-6,9,14,17-21,23H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human beta-Secretase (BACE) |
J Med Chem 46: 4625-30 (2003)
Article DOI: 10.1021/jm030247h
BindingDB Entry DOI: 10.7270/Q2PR7VCB |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50134705

(5-{2-[4-(2-Biphenyl-2-yl-2-hydroxy-acetylamino)-5-...)Show SMILES COC(=O)c1cc(NC(=O)[C@@H](NC(=O)C[C@H](O)[C@H](Cc2cc(F)cc(F)c2)NC(=O)C(O)c2ccccc2-c2ccccc2)C(C)C)cc(c1)C(=O)OC Show InChI InChI=1S/C40H41F2N3O9/c1-22(2)35(37(49)43-29-18-25(39(51)53-3)17-26(19-29)40(52)54-4)45-34(47)21-33(46)32(16-23-14-27(41)20-28(42)15-23)44-38(50)36(48)31-13-9-8-12-30(31)24-10-6-5-7-11-24/h5-15,17-20,22,32-33,35-36,46,48H,16,21H2,1-4H3,(H,43,49)(H,44,50)(H,45,47)/t32-,33-,35-,36?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for its effective concentration against human beta-Secretase (BACE) |
J Med Chem 46: 4625-30 (2003)
Article DOI: 10.1021/jm030247h
BindingDB Entry DOI: 10.7270/Q2PR7VCB |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50134706

(5-{2-[4-(2-Biphenyl-2-yl-2-hydroxy-acetylamino)-5-...)Show SMILES CC(C)[C@H](NC(=O)C[C@H](O)[C@H](Cc1cc(F)cc(F)c1)NC(=O)C(O)c1ccccc1-c1ccccc1)C(=O)Nc1cc(cc(c1)C(O)=O)C(O)=O Show InChI InChI=1S/C38H37F2N3O9/c1-20(2)33(35(47)41-27-16-23(37(49)50)15-24(17-27)38(51)52)43-32(45)19-31(44)30(14-21-12-25(39)18-26(40)13-21)42-36(48)34(46)29-11-7-6-10-28(29)22-8-4-3-5-9-22/h3-13,15-18,20,30-31,33-34,44,46H,14,19H2,1-2H3,(H,41,47)(H,42,48)(H,43,45)(H,49,50)(H,51,52)/t30-,31-,33-,34?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for its effective concentration against human beta-Secretase (BACE) |
J Med Chem 46: 4625-30 (2003)
Article DOI: 10.1021/jm030247h
BindingDB Entry DOI: 10.7270/Q2PR7VCB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data