

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B2 bradykinin receptor (Cavia porcellus) | BDBM50075000 ((E)-N-({[4-Chloro-2-cyano-3-(2-methyl-quinolin-8-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074978 (2-[3-(4-Amino-butyl)-ureido]-N-[4-chloro-2-cyano-3...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074986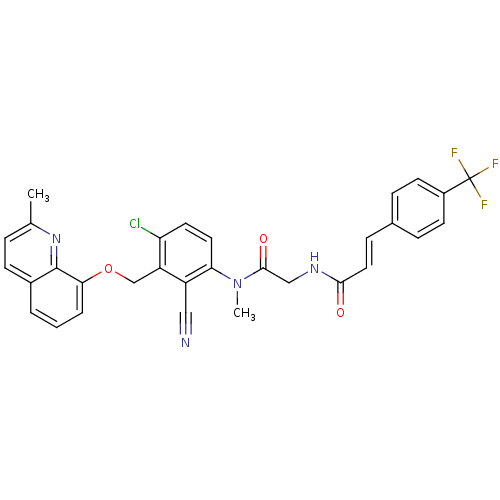 ((E)-N-({[4-Chloro-2-cyano-3-(2-methyl-quinolin-8-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074997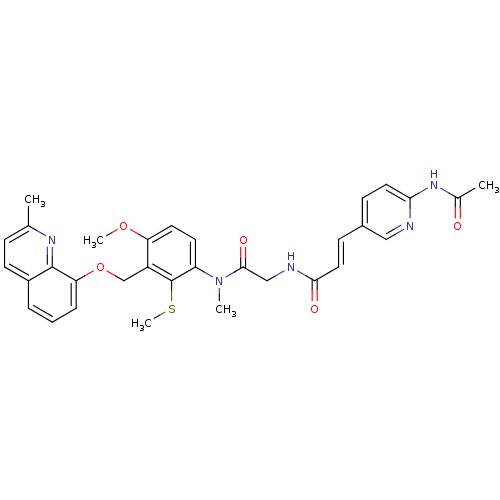 ((E)-3-(6-Acetylamino-pyridin-3-yl)-N-({[4-methoxy-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074993 ((E)-N-({[2,4-Dimethyl-3-(2-methyl-quinolin-8-yloxy...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074982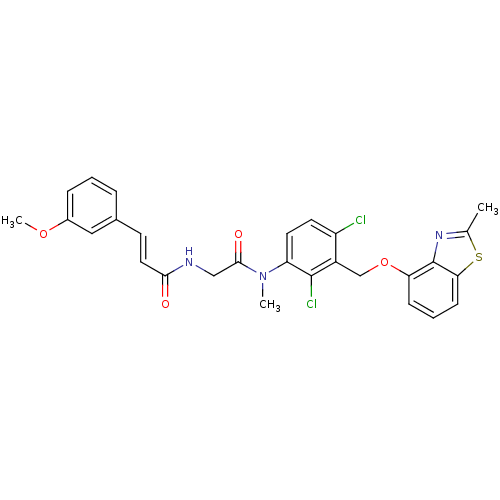 ((E)-N-({[2,4-Dichloro-3-(2-methyl-benzothiazol-4-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074983 ((E)-N-({[2-Chloro-4-methoxy-3-(2-methyl-quinolin-8...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074984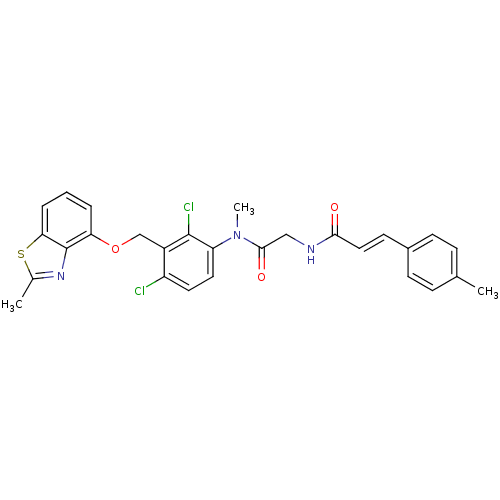 ((E)-N-({[2,4-Dichloro-3-(2-methyl-benzothiazol-4-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074992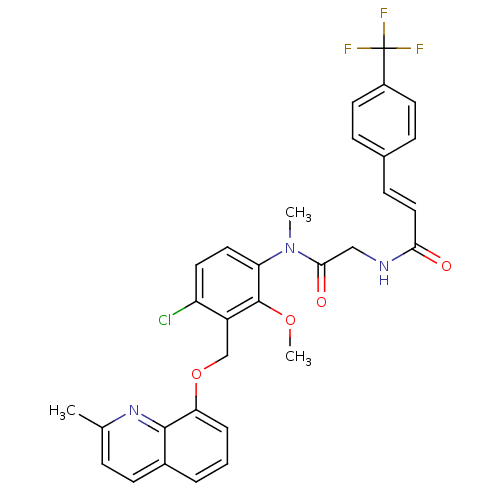 ((E)-N-({[4-Chloro-2-methoxy-3-(2-methyl-quinolin-8...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074980 ((E)-N-({[2,4-Dichloro-3-(2-methyl-benzothiazol-4-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074990 (4-[3-({[4-Chloro-2-cyano-3-(2-methyl-quinolin-8-yl...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074991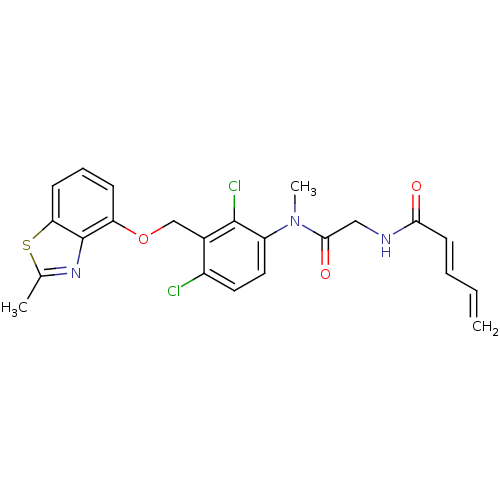 ((E)-Penta-2,4-dienoic acid ({[2,4-dichloro-3-(2-me...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074985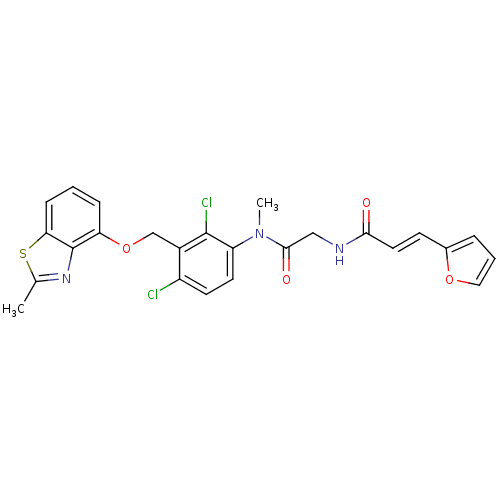 ((E)-N-({[2,4-Dichloro-3-(2-methyl-benzothiazol-4-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074998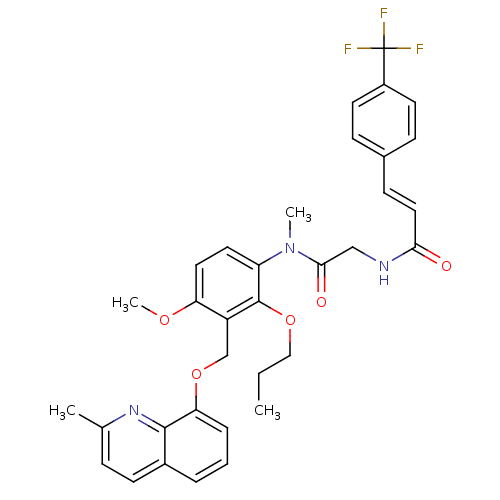 ((E)-N-({[4-Methoxy-3-(2-methyl-quinolin-8-yloxymet...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074994 (5-[({[4-Methoxy-3-(2-methyl-quinolin-8-yloxymethyl...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074987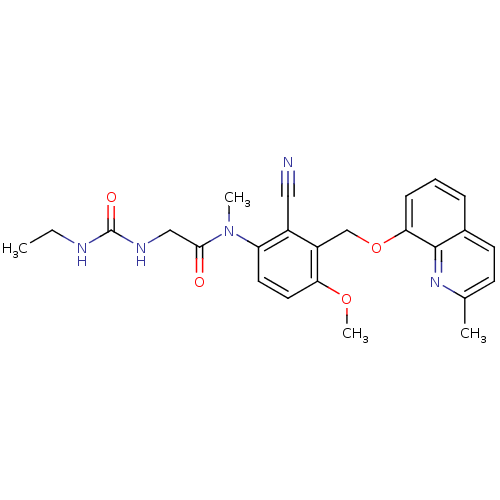 (CHEMBL113502 | N-[2-Cyano-4-methoxy-3-(2-methyl-qu...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074979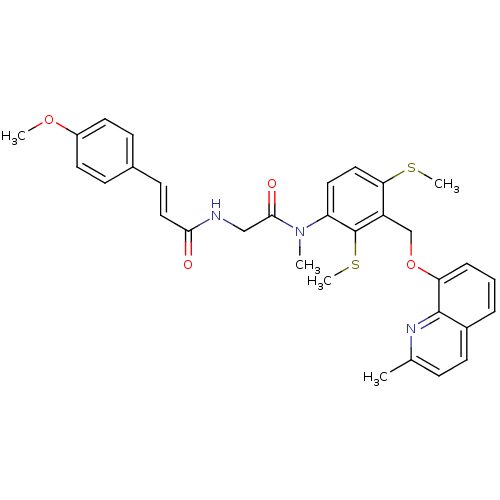 ((E)-3-(4-Methoxy-phenyl)-N-({methyl-[3-(2-methyl-q...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074999 (CHEMBL114180 | N-[4-Chloro-2-cyano-3-(2-methyl-qui...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074996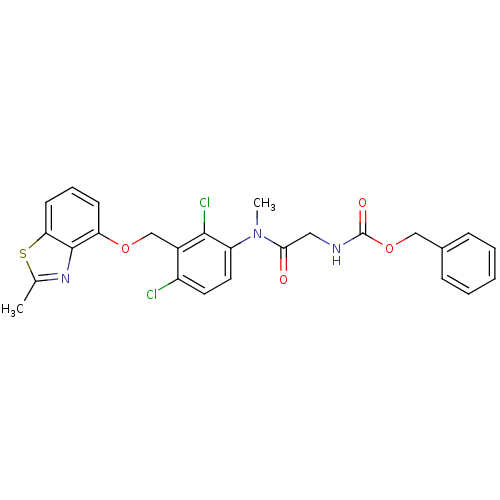 (({[2,4-Dichloro-3-(2-methyl-benzothiazol-4-yloxyme...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074981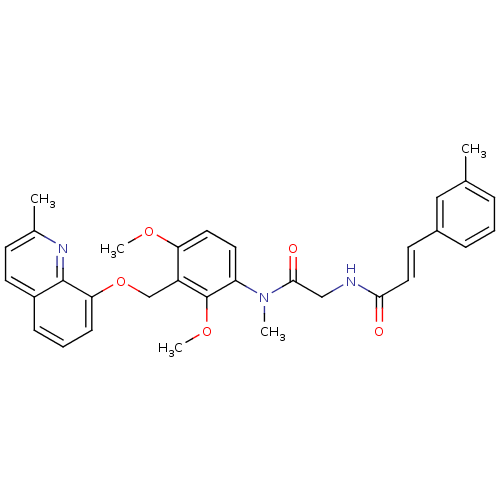 ((E)-N-({[2,4-Dimethoxy-3-(2-methyl-quinolin-8-ylox...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074995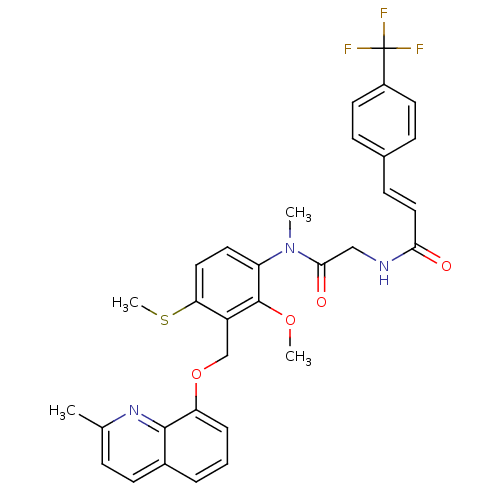 ((E)-N-({[2-Methoxy-3-(2-methyl-quinolin-8-yloxymet...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074988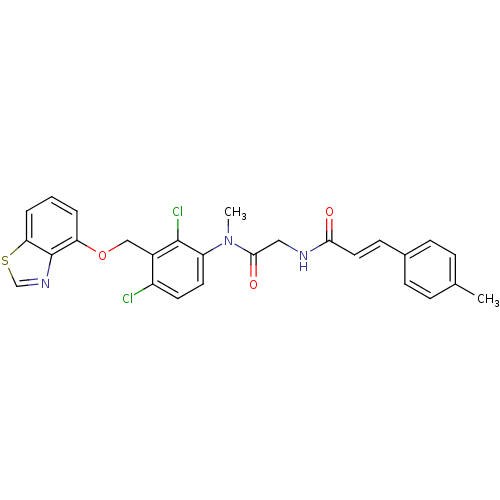 ((E)-N-({[3-(Benzothiazol-4-yloxymethyl)-2,4-dichlo...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074989 ((E)-N-({[2,4-Dichloro-3-(2-phenyl-benzothiazol-4-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470233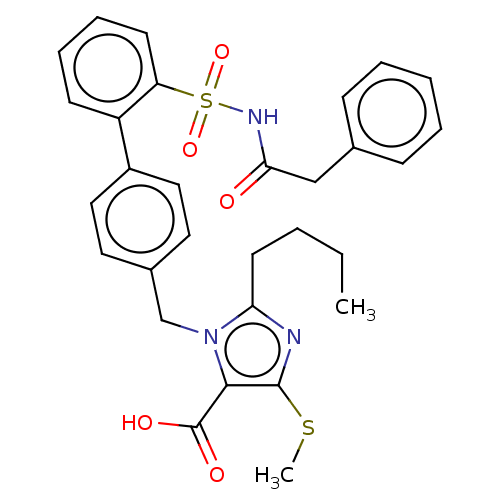 (CHEMBL80177) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470229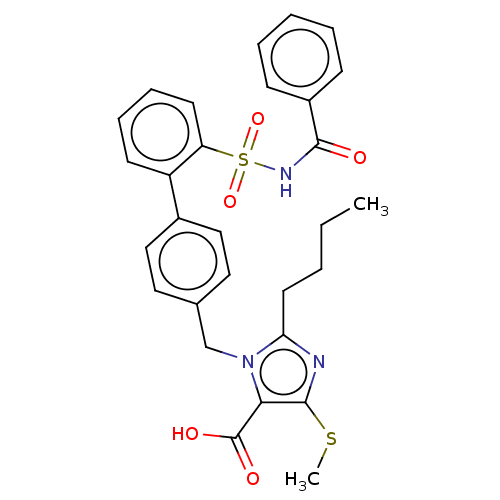 (CHEMBL76870) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470239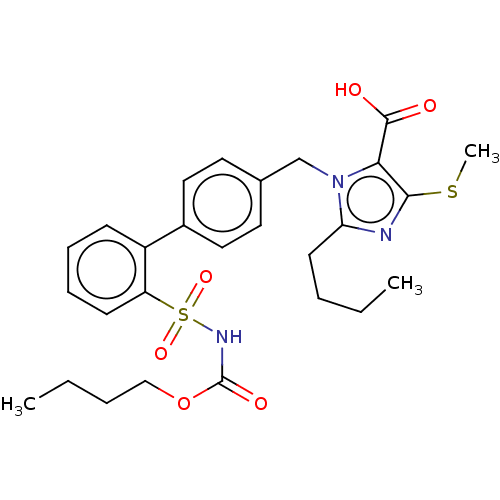 (CHEMBL311312) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470236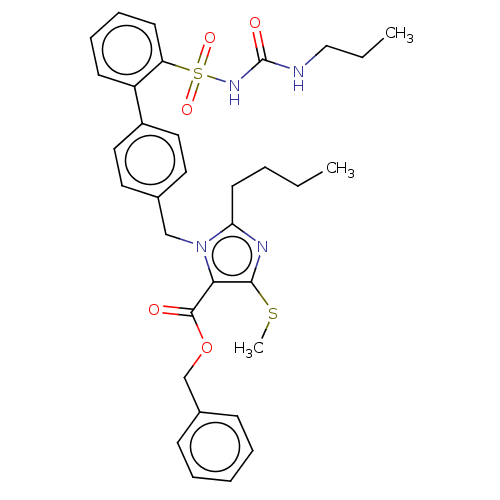 (CHEMBL309313) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045686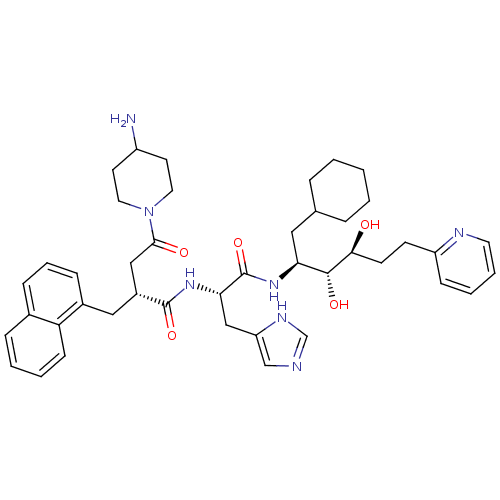 (4-(4-Amino-piperidin-1-yl)-N-[1-(1-cyclohexylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045691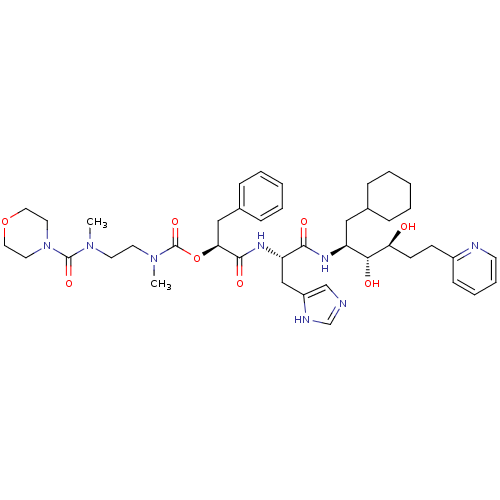 (CHEMBL94827 | Methyl-{2-[methyl-(morpholine-4-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045691 (CHEMBL94827 | Methyl-{2-[methyl-(morpholine-4-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of renin in human plasma | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045687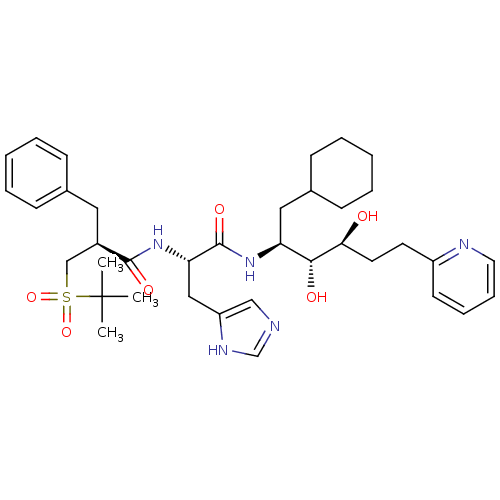 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of renin in human plasma | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470250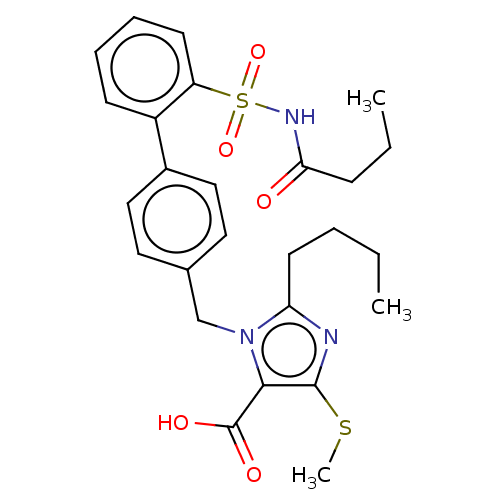 (CHEMBL77308) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213569 (CHEMBL78866) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470225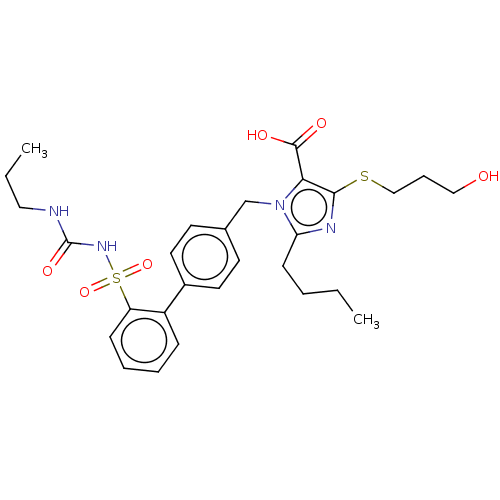 (CHEMBL80700) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470226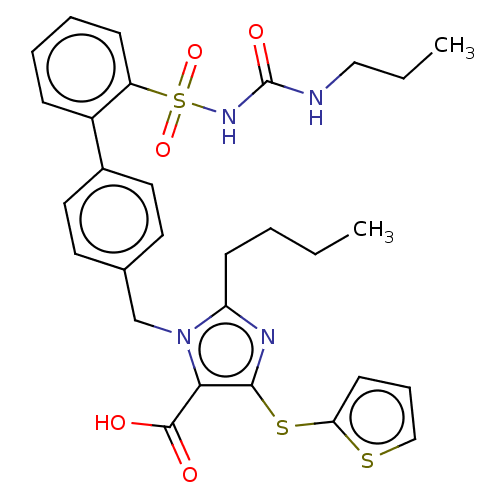 (CHEMBL311827) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470235 (CHEMBL308135) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470206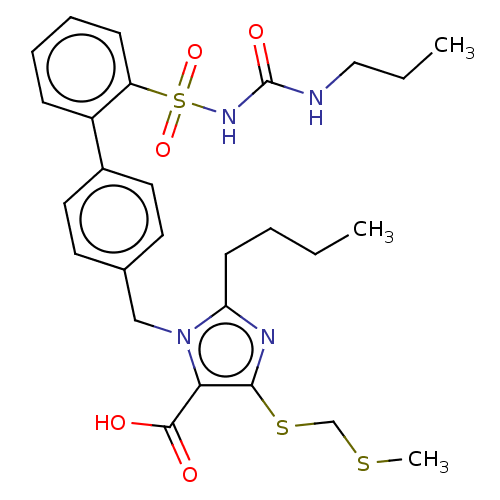 (CHEMBL445365) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213557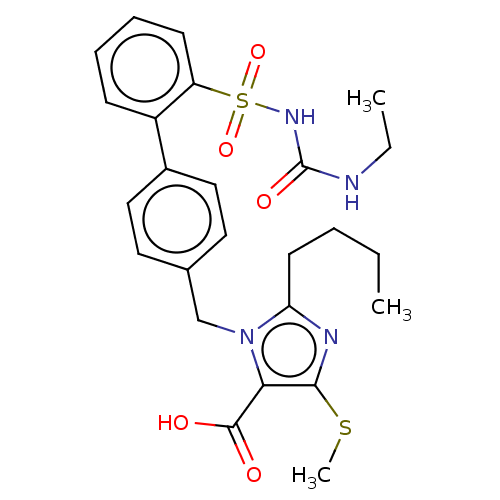 (CHEMBL309602) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50031491 (2-propyl-4-(methythio)-1-[[[2'-[(propylamino)carbo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045683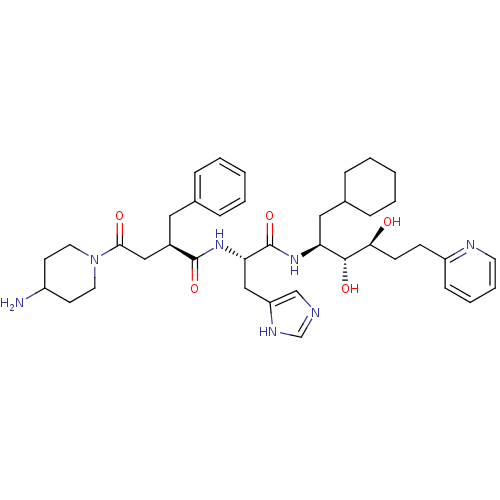 (4-(4-Amino-piperidin-1-yl)-2-benzyl-N-[1-(1-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of renin in human plasma | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045685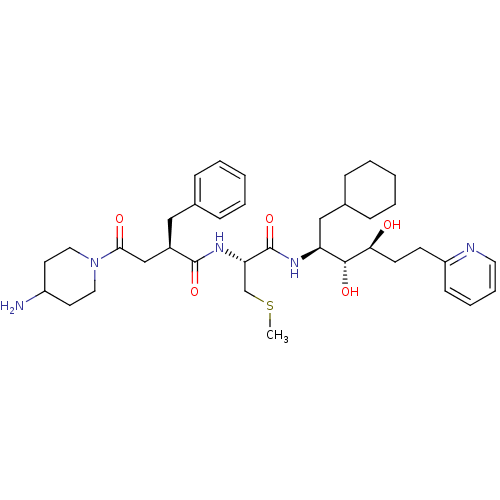 (4-(4-Amino-piperidin-1-yl)-2-benzyl-N-[1-(1-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045685 (4-(4-Amino-piperidin-1-yl)-2-benzyl-N-[1-(1-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470249 (CHEMBL309845) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470241 (CHEMBL305638) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50031537 (2-Butyl-3-(N'-propylureidylsulfonyl-biphenyl-4-ylm...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470227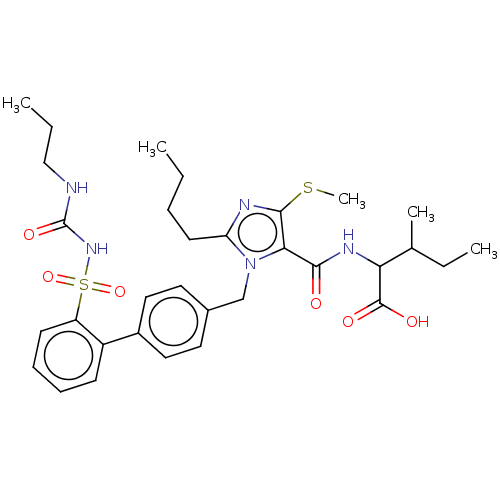 (CHEMBL76538) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470223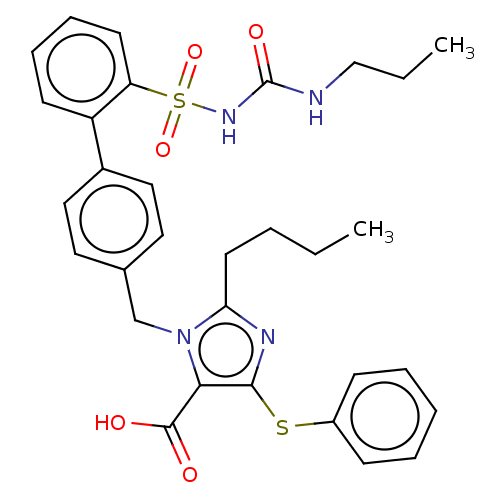 (CHEMBL312386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045692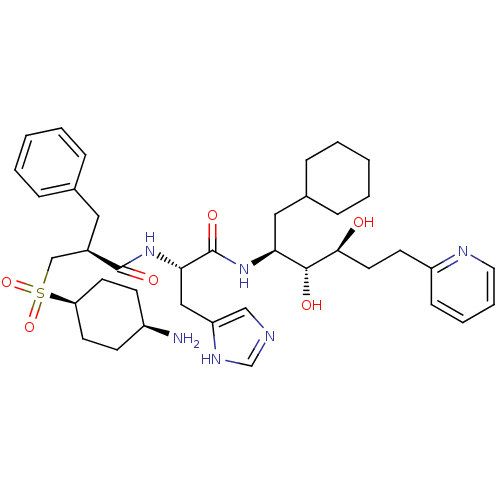 (3-(4-Amino-cyclohexanesulfonyl)-2-benzyl-N-[1-(1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045692 (3-(4-Amino-cyclohexanesulfonyl)-2-benzyl-N-[1-(1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of purified human kidney renin | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045693 (4-Amino-piperidine-1-carboxylic acid 1-[1-(1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description In vitro inhibition of renin in human plasma | J Med Chem 36: 2788-800 (1993) BindingDB Entry DOI: 10.7270/Q24J0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 192 total ) | Next | Last >> |