

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561628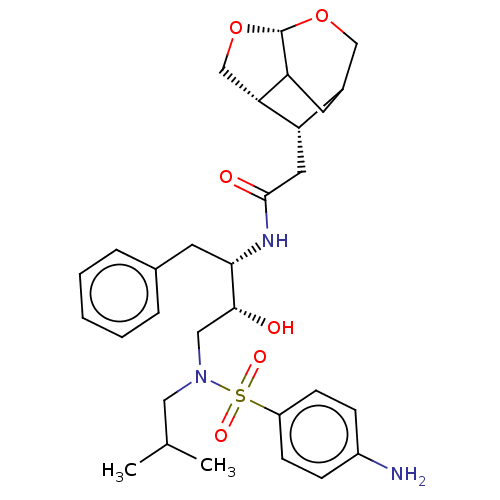 (CHEMBL4800615) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561630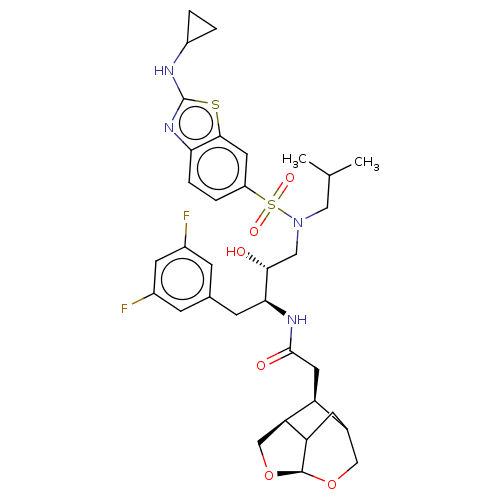 (CHEMBL4743665) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561627 (CHEMBL4744116) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561629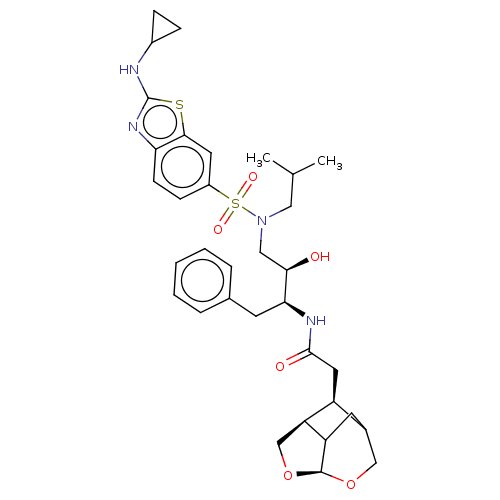 (CHEMBL4740468) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561623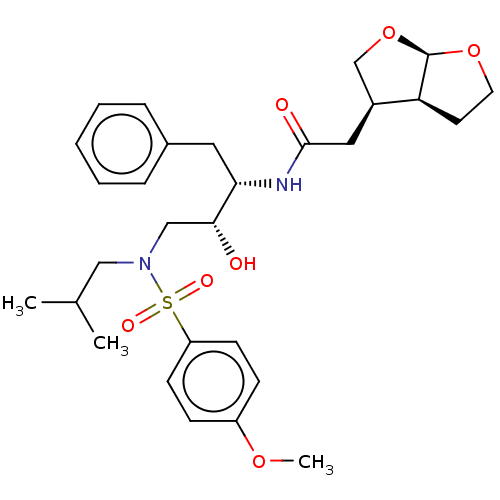 (CHEMBL256107 | GRL-0026A) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561626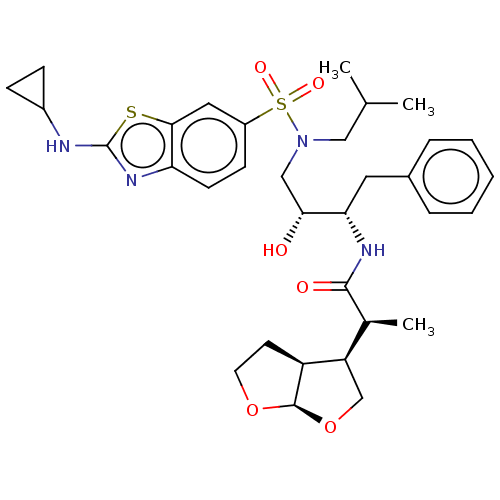 (CHEMBL4763723) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561624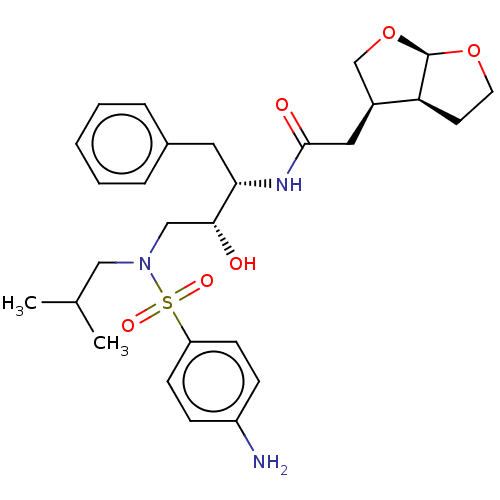 (CHEMBL4764958) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496891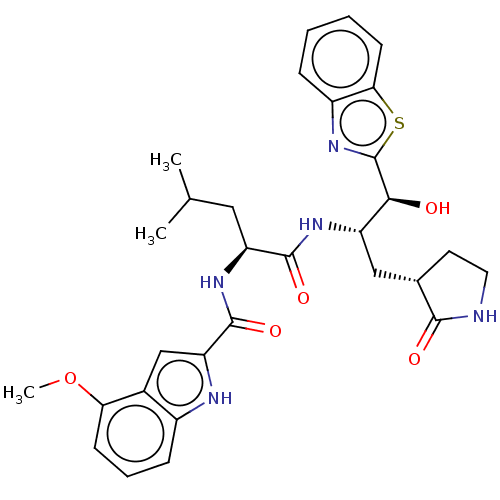 (GRL-2420) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561625 (CHEMBL4784475) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 284 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496875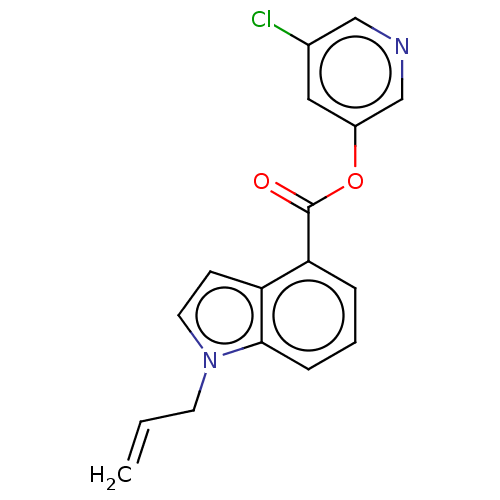 (indole chloropyridinyl-ester derived, 7d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496873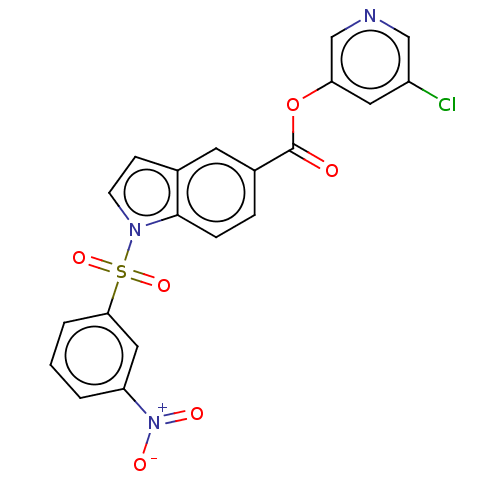 (indole chloropyridinyl-ester derived, 7b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM429304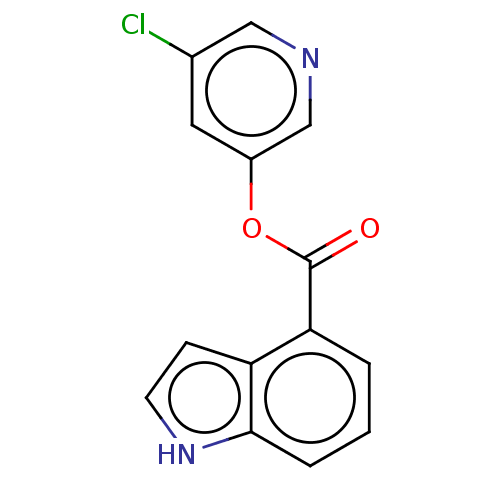 (CVD-0006354 | acs.jmedchem.1c00409_ST.426 | indole...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM429306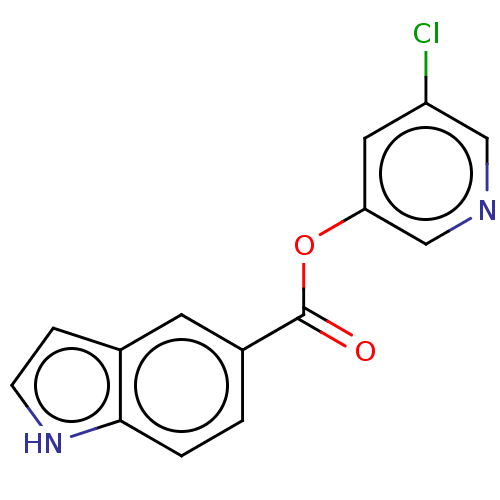 (acs.jmedchem.1c00409_ST.427 | indole chloropyridin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496871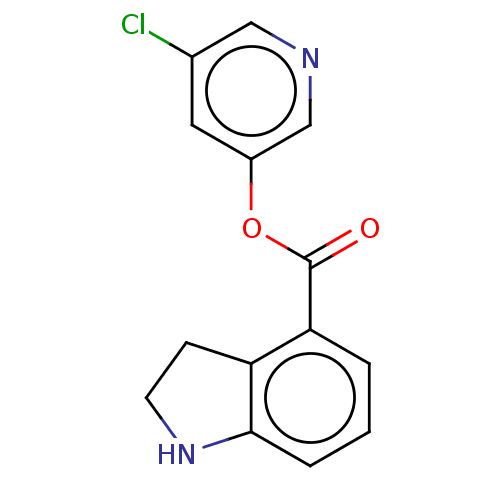 (indole chloropyridinyl-ester derived, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496881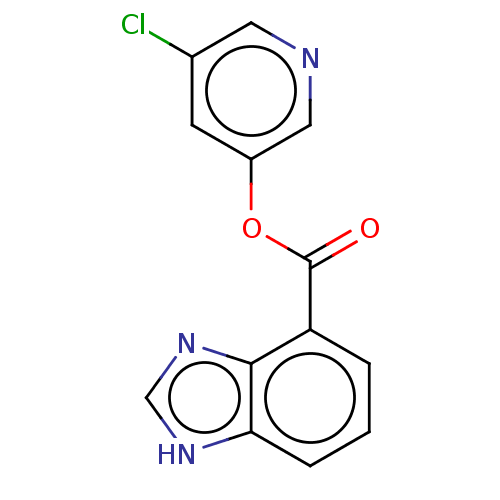 (indole chloropyridinyl-ester derived, 7j) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496876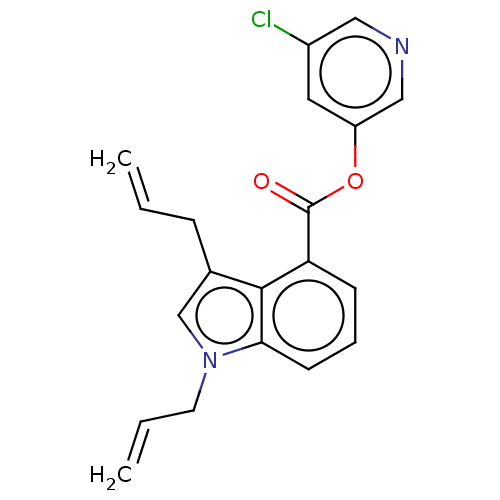 (indole chloropyridinyl-ester derived, 7e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496884 (indole chloropyridinyl-ester derived, 7m) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 419 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496877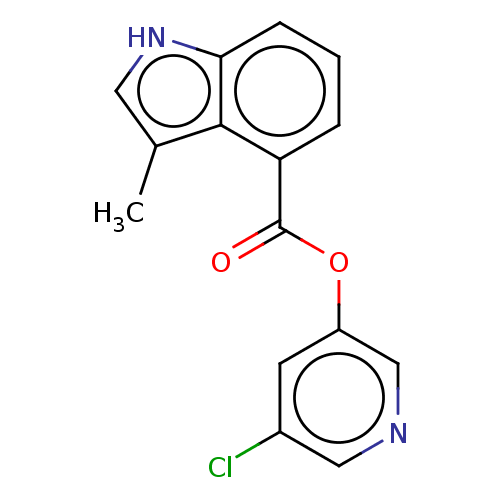 (indole chloropyridinyl-ester derived, 7f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496882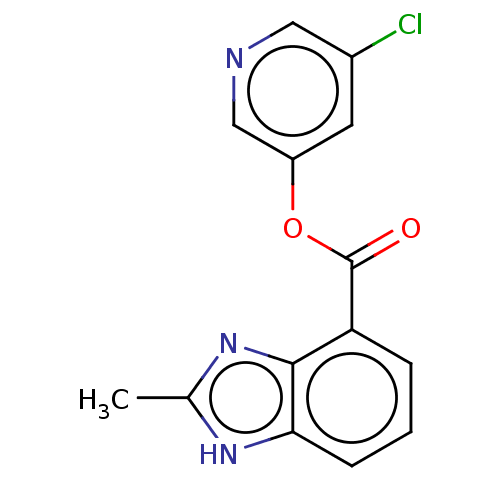 (indole chloropyridinyl-ester derived, 7k) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 489 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496879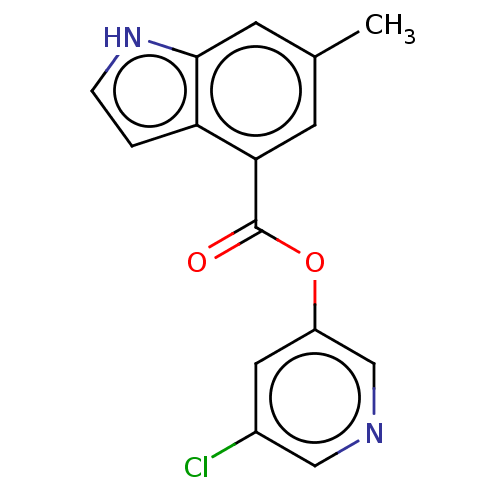 (indole chloropyridinyl-ester derived, 7h) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496880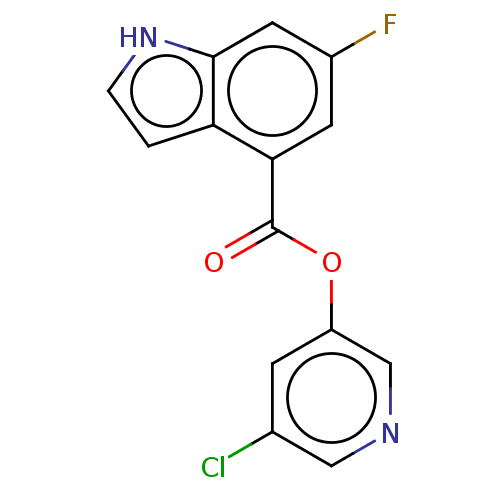 (indole chloropyridinyl-ester derived, 7i) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496874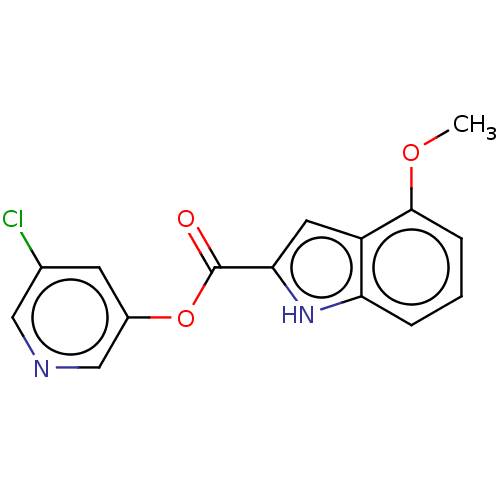 (indole chloropyridinyl-ester derived, 7c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496883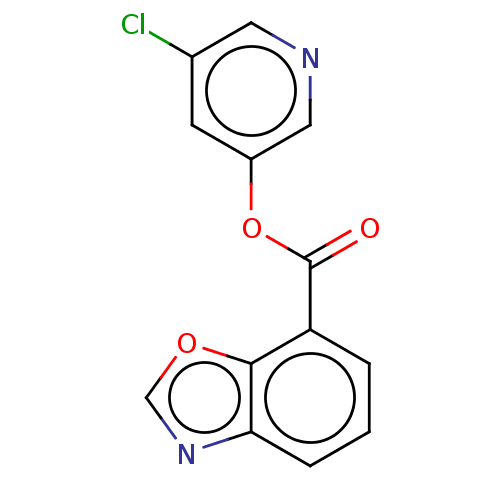 (indole chloropyridinyl-ester derived, 7l) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496888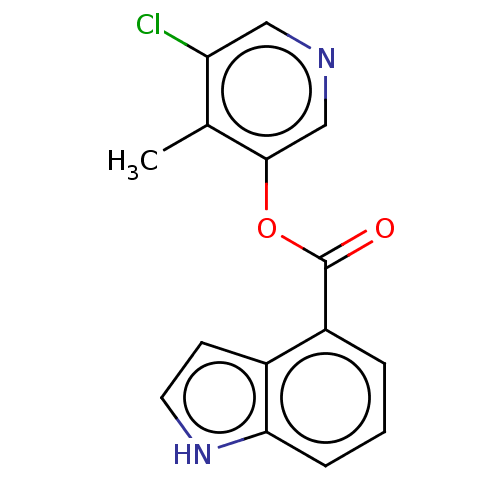 (indole chloropyridinyl-ester derived, 9d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496890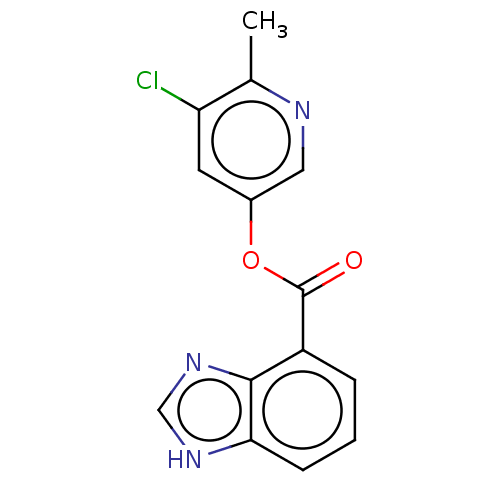 (indole chloropyridinyl-ester derived, 9f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496878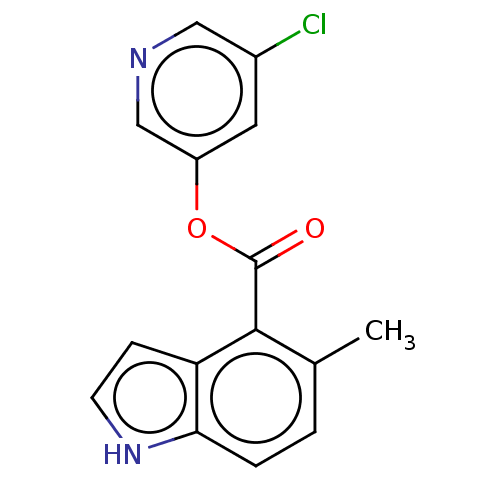 (indole chloropyridinyl-ester derived, 7g) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496889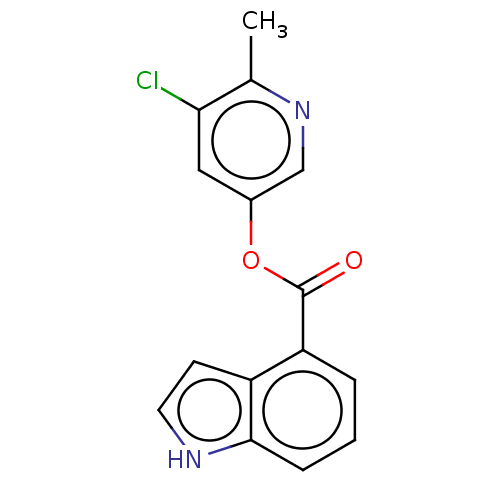 (indole chloropyridinyl-ester derived, 9e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496887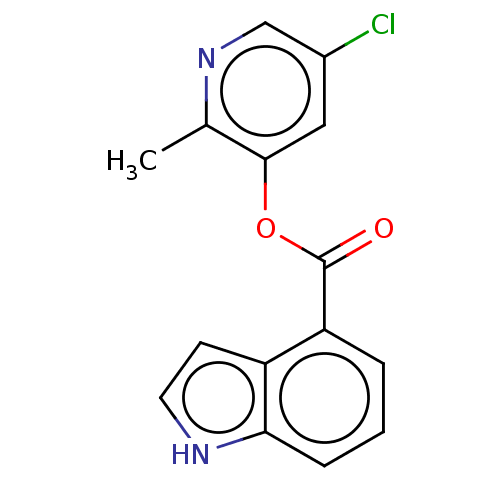 (indole chloropyridinyl-ester derived, 9c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496885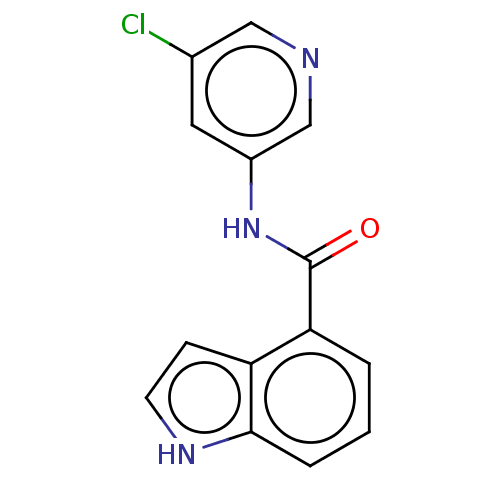 (indole chloropyridinyl-ester derived, 9a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496886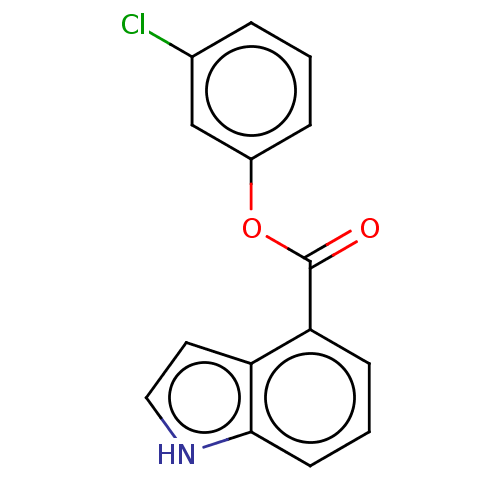 (indole chloropyridinyl-ester derived, 9b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University | Assay Description IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496891 (GRL-2420) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496890 (indole chloropyridinyl-ester derived, 9f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496889 (indole chloropyridinyl-ester derived, 9e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496888 (indole chloropyridinyl-ester derived, 9d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496887 (indole chloropyridinyl-ester derived, 9c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496886 (indole chloropyridinyl-ester derived, 9b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496885 (indole chloropyridinyl-ester derived, 9a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496884 (indole chloropyridinyl-ester derived, 7m) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496883 (indole chloropyridinyl-ester derived, 7l) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496882 (indole chloropyridinyl-ester derived, 7k) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.71E+4 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496881 (indole chloropyridinyl-ester derived, 7j) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496880 (indole chloropyridinyl-ester derived, 7i) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496879 (indole chloropyridinyl-ester derived, 7h) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496878 (indole chloropyridinyl-ester derived, 7g) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496877 (indole chloropyridinyl-ester derived, 7f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.67E+4 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496876 (indole chloropyridinyl-ester derived, 7e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496875 (indole chloropyridinyl-ester derived, 7d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496874 (indole chloropyridinyl-ester derived, 7c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496873 (indole chloropyridinyl-ester derived, 7b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 6.98E+4 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM429306 (acs.jmedchem.1c00409_ST.427 | indole chloropyridin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.37E+4 | n/a | n/a | n/a | n/a |
Purdue University | Assay Description VeroE6 cells and TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells were obtained from the Japanese Collection of Research Bioresource... | J Med Chem 64: 14702-14714 (2021) Article DOI: 10.1021/acs.jmedchem.1c01214 BindingDB Entry DOI: 10.7270/Q2CC13T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |