

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM50180775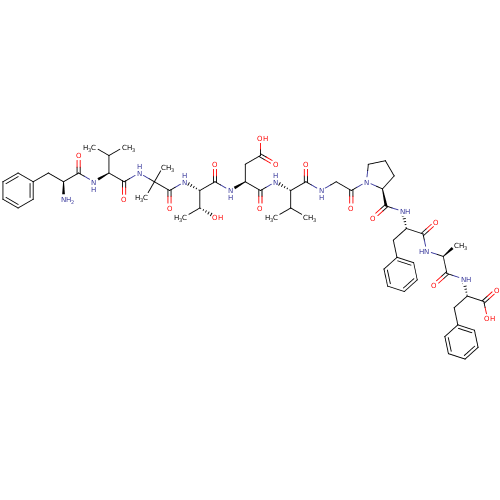 (CHEMBL386763 | FV-Aib-TDVGPFAF | [Aib29,Asp31,Pro3...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Displacement of [3H-propionyl-K24] from halphaCGRP expressed in human neuroblastoma SK-N-MC cells | J Med Chem 49: 616-24 (2006) Article DOI: 10.1021/jm050613s BindingDB Entry DOI: 10.7270/Q280526H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22925 ((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of human erythrocytic adenosine deaminase. | J Med Chem 26: 1478-82 (1983) Checked by Author BindingDB Entry DOI: 10.7270/Q29Z95GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM50180778 (CHEMBL2371891 | FV-Hyp-TDVGPFAF) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Displacement of [3H-propionyl-K24] from halphaCGRP expressed in human neuroblastoma SK-N-MC cells | J Med Chem 49: 616-24 (2006) Article DOI: 10.1021/jm050613s BindingDB Entry DOI: 10.7270/Q280526H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50398494 (CHEMBL2177429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay | Bioorg Med Chem 21: 6657-64 (2013) Article DOI: 10.1016/j.bmc.2013.08.015 BindingDB Entry DOI: 10.7270/Q26Q1ZPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM50367032 (COFORMYCIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of human erythrocytic adenosine deaminase | J Med Chem 26: 1478-82 (1983) Checked by Author BindingDB Entry DOI: 10.7270/Q29Z95GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50398494 (CHEMBL2177429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay | J Med Chem 55: 8392-408 (2012) Article DOI: 10.1021/jm300780p BindingDB Entry DOI: 10.7270/Q2TX3GH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM9236 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0150 | -61.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
University of Illinois at Chicago | Assay Description The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... | Bioorg Med Chem Lett 12: 1993-6 (2002) Article DOI: 10.1016/s0960-894x(02)00300-1 BindingDB Entry DOI: 10.7270/Q2SJ1HS5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM50062162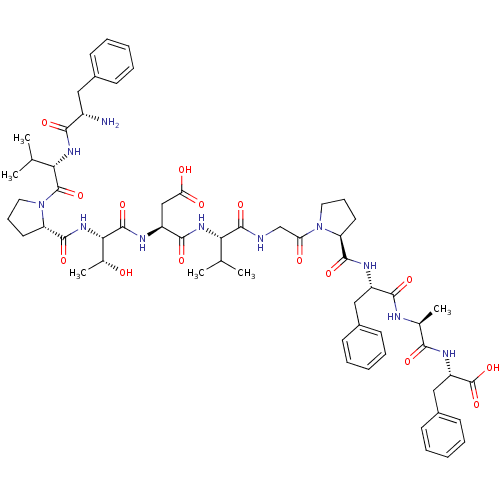 (CHEMBL264010 | FVPTDVGPFAF) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Displacement of [3H-propionyl-K24] from halphaCGRP expressed in human neuroblastoma SK-N-MC cells | J Med Chem 49: 616-24 (2006) Article DOI: 10.1021/jm050613s BindingDB Entry DOI: 10.7270/Q280526H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM50180781 (CHEMBL2371890 | FV-Tic-TDVGPFAF) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Displacement of [3H-propionyl-K24] from halphaCGRP expressed in human neuroblastoma SK-N-MC cells | J Med Chem 49: 616-24 (2006) Article DOI: 10.1021/jm050613s BindingDB Entry DOI: 10.7270/Q280526H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50059585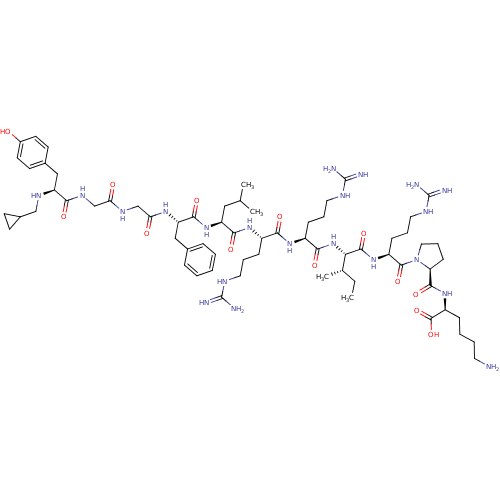 (CHEMBL442323 | N-CPM[D-Pro-10]Dyn A-(1-11)) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Affinity against Opioid receptor kappa 1 was determined by measuring the inhibition of [3H]-bremazocine binding to guinea pig cerebellar membranes | J Med Chem 40: 2733-9 (1997) Article DOI: 10.1021/jm960747t BindingDB Entry DOI: 10.7270/Q2D50M2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002347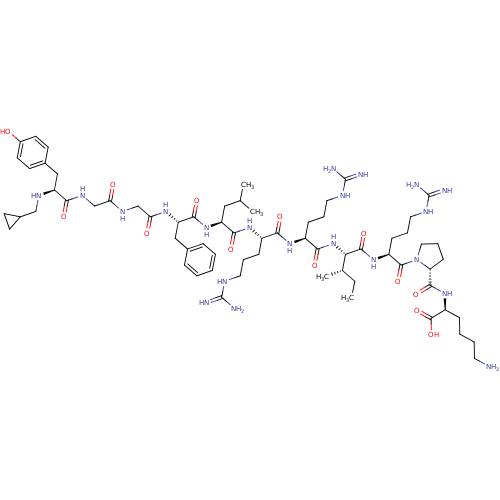 (CHEMBL425806 | [N-cyclopropyl methylTyr1, D-pro10]...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Tested for inhibitory effect on binding of [3H]bremazocine to opioid receptor kappa in guinea pig cerebellum membranes | J Med Chem 35: 4638-9 (1993) BindingDB Entry DOI: 10.7270/Q2416XPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50049553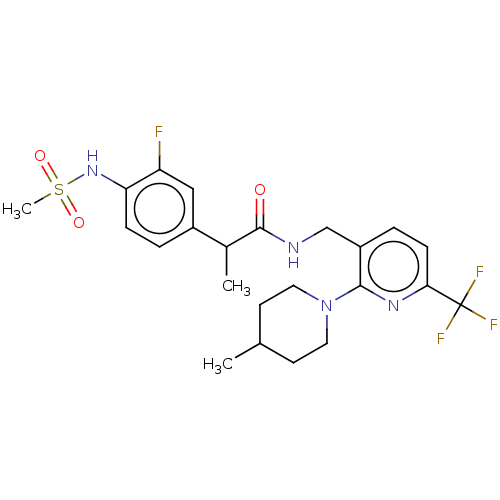 (CHEMBL2177428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activation by FLIPR assay | Bioorg Med Chem Lett 25: 2326-30 (2015) Article DOI: 10.1016/j.bmcl.2015.04.024 BindingDB Entry DOI: 10.7270/Q2Z60QSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576898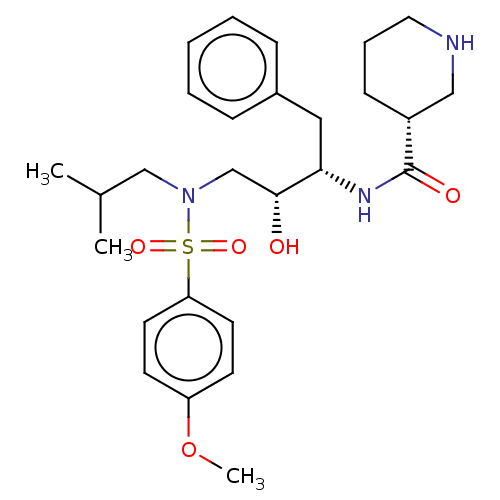 (CHEMBL4877646) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002345 (CHEMBL407529 | PhCH2Tyr-Gly-Gly-Phe-Leu-Arg-Arg-ll...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Tested for inhibitory effect on binding of [3H]bremazocine to opioid receptor kappa in guinea pig cerebellum membranes | J Med Chem 35: 4638-9 (1993) BindingDB Entry DOI: 10.7270/Q2416XPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50059583 (CHEMBL268818 | N-benzyl[D-Pro-10]Dyn A-(1-11) | Ph...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Affinity against Opioid receptor kappa 1 was determined by measuring the inhibition of [3H]-bremazocine binding to guinea pig cerebellar membranes | J Med Chem 40: 2733-9 (1997) Article DOI: 10.1021/jm960747t BindingDB Entry DOI: 10.7270/Q2D50M2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002346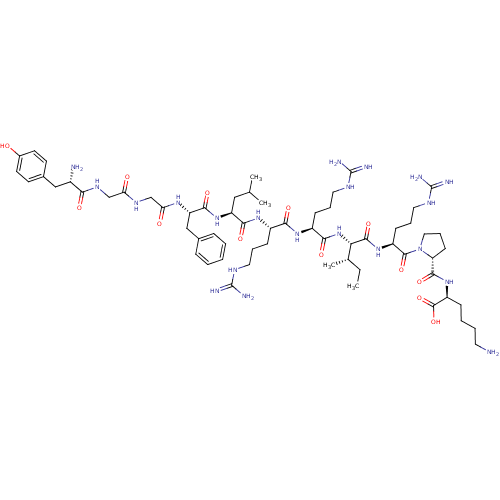 (CHEMBL384584 | [D-pro10]Dynorphin A(1-11)) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Tested for inhibitory effect on binding of [3H]bremazocine to opioid receptor kappa in guinea pig cerebellum membranes | J Med Chem 35: 4638-9 (1993) BindingDB Entry DOI: 10.7270/Q2416XPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002346 (CHEMBL384584 | [D-pro10]Dynorphin A(1-11)) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Affinity against Opioid receptor kappa 1 was determined by measuring the inhibition of [3H]-bremazocine binding to guinea pig cerebellar membranes | J Med Chem 40: 2733-9 (1997) Article DOI: 10.1021/jm960747t BindingDB Entry DOI: 10.7270/Q2D50M2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50578268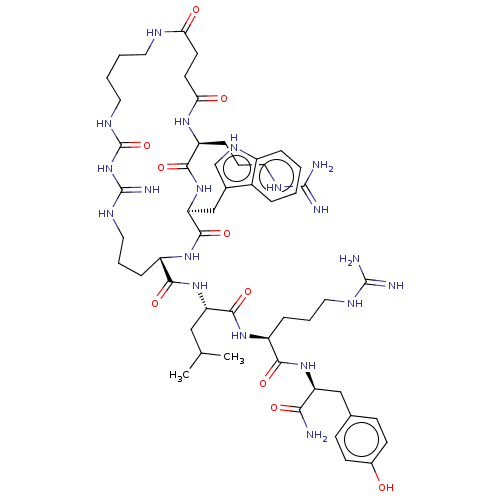 (CHEMBL4856311) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]UR-KK200 from human neuropeptide Y Y4 receptor expressed in CHO-mtAEQ cells co-expressing Gqi5 measured after 90 mins by scintill... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01574 BindingDB Entry DOI: 10.7270/Q2VH5SNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50368278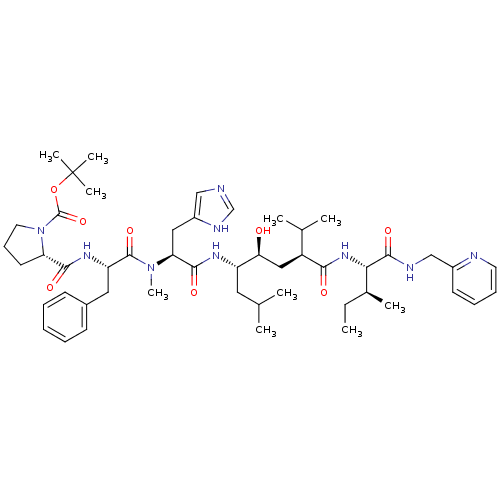 (CHEMBL1790497) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibition constants using recombinant human renin assay | J Med Chem 34: 2107-12 (1991) BindingDB Entry DOI: 10.7270/Q2HQ40J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576910 (CHEMBL4862758) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50366620 (RESINIFERATOXIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0426 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50368274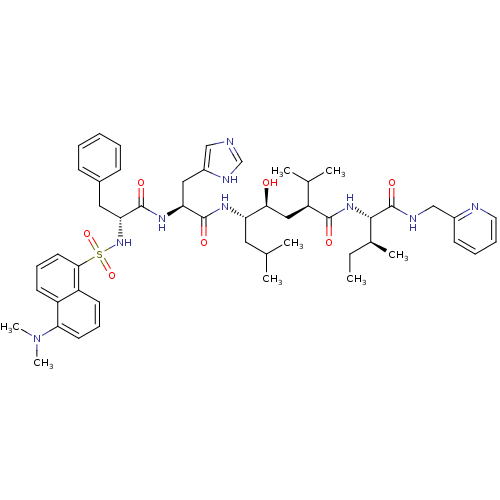 (CHEMBL1790492) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibition constants using recombinant human renin assay | J Med Chem 34: 2107-12 (1991) BindingDB Entry DOI: 10.7270/Q2HQ40J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50578262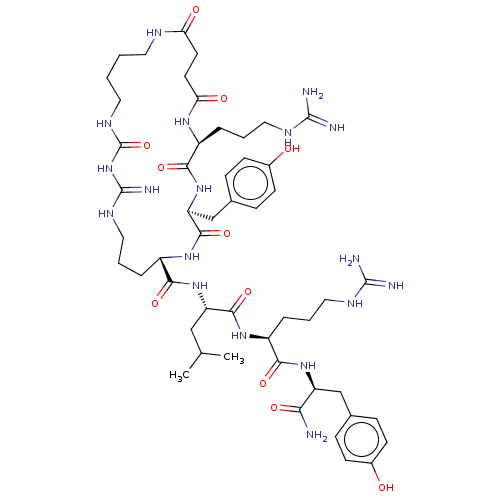 (CHEMBL4873376) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]UR-KK200 from human neuropeptide Y Y4 receptor expressed in CHO-mtAEQ cells co-expressing Gqi5 measured after 90 mins by scintill... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01574 BindingDB Entry DOI: 10.7270/Q2VH5SNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002350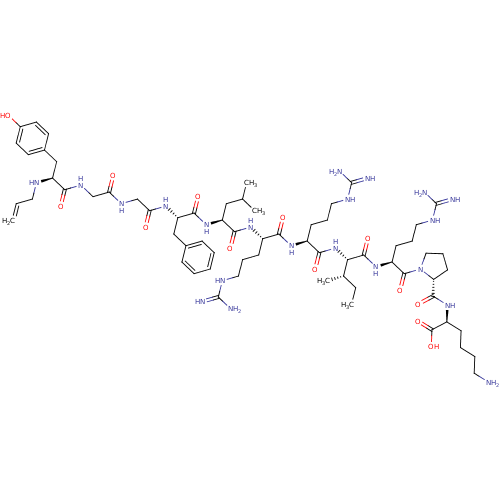 (CHEMBL265387 | [N-allylTyr1, D-pro10]Dynorphin A(1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Tested for inhibitory effect on binding of [3H]bremazocine to opioid receptor kappa in guinea pig cerebellum membranes | J Med Chem 35: 4638-9 (1993) BindingDB Entry DOI: 10.7270/Q2416XPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50059586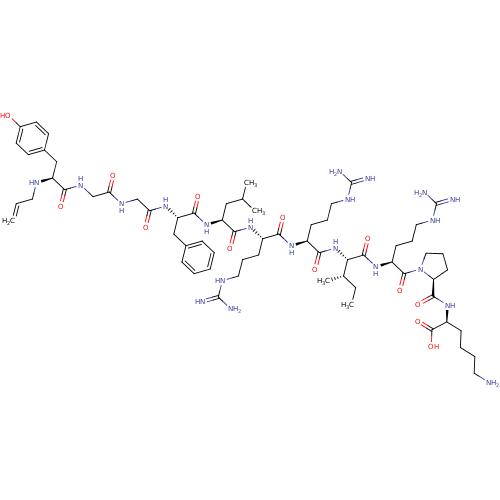 (CHEMBL406338 | N-allyl[D-Pro-10]Dyn A-(1-11)) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Affinity against Opioid receptor kappa 1 was determined by measuring the inhibition of [3H]-bremazocine binding to guinea pig cerebellar membranes | J Med Chem 40: 2733-9 (1997) Article DOI: 10.1021/jm960747t BindingDB Entry DOI: 10.7270/Q2D50M2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50442379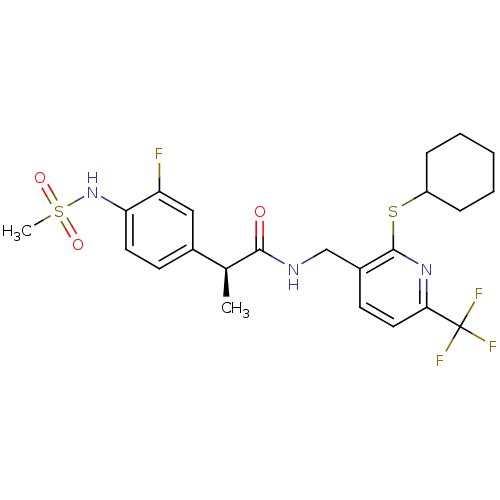 (CHEMBL2442912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay | Bioorg Med Chem 21: 6657-64 (2013) Article DOI: 10.1016/j.bmc.2013.08.015 BindingDB Entry DOI: 10.7270/Q26Q1ZPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012322 (CHEMBL264524 | N-(2-Hydroxy-1,1-bis-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of human renin by radio-immuno assay of angiotensin I (ANG I) | J Med Chem 34: 2107-12 (1991) BindingDB Entry DOI: 10.7270/Q2HQ40J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50334454 (CHEMBL1643895 | Ramosetron | US9045501, Ramosetron) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Binding affinity to human 5HT3A receptor | Bioorg Med Chem Lett 21: 58-61 (2010) Article DOI: 10.1016/j.bmcl.2010.11.080 BindingDB Entry DOI: 10.7270/Q2474B4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8965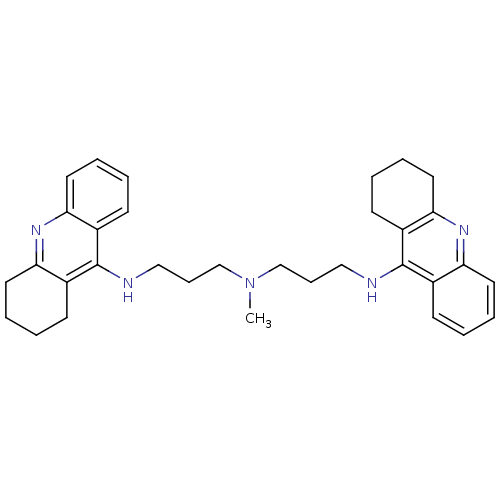 (CHEMBL338755 | Tacrine Dimer 4a | methylbis[3-(1,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | -58.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247744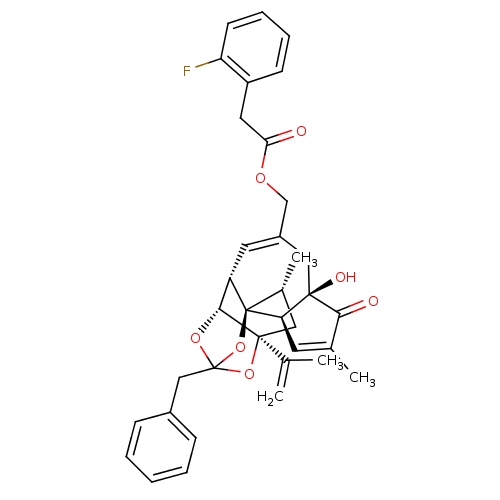 (CHEMBL504725 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50062858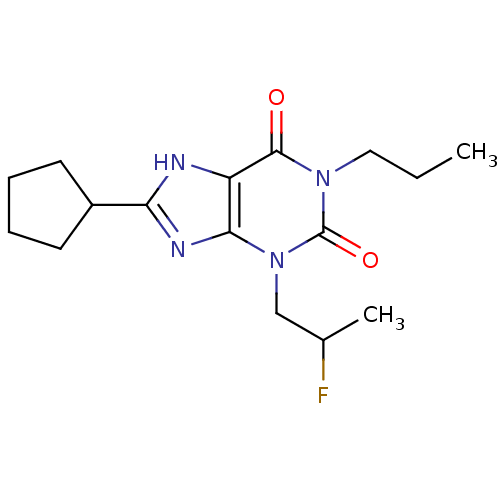 (8-Cyclopentyl-3-(2-fluoro-propyl)-1-propyl-3,7-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forschungszentrum Jülich GmbH Curated by ChEMBL | Assay Description Displacement of [3H]-CPX from Adenosine A1 receptor of bovine brain cerebral cortex membranes | J Med Chem 41: 555-63 (1998) Article DOI: 10.1021/jm9705465 BindingDB Entry DOI: 10.7270/Q2RX9B60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50046377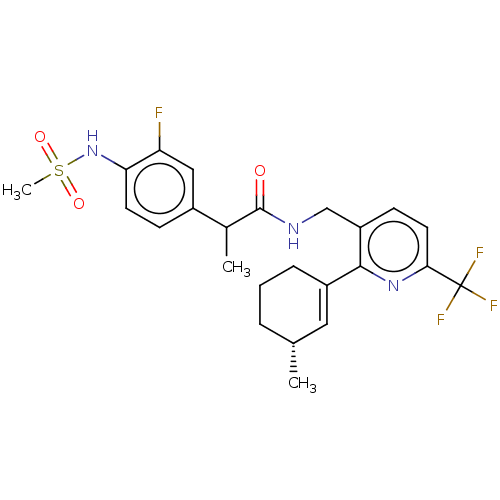 (CHEMBL3314406) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced increase in intracellular Ca2+ level by FLIPR a... | Bioorg Med Chem Lett 24: 4039-43 (2014) Article DOI: 10.1016/j.bmcl.2014.05.074 BindingDB Entry DOI: 10.7270/Q2B859RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50046377 (CHEMBL3314406) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced increase in intracellular Ca2+ level by FLIPR a... | Bioorg Med Chem Lett 24: 4039-43 (2014) Article DOI: 10.1016/j.bmcl.2014.05.074 BindingDB Entry DOI: 10.7270/Q2B859RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50046379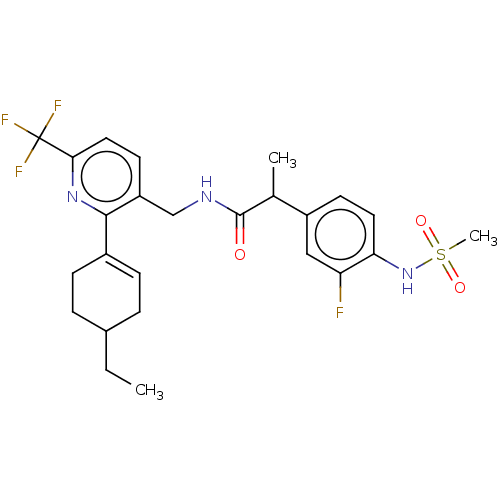 (CHEMBL3314407) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced increase in intracellular Ca2+ level by FLIPR a... | Bioorg Med Chem Lett 24: 4039-43 (2014) Article DOI: 10.1016/j.bmcl.2014.05.074 BindingDB Entry DOI: 10.7270/Q2B859RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50046379 (CHEMBL3314407) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced increase in intracellular Ca2+ level by FLIPR a... | Bioorg Med Chem Lett 24: 4039-43 (2014) Article DOI: 10.1016/j.bmcl.2014.05.074 BindingDB Entry DOI: 10.7270/Q2B859RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50046394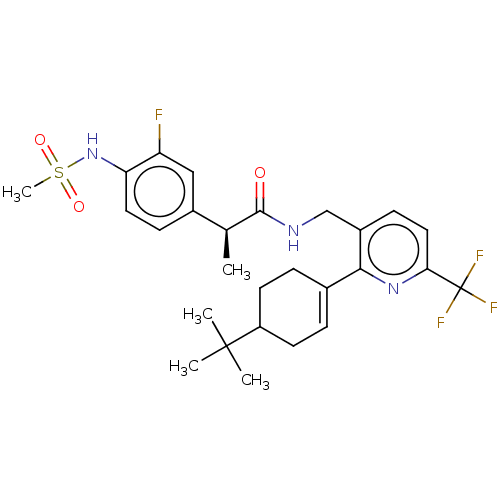 (CHEMBL3314409) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced increase in intracellular Ca2+ level by FLIPR a... | Bioorg Med Chem Lett 24: 4039-43 (2014) Article DOI: 10.1016/j.bmcl.2014.05.074 BindingDB Entry DOI: 10.7270/Q2B859RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50046394 (CHEMBL3314409) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced increase in intracellular Ca2+ level by FLIPR a... | Bioorg Med Chem Lett 24: 4039-43 (2014) Article DOI: 10.1016/j.bmcl.2014.05.074 BindingDB Entry DOI: 10.7270/Q2B859RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576913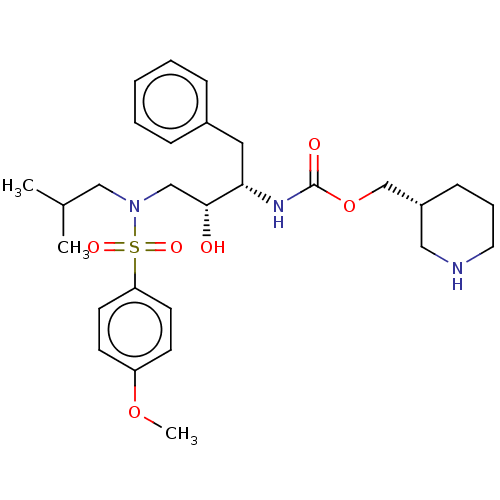 (CHEMBL4868812) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50046396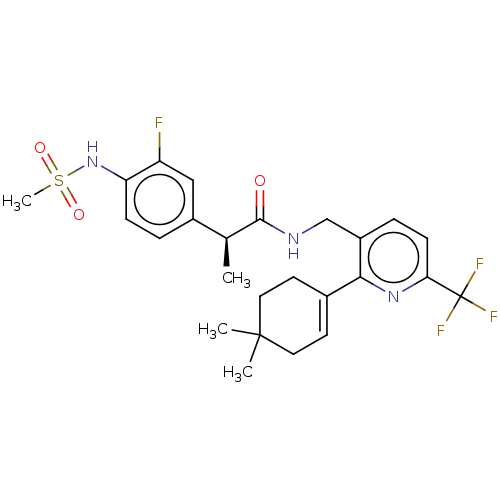 (CHEMBL3314411) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced increase in intracellular Ca2+ level by FLIPR a... | Bioorg Med Chem Lett 24: 4039-43 (2014) Article DOI: 10.1016/j.bmcl.2014.05.074 BindingDB Entry DOI: 10.7270/Q2B859RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284986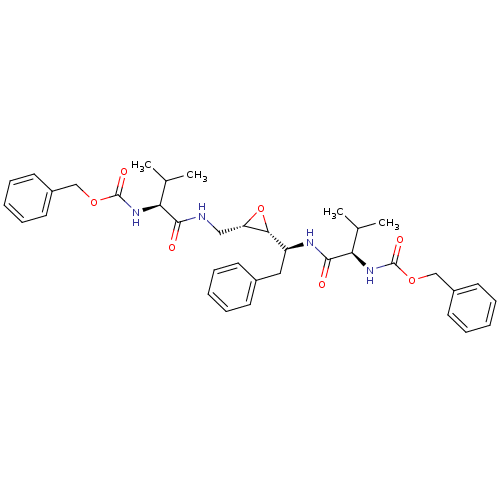 (CHEMBL55197 | [(R)-1-((S)-1-{(2R,3S)-3-[((S)-2-Ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition activity against HIV-1 protease | Bioorg Med Chem Lett 5: 1843-1848 (1995) Article DOI: 10.1016/0960-894X(95)00306-E BindingDB Entry DOI: 10.7270/Q2KW5G0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50088020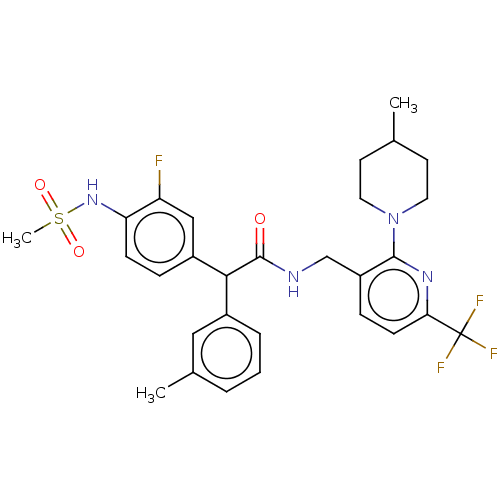 (CHEMBL3427109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay | Bioorg Med Chem Lett 25: 2326-30 (2015) Article DOI: 10.1016/j.bmcl.2015.04.024 BindingDB Entry DOI: 10.7270/Q2Z60QSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50463772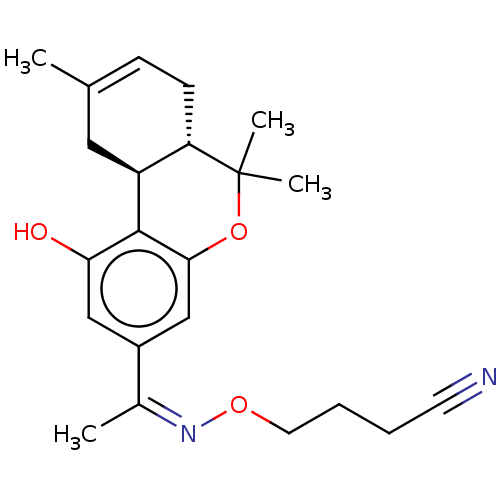 (CHEMBL4239190) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant mouse Cb2 receptor expressed in HEK293 cells | Bioorg Med Chem 26: 4963-4970 (2018) Article DOI: 10.1016/j.bmc.2018.08.003 BindingDB Entry DOI: 10.7270/Q2BG2RNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50576900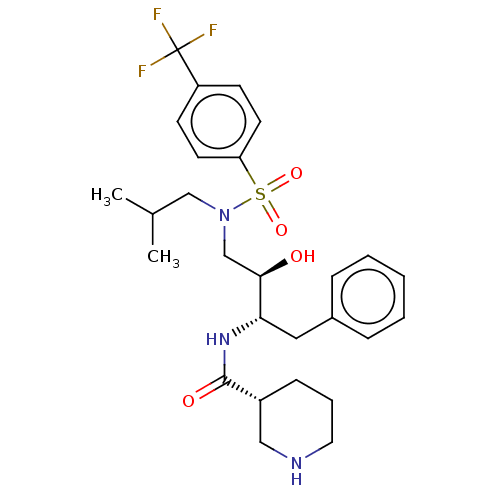 (CHEMBL4852688) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113450 BindingDB Entry DOI: 10.7270/Q2571GTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50046396 (CHEMBL3314411) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced increase in intracellular Ca2+ level by FLIPR a... | Bioorg Med Chem Lett 24: 4039-43 (2014) Article DOI: 10.1016/j.bmcl.2014.05.074 BindingDB Entry DOI: 10.7270/Q2B859RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8977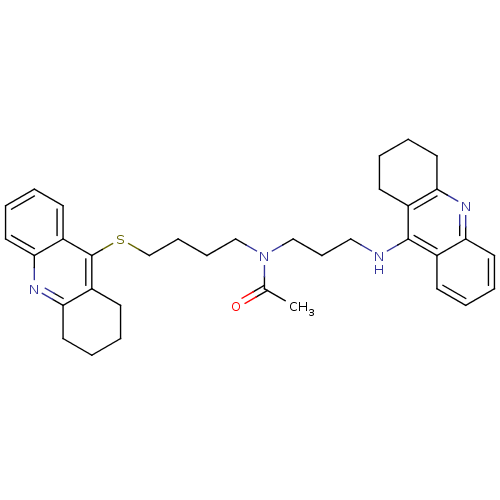 (CHEMBL175949 | N-[3-(1,2,3,4-Tetrahydroacridin-9-y...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50604026 (CHEMBL5192384) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01670 BindingDB Entry DOI: 10.7270/Q2VH5SX2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50231952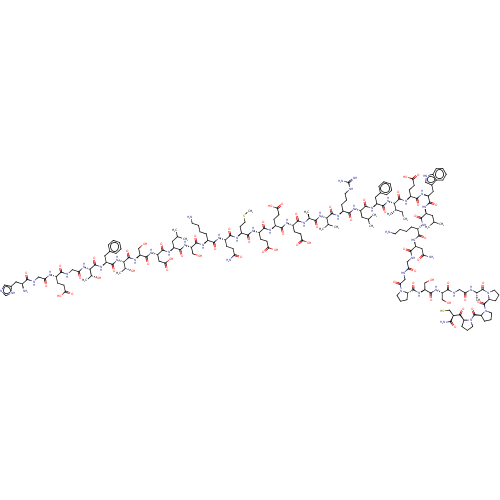 (CHEMBL4081554) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Displacement of [125I]-GLP (7 to 36 residues) from human GLP1R expressed in CHO cell membranes incubated for 30 mins measured after 10 hrs by scintil... | Eur J Med Chem 127: 703-714 (2017) Article DOI: 10.1016/j.ejmech.2016.10.044 BindingDB Entry DOI: 10.7270/Q2T155W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288943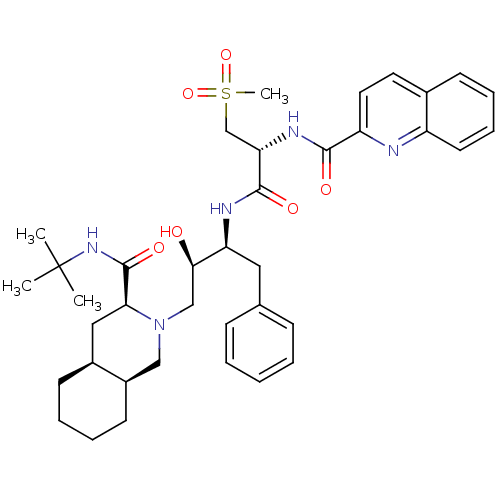 (CHEMBL154519 | Quinoline-2-carboxylic acid {(R)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052442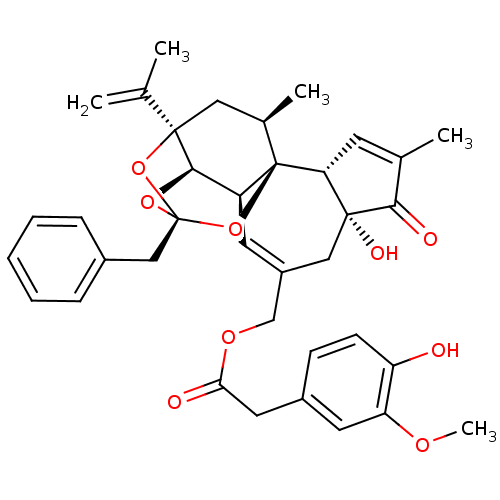 ((4-Hydroxy-3-methoxy-phenyl)-acetic acid (2R,3S,3a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding to Rat Vanilloid receptor 1 (VR1) expressing CHO cells compared to capsacin | J Med Chem 46: 3116-26 (2003) Article DOI: 10.1021/jm030089u BindingDB Entry DOI: 10.7270/Q2SB4551 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032698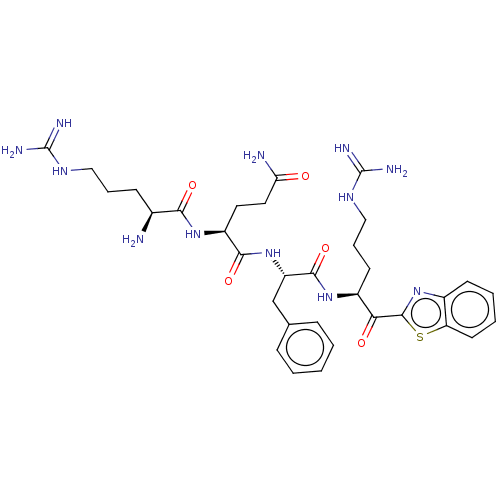 (CHEMBL3354676 | N-0130) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... | Citation and Details Article DOI: 10.1038/s41586-022-04661-w BindingDB Entry DOI: 10.7270/Q2NP27M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 55896 total ) | Next | Last >> |