

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM97445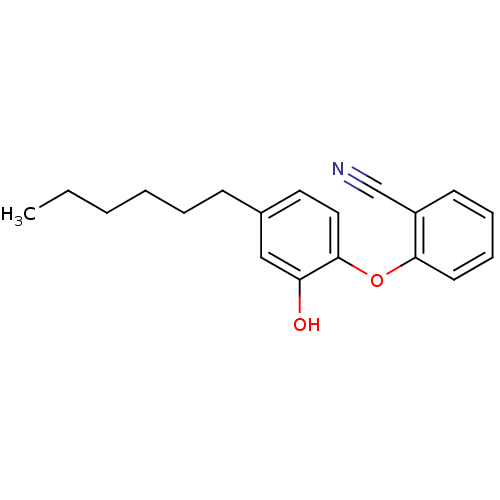 (PT119) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus enoyl ACP reductase | Eur J Med Chem 88: 66-73 (2014) Article DOI: 10.1016/j.ejmech.2014.09.008 BindingDB Entry DOI: 10.7270/Q25T3N3S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <0.200 | <-55.4 | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50434481 (CHEMBL2385132 | CHEMBL2385133 | CHEMBL2385134) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Antagonist activity at OX2 receptor (unknown origin) | Bioorg Med Chem Lett 23: 3389-92 (2013) Article DOI: 10.1016/j.bmcl.2013.03.079 BindingDB Entry DOI: 10.7270/Q279461B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.5 | -50.4 | 13 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM178095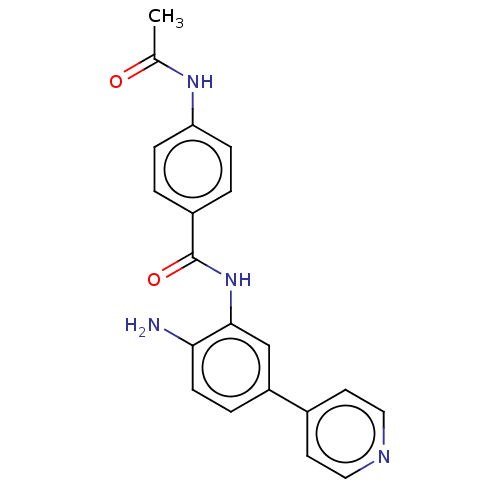 (BRD2492) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.6 | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50076216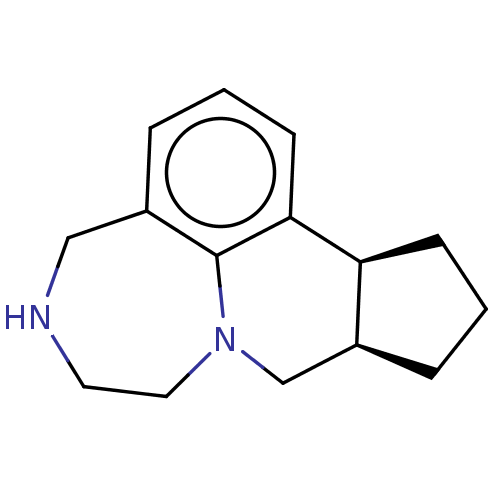 (Vabicaserin) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [125I]DOI from human 5-HT2C receptor | J Med Chem 57: 1488-94 (2014) Article DOI: 10.1021/jm401802f BindingDB Entry DOI: 10.7270/Q2PC35CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50401014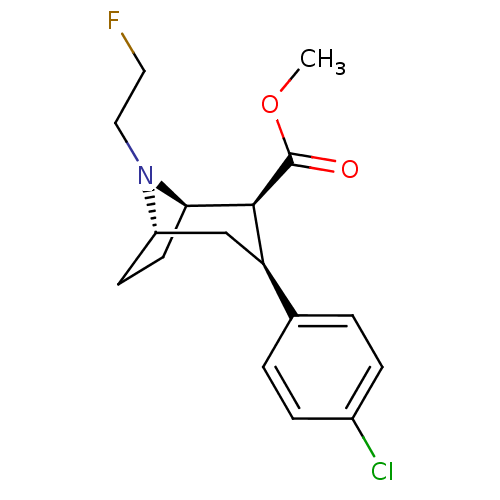 (CHEMBL2206307) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University Curated by ChEMBL | Assay Description Binding affinity to DAT | Bioorg Med Chem Lett 22: 679-82 (2011) Article DOI: 10.1016/j.bmcl.2011.10.053 BindingDB Entry DOI: 10.7270/Q2ZW1N2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50076245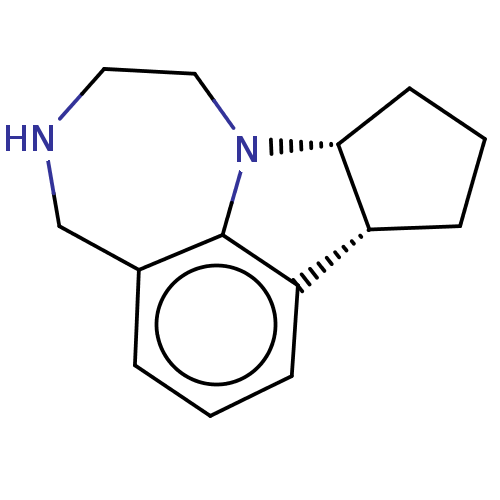 (CHEMBL1628670 | US10544152, Cpd # 1 | WAY-163909) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [125I]DOI from human 5-HT2C receptor | J Med Chem 57: 1488-94 (2014) Article DOI: 10.1021/jm401802f BindingDB Entry DOI: 10.7270/Q2PC35CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM178095 (BRD2492) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | -44.8 | 19 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19422 (4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | -43.4 | 46 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM178100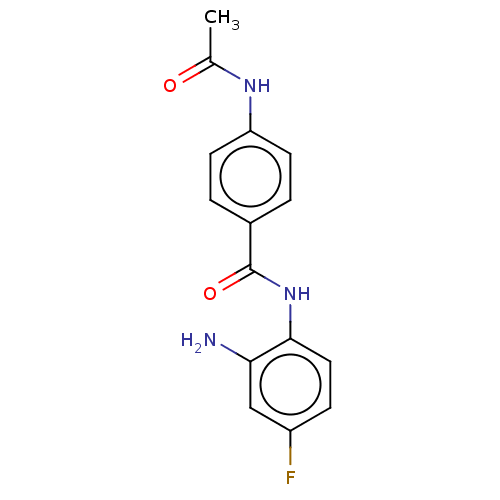 (BRD3308 | US11377423, Cmpd 1) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 29 | -43.0 | 64 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19422 (4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | -42.4 | 41 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19422 (4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 223 | -38.0 | 147 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 500 | -36.0 | 398 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM178100 (BRD3308 | US11377423, Cmpd 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 5.10E+3 | -30.2 | 1.08E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM178100 (BRD3308 | US11377423, Cmpd 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 6.30E+3 | -29.7 | 1.15E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM178095 (BRD2492) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.40E+4 | -27.7 | 2.08E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM323704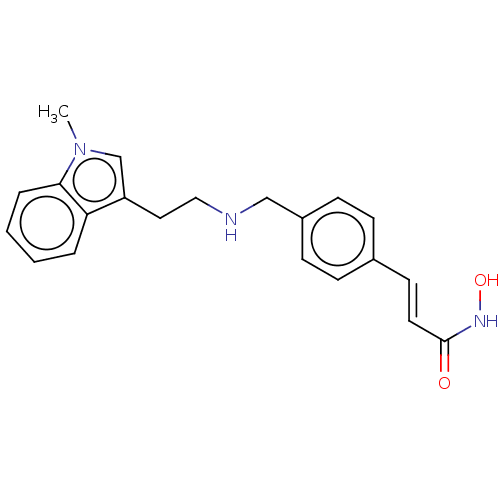 (US10188756, Compound CN107) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM323709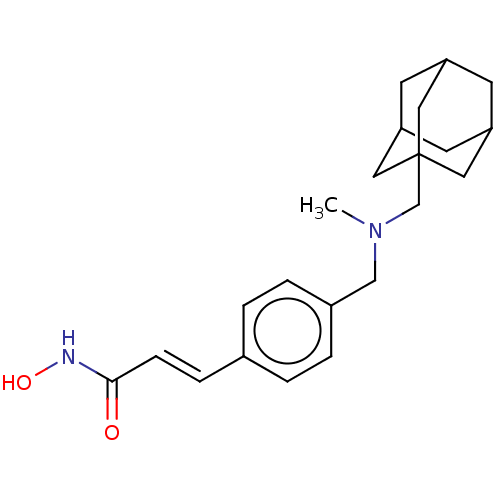 (US10188756, Compound CN133 | US11207431, Martinost...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM323704 (US10188756, Compound CN107) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM323717 (US10188756, Compound CN148) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM323720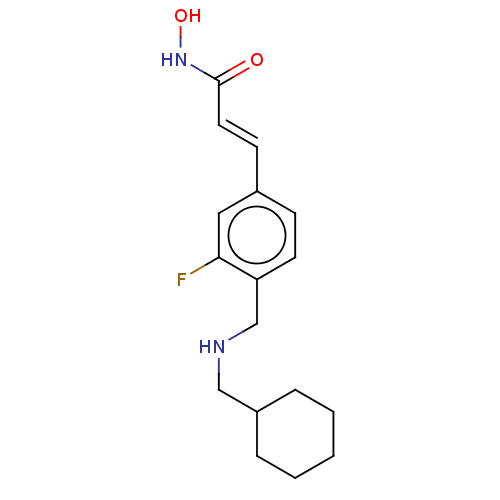 (US10188756, Compound CN166) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM323709 (US10188756, Compound CN133 | US11207431, Martinost...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM323718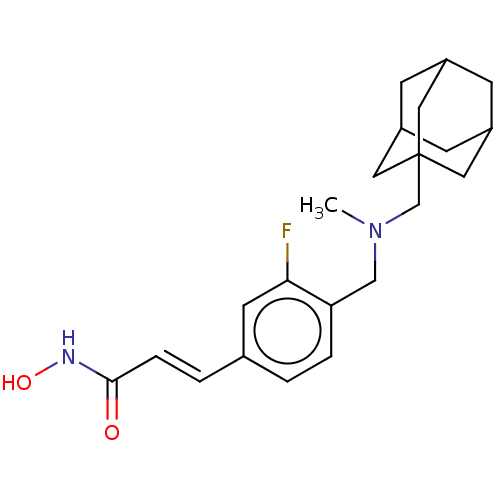 (US10188756, Compound CN149 | US11207431, Example B) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM323718 (US10188756, Compound CN149 | US11207431, Example B) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM323717 (US10188756, Compound CN148) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM323707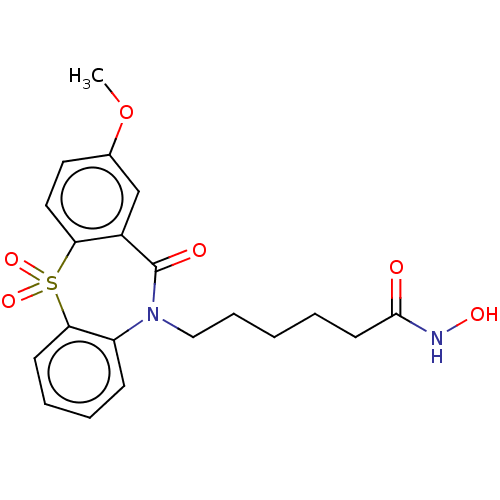 (US10188756, Compound CN113) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM323709 (US10188756, Compound CN133 | US11207431, Martinost...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM323708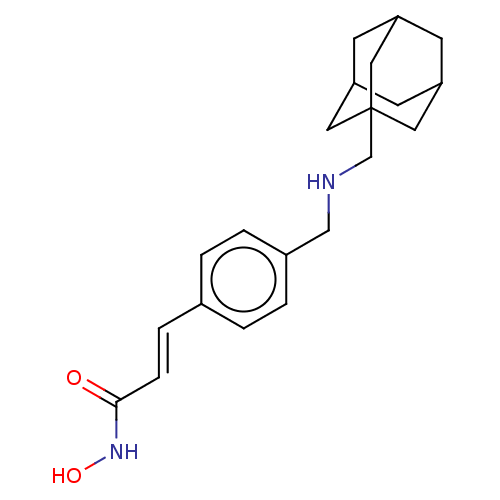 (US10188756, Compound CN132) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM323706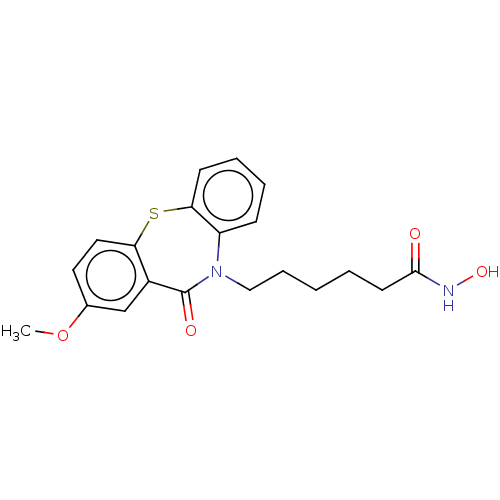 (US10188756, Compound CN112) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM323708 (US10188756, Compound CN132) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM323707 (US10188756, Compound CN113) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM323706 (US10188756, Compound CN112) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM323704 (US10188756, Compound CN107) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM323707 (US10188756, Compound CN113) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM323704 (US10188756, Compound CN107) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM323720 (US10188756, Compound CN166) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM323708 (US10188756, Compound CN132) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM323712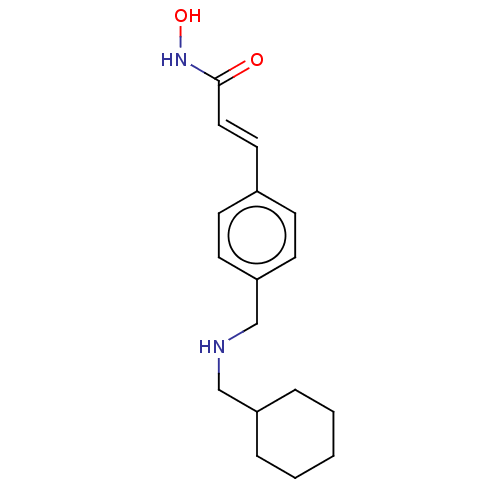 (US10188756, Compound CN143) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50353238 (CHEMBL1830399 | US10188756, Compound CN101) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50296249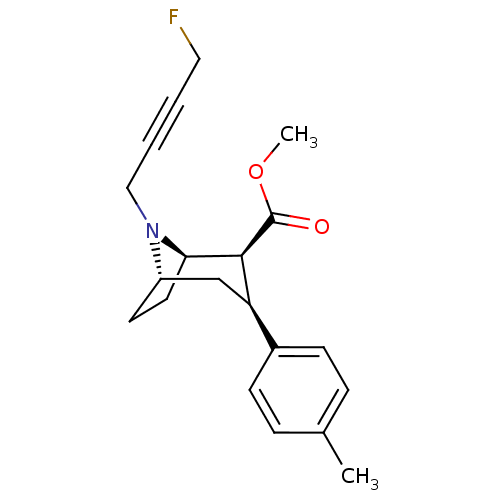 ((1R,2S,3S,5S)-methyl 8-(4-fluorobut-2-ynyl)-3-p-to...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University Curated by ChEMBL | Assay Description Displacement of [3H]WIN35,428 from human DAT expressed in HEK293 cells | Bioorg Med Chem Lett 22: 679-82 (2011) Article DOI: 10.1016/j.bmcl.2011.10.053 BindingDB Entry DOI: 10.7270/Q2ZW1N2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50434481 (CHEMBL2385132 | CHEMBL2385133 | CHEMBL2385134) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Antagonist activity at OX2 receptor (unknown origin) | Bioorg Med Chem Lett 23: 3389-92 (2013) Article DOI: 10.1016/j.bmcl.2013.03.079 BindingDB Entry DOI: 10.7270/Q279461B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM323707 (US10188756, Compound CN113) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM323719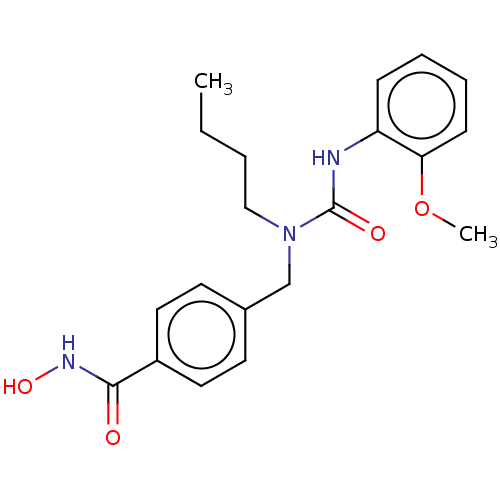 (US10188756, Compound CN165) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.13 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 283 total ) | Next | Last >> |